Summary
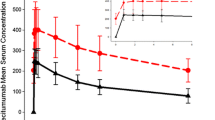
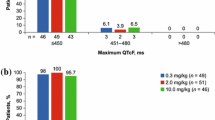
Purpose The study evaluated the potential effect of dacomitinib, a small molecule epidermal growth factor receptor (EGFR) inhibitor, on the electrocardiogram (ECG) parameters in adult patients with advanced non-small cell lung cancer enrolled in a multicenter, open-label, phase 2 study. Methods Patients received dacomitinib for six doses of 45 mg every 12 h in a 7-day lead-in cycle (cycle 0), then 60 mg every 12 h for six doses in a 14-day cycle (cycle 1). Clock time-matched triplicate ECGs were performed at 0, 2, 4, 6, 8 and 10 h on day 1 (baseline) and day 4 of cycle 0, and prior to dose on days 1 and 4 of cycle 1. The QT interval was corrected for heart rate using Fridericia’s correction (QTcF) and a study specific correction factor (QTcS). Results Thirty-two patients in the study comprised the QTc-evaluable population. Dacomitinib had no effect on the heart rate. The upper limits of the 95% confidence interval (CI) for the mean change from baseline in QTcF and QTcS were < 10 ms at all time points. A lack of relationship between plasma concentrations of dacomitinib or total active moiety on QTcF and QTcS was evidenced. All upper 90% CIs of the PR intervals were < 200 ms, although a small mean increase from baseline (2.7–6.6 ms) was observed. Conclusions There was a lack of a clinically relevant effect of dacomitinib on ECG parameters at dacomitinib concentrations comparable to those obtained at its highest therapeutic dosing regimen of 45 mg once daily. ClinicalTrials.gov identifier: NCT01858389.


Similar content being viewed by others
References
Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE, Althaus IW, Gandhi L, Shapiro GI, Nelson JM, Heymach JV, Meyerson M, Wong KK, Janne PA (2007) PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 67(24):11924–11932. https://doi.org/10.1158/0008-5472.CAN-07-1885
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 18(11):1454–1466. https://doi.org/10.1016/S1470-2045(17)30608-3
Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Lee M, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Wu YL (2018) Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 36(22):2244–2250. https://doi.org/10.1200/JCO.2018.78.7994
VIZIMPRO® (dacomitinib) tablets: US prescribing information (2018) Pfizer, Inc.; 2018
Kloth JS, Pagani A, Verboom MC, Malovini A, Napolitano C, Kruit WH, Sleijfer S, Steeghs N, Zambelli A, Mathijssen RH (2015) Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer 112(6):1011–1016. https://doi.org/10.1038/bjc.2015.82
Sager PT, Nebout T, Darpo B (2005) ICH E14: a new regulatory guidance on the clinical evaluation of QT/QTc internal prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Drug Inf J 39(4):387–394. https://doi.org/10.1177/009286150503900407
Darpo B, Sarapa N, Garnett C, Benson C, Dota C, Ferber G, Jarugula V, Johannesen L, Keirns J, Krudys K, Ortemann-Renon C, Riley S, Rogers-Subramaniam D, Stockbridge N (2014) The IQ-CSRC prospective clinical phase 1 study: “can early QT assessment using exposure response analysis replace the thorough QT study?”. Ann Noninvasive Electrocardiol 19(1):70–81. https://doi.org/10.1111/anec.12128
Yu HA, Ahn MJ, Cho BC, Gerber DE, Natale RB, Socinski MA, Giri N, Quinn S, Sbar E, Zhang H, Giaccone G (2017) Phase 2 study of intermittent pulse dacomitinib in patients with advanced non-small cell lung cancers. Lung Cancer 112:195–199. https://doi.org/10.1016/j.lungcan.2017.08.017
Janne PA, Ou SH, Kim DW, Oxnard GR, Martins R, Kris MG, Dunphy F, Nishio M, O'Connell J, Paweletz C, Taylor I, Zhang H, Goldberg Z, Mok T (2014) Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial. Lancet Oncol 15(13):1433–1441. https://doi.org/10.1016/S1470-2045(14)70461-9
Ruiz-Garcia A, Masters JC, Mendes da Costa L, LaBadie RR, Liang Y, Ni G, Ellery CA, Boutros T, Goldberg Z, Bello CL (2016) Effect of food or proton pump inhibitor treatment on the bioavailability of dacomitinib in healthy volunteers. J Clin Pharmacol 56(2):223–230. https://doi.org/10.1002/jcph.588
Janne PA, Boss DS, Camidge DR, Britten CD, Engelman JA, Garon EB, Guo F, Wong S, Liang J, Letrent S, Millham R, Taylor I, Eckhardt SG, Schellens JH (2011) Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res 17(5):1131–1139. https://doi.org/10.1158/1078-0432.CCR-10-1220
Acknowledgments
The authors acknowledge all of the A7471047 patients and their families, the investigators and the study sites staff.
Editorial support was provided by Michelle Daniels, inScience Communications, Springer Healthcare (Philadelphia, PA, USA) and funded by Pfizer.
Funding
This study was sponsored by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
WT, KW and KP are employees of and own stock in Pfizer Inc. NG and SQ were employees of Pfizer Inc. during the analysis of the manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 299 kb)
Rights and permissions
About this article
Cite this article
Tan, W., Giri, N., Quinn, S. et al. Evaluation of the potential effect of dacomitinib, an EGFR tyrosine kinase inhibitor, on ECG parameters in patients with advanced non-small cell lung cancer. Invest New Drugs 38, 874–884 (2020). https://doi.org/10.1007/s10637-019-00887-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00887-0