Abstract
Introduction
Dapagliflozin is a first-in-class sodium-glucose transporter 2 (SGLT2) inhibitor under investigation for the treatment of type 2 diabetes mellitus. A thorough QTc study was conducted, according to International Conference on Harmonization E14 guidelines, to characterize the effect of dapagliflozin on cardiac repolarization.
Methods
The present study was a double-blind, four-period, placebo-controlled crossover study at a single-center inpatient clinical pharmacology unit. The study enrolled 50 healthy men who were randomized to receive sequences of single doses of dapagliflozin 150 mg, dapagliflozin 20 mg, moxifloxacin 400 mg, and placebo. The sequences were randomized based on the Williams design for a cross-over study to reduce the “carryover” effects from drug-to-drug even with sufficient washout periods. Digital 12-lead electrocardiograms were recorded at nine time points over 24 hours in each period. QT intervals were corrected for heart rate using a study-specific correction factor (QTcX) and Fridericia’s formula.
Results
For dapagliflozin, the upper bound of the one-sided 95% confidence interval (CI) for time-matched, placebo-subtracted, baseline adjusted QTc intervals (ΔΔQTc) was <10 ms. ΔΔQTc was independent of dapagliflozin concentrations. No QTc thresholds >450 ms or QTc increases >30 ms were observed. Moxifloxacin increased the mean QTcX interval by 7.7 ms (lower bound 90% CI, 6.2 ms) over 1–4 hours after dosing, confirming assay sensitivity.
Conclusion
Dapagliflozin, at supratherapeutic doses, does not have a clinically significant effect on the QT interval in healthy subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a worldwide epidemic, with approximately 285 million patients at present and a projected rise to 439 million by 2030.1 This progressive disease typically requires chronic lifestyle and pharmacologic management to maintain effective glycemic control. Given the potential for extensive exposure and continued therapy, investigational drugs for T2DM demand rigorous evaluation for potential long-term safety concerns, including potential effects on cardiac repolarization. Delayed ventricular repolarization, as measured by a prolonged QT interval, has been associated with an increased risk of arrhythmias, including torsades de pointes.2
Dapagliflozin is a first-in-class oral, oncedaily, potent, and highly selective sodium glucose cotransporter 2 (SGLT2) inhibitor being developed for the treatment of T2DM.3,4 Treatment with dapagliflozin promotes urinary glucose excretion by preventing up to 50% of the filtered glucose from being reabsorbed.5,6 Thus, by inhibiting renal glucose reabsorption, SGLT2 inhibition provides a mechanism for improving glycemic control in patients with T2DM that is independent of insulin secretion or insulin action.
The pharmacokinetics of dapagliflozin have been assessed in both healthy volunteers and patients with T2DM.5,6 Dapagliflozin is rapidly absorbed after oral administration, has a terminal elimination half-life (t½) of approximately 16 hours, and is primarily metabolized by UGT1A9 to an inactive O-glucuronide that is eliminated renally. Results from placebo-controlled clinical trials have demonstrated that dapagliflozin improves glycemic control in patients with T2DM and is well tolerated.6–8
The potential effect of dapagliflozin on ventricular repolarization (QT/QTc) was previously investigated in nonclinical assays. Dapagliflozin minimally inhibited the human cardiac delayed rectifier potassium current, coded by the human ether-a-go-go-related gene, by 3.7% at 4 μg/mL, a concentration approximately 800 times greater than the maximum observed plasma drug concentration (Cmax) of a 10 mg dose in humans. The 10 mg dose has been the largest dose assessed in phase 3 dapagliflozin clinical trials.9,10 Likewise, no increase in QTc was observed in dogs given a dose of 120 mg/kg per day (Bristol-Myers Squibb, unpublished results). While the preclinical results suggest there is little risk for QT interval prolongation in humans, concerns about the cardiovascular risk of antidiabetic drugs have recently been raised.11,12 Current recommendations require that all nonarrhythmic compounds undergo clinical evaluation of QT/QTc interval prolongation by means of a thorough QT (TQT) study.2 The purpose of this TQT study was to provide an assessment of the potential for a single dose of dapagliflozin to prolong ventricular repolarization by testing the hypothesis that dapagliflozin does not prolong the QTc interval while ensuring the rigorousness of the conclusion by assessing the ability of moxifloxacin to increase the QTc interval.
Methods
This study was designed and monitored in accordance with ethical principles of Good Clinical Practice as defined by the International Conference on Harmonisation and the Declaration of Helsinki. An Institutional Review Board (Chesapeake Research Review, Inc, Columbia, MD, USA) approved the protocol before trial commencement, and all subjects gave written, informed consent.
Subjects
Inclusion criteria were: healthy men between the ages of 18 and 45 years, with a body mass index between 20 and 28 kg/m2. Exclusion criteria were: an abnormal electrocardiogram (ECG); history of arrhythmia; QT interval corrected for heart rate using Fridericia’s formula (QTcF) >450 ms; use of chronic prescription medication; history or presence of neurologic, hematologic, psychiatric, gastrointestinal, hepatic, or renal disease; consumption of caffeine-containing products within 24 hours of dosing; or history of hypoglycemia. Subjects were to refrain from alcohol consumption during the entire study, strenuous exercise 48 hours before study day 1 of each period, and over-the-counter preparations, including herbal remedies.
Study Design
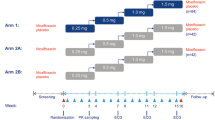
This study, conducted at PAREXEL Clinical Research Unit (Baltimore, MD, USA), was a double-blind, double-dummy, randomized, fourperiod crossover study with an active control. There were four single-dose treatment periods, with a 7–10-day washout period between each dose. Treatment sequences were randomized based on the Williams design for a cross-over study to reduce the potential “carryover” effects from drug to drug even with sufficient washout periods.
Subjects were screened within 21 days of randomization. Each subject received the following four treatments, administered in the order prescribed by the sequence to which the subject had been randomly assigned: Treatment A, dapagliflozin 150 mg; Treatment B, dapagliflozin 20 mg; Treatment C, over encapsulated moxifloxacin 400 mg; and Treatment D, placebo. Subjects underwent a 10-hour fast before dosing, and no food was allowed until 4 hours afterward. Each dose was administered with 240 mL of water.
Safety
Subjects were confined to the clinical research unit for 72 hours after dosing. Vital signs, 12-lead safety ECG, physical examinations, and safety laboratory analyses involving routine hematology, serum chemistry, and urinalysis were obtained throughout the study and at a follow-up examination 5–7 days after the last treatment period. All adverse events (AEs) were evaluated by the investigator and characterized with respect to intensity, duration, relationship to study drug, and outcome.
Pharmacodynamic Measurements
Recording of Digital Electrocardiogram
Twelve-lead continuous digital ECG (dECG) recordings were obtained using a Schiller Cardiovit CS-200 recorder (Schiller AG, Baar, Switzerland) and analyzed by EClysis® (ECG Analysis, AstraZeneca proprietary tool, Wilmington, DE, USA) an automated reading method for dECG intervals with manual adjudication.13 Recordings were taken for 10 minutes before dosing and then resumed 15 minutes after dosing until 3 hours after dosing. From the 0–3 hour recording, 5 minute recordings were selected at 0.5, 1, 2, and 3 hours. Thereafter, 5 minute recordings were taken at 4, 6, 8, 12, and 24 hours after dosing. All dECG measurements were obtained just before blood draws for pharmacokinetic assessment.
Determination of Digital Electrocardiogram Parameters
The following dECG variables were reported: RR interval, PR interval, QRS interval, QTtang interval, and QT interval corrected for heart rate using a study-specific factor (QTcX), QTcF, and Bazett’s correction (QTcB). Ten-second dECGs were extracted every 30 seconds from the predefined 5-minute continuous recording. The extracted data were averaged to arrive at a mean for each time point. The QTtang interval is the QT interval measured by Eclysis® from the beginning of the Q wave to the intercept between the isoelectric line and the regression line, derived on the T-wave downstroke for values between 80% and 20% of the T-top amplitude.
The primary variable was QTcX, which was derived from the dECG using a study-specific correction factor. QTcX was calculated by the equation QTcX=QTtang/RRb, with the QTtang interval expressed in milliseconds and the RR interval in seconds.14 The correction factor b was estimated using a linear mixed-effect model with volunteer as a random effect. The dependency between the QTtang interval and the RR interval was assumed to be described by:

where a was a random subject effect. The estimate was based on all predose measurements from all periods. The QTc interval calculated by QTcF used b=1/3 and by QTcB used b=1/2.
Pharmacokinetic Measurements
For dapagliflozin and its metabolite (BMS-801576), the following single-dose pharmacokinetic parameters were derived from the plasma concentration versus time data: area under the plasma concentration versus time curve from time zero to infinity (AUC), Cmax, time to Cmax (tmax), and t½. AUC was determined using the linear trapezoidal rule, while Cmax and tmax were determined by visual inspection of the plasma concentration versus time curve. The t½ was calculated as 0.693/λz, where λz was the terminal elimination rate constant derived from the log-linear regression of the terminal portion of the plasma concentration versus time curve.
Bioanalytical Methods
Assays for plasma concentrations of dapagliflozin and BMS-801576 were performed by ATLANBIO (Saint-Nazarine, France) using liquid chromatography tandem mass spectrometry detection. The between-run variability and within-run variability for the analytical quality controls of dapagliflozin were 0.0% and ≤8.7%, respectively, for the coefficient of variation, with deviations from the nominal concentrations of ±3.6%. The respective numbers for BMS-801576 were ≤3.6% and ≤9.5%, and ±7.3%. For both dapagliflozin and BMS-801576, the assay range representing the lower and upper limits of quantification in plasma was 1.0–500 ng/mL.
Statistical Analysis
The primary comparison was a test of noninferiority of dapagliflozin 150 mg compared with placebo for the mean, time-matched differences in change from baseline in QTcX. All one-sided 95% confidence intervals (CIs) needed to be <10 ms at each of the nine time points examined. Assuming a drug effect of 2 ms and a standard deviation of within-subject changes from baseline (ΔQTc) of 8.5 ms, 36 subjects were required for 90% power for each of the nine time points to be <10 ms. The QTcX analysis set consisted of all volunteers who had data available for all four periods, including the baseline.
Three different contrasts (dapagliflozin 150 mg vs. placebo; dapagliflozin 20 mg vs. placebo; and moxifloxacin 400 mg vs. placebo) were estimated in the same model. The QTcX at time points 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 hours was analyzed by repeated measures analysis of covariance. The treatment effect of dapagliflozin with its upper bound of a two-sided 90% CI was evaluated against the margin of 10 ms at each of the nine time points.
To test for assay sensitivity, a linear contrast comparing moxifloxacin versus placebo from the primary analysis model on mean QTcX over 1, 2, 3, and 4 hours after dosing was analyzed. A two-sided 90% CI was constructed for this mean time period. The lower bound of the two-sided 90% CI was evaluated against the threshold of 5 ms.
The QT versus dapagliflozin concentration analysis used a linear mixed-effect model. The null hypothesis of zero-slope was tested with a two-sided t-test at the 5% significance level. If the slope was significant, the predicted ΔΔQTcX and its corresponding upper 90% two-sided CI bound were to be computed at the Cmax of the therapeutic dose of dapagliflozin.
Results
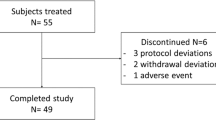
A total of 50 healthy men were randomized and received study drug. Enrolled subjects ranged in age from 19 to 44 years (mean, 32 years) and in BMI from 20 to 28 kg/m2 (mean 25 kg/m2). They were classified by race as white, 14 (28%); black, 34 (68%); Asian, 1 (2%), and American Indian or Alaska Native, 1 (2%). Thirteen subjects discontinued from the study. Of those 13 subjects, six were unwilling to continue the protocol, and three were removed for severe noncompliance with the protocol. One subject was withdrawn for AEs (pharyngeal pain, myalgias, and headache). Two subjects were withdrawn at the request of the investigator, one for pruritus and urticaria, and the other because he developed a resting heart rate <50 beats/min before dosing in one period. The final subject withdrawn from the study developed hypertension; he later admitted to stopping hypertension medications before randomization, and thus was ineligible for inclusion in the study.
QTc Interval
The value of the study-specific correction factor (b) for QTcX was 0.209. The placebo-subtracted, baseline-adjusted mean QTcX for dapagliflozin (20 mg and 150 mg) was <5 ms at each time point, and all upper bounds of the two-sided 90% CI for the contrasts were <10 ms (Figure 1). The maximum placebo-subtracted, baseline-adjusted mean effect of dapagliflozin 150 mg for QTcX was 1.2 ms at 3 hours after dosing, and the upper bound of the CI was 3.4 ms. For dapagliflozin 20 mg, the maximum placebo-corrected, baseline-adjusted mean effect for QTcX was 2.3 ms at 8 hours after dosing (90% CI, 0.0 to 4.6) (Table 1).
Similar findings were observed using QTcF. The maximum placebo-subtracted, baseline-adjusted mean effect for QTcF was 2.8 ms (90% CI, 0.6 to 5.1) and 1.6 ms (90% CI, −0.7 to 3.9) for the 20 mg and 150 mg dapagliflozin doses, respectively (Table 1). The findings for QTcB were consistent with those for QTcF.
For moxifloxacin, with the exception of the contrast at 0.5 and 12 hours after dosing, all point estimates for the contrasts between moxifloxacin 400 mg and placebo for QTcX were >5 ms, and the lower bounds of the 90% CI were >5 ms at 3 and 4 hours. The maximum placebo-corrected, baseline-adjusted mean effect of moxifloxacin 400 mg for QTcX was 9.7 ms at 4 hours after dosing, and the lower bound of the two-sided 90% CI was 7.5 ms (Table 1).
Assay sensitivity was demonstrated by comparing the moxifloxacin and placebo treatments using the primary analysis model for QTcX averaged over the 1-, 2-, 3-, and 4-hour postdose time points. The average 1–4 hour effect of moxifloxacin on the placebo-corrected, baseline-adjusted QTcX was 7.7 ms, with a lower bound of the 90% CI of 6.2 ms.
QTc Interval Increase From Baseline and Absolute QTc Interval Prolongation
Figure 2 presents a plot of the shift from baseline to the maximum observed QTcX interval by treatment.There was no increase from baseline >30 ms for QTcX over the 24-hour period following dapagliflozin or placebo administration. Only one subject had an increase from baseline >30 ms for QTcX after administration of moxifloxacin 400 mg (3-hour postdose time point).
Two subjects had an increase from baseline of 30 ms for QTcF over the 24 hours of treatment with moxifloxacin. These values, 30.2 and 31.1 ms, occurred at 4 and 3 hours after dosing, respectively. No subject had an increase from baseline for QTcF >30 ms for any dose of dapagliflozin. No subjects had a QTcX or QTcF value >450 ms during the study.
Concentration-QT Relationship
Mean individual QTcX intervals versus dapagliflozin (20 mg and 150 mg) plasma concentration are presented in Figure 3. There was no apparent concentration-dependent effect of dapagliflozin on QTcX. The estimated slope was −0.29 ms/μg per mL, and the test of the null hypothesis of zero slopes was not statistically significant (P=0.51).
Effect on RR, QRS, and PR Intervals
There was little effect of dapagliflozin on heart rate. The mean change from baseline in the RR interval at each time point ranged from 14.8 to −138.4 ms for dapagliflozin 150 mg, 2.9 to −135.9 ms for dapagliflozin 20 mg, 8.4 to −127.8 ms for moxifloxacin 400 mg, and 20.4 to −128.1 ms for placebo. Results were similar for QRS and PR intervals regardless of treatment (Table 2).
Pharmacokinetics
Pharmacokinetic parameters of dapagliflozin and BMS-801576 are presented in Table 3. Dapagliflozin was rapidly absorbed after oral administration, with a median time to Cmax of 1 hour for both the 20 mg and 150 mg doses. The geometric Cmax and AUC values appeared to increase in a dose-proportional manner. The geometric mean t½ was 14.8 hours after dapagliflozin 150 mg administration and 13.8 hours after 20 mg administration. Maximum plasma concentrations of BMS-801576 were reached at a median time of 2 hours after dapagliflozin administration.
Safety
There were no deaths during this study. One subject experienced a serious AE, a transient ischemic attack, approximately 8 days after receiving moxifloxacin in the final period. This AE was not considered by the investigator to be drug related.
Nineteen subjects had AEs during the study, including nine subjects after dapagliflozin administration. Headache was the only AE reported by more than one subject after dapagliflozin administration, occurring in three and two subjects who received the 150 mg and 20 mg dose, respectively. Other AEs reported after administration of dapagliflozin 150 mg were conjunctivitis, diarrhea, myalgia, pharyngeal pain, and tinea versicolor; those after the 20 mg administration were nausea, palpitations, paresthesia, pruritus, and urticaria. All AEs were of mild intensity. Overall, 11.4%, 12.2%, 12.2%, and 10.9% of subjects experienced an AE after administration of dapagliflozin 150 mg, dapagliflozin 20 mg, moxifloxacin 400 mg, and placebo, respectively.
Discussion
The assessment of a drug to delay cardiac repolarization, as assessed by the QT/QTc interval, is now required for compounds in development.19 The objective of this study was to provide a rigorous assessment of the potential for dapagliflozin to prolong ventricular repolarization in human subjects at both presumed therapeutic and supratherapeutic doses. The primary endpoint compared the change in QTc interval from predose baseline values between active and placebo treatment.
The mean QTc intervals were not prolonged using a study-specific correction method (QTcX) or the standard heart rate correction method (Fridericia’s). With both methods, all upper bounds of the 90% CI for the difference in mean QTc interval between either dose of dapagliflozin and placebo were <10 ms. Therefore, either correction method resulted in a negative TQT study, defined as one in which the upper bound of the 95% one-sided CI for the largest time-matched mean effect of the drug on the QTc interval excludes 10 ms. This definition is meant to imply that the mean effect of a study drug on the QTc interval is not >5 ms.15 Both doses of dapagliflozin, using either heart rate correction method, met this requirement, as the largest placebo-subtracted, baseline-adjusted mean QTc interval for any dose or method of heart rate correction was only 2.8 ms. No subject treated with dapagliflozin had outlier values, namely an increase in QTcX or QTcF from baseline >30 ms or a QTcX or QTcF value >450 ms. The lack of outliers provides further confidence that dapagliflozin does not prolong the QTc interval.
In addition to the standard analysis of a TQT study, this study also incorporated concentration-QT modeling.15 The QTc versus concentration plot for dapagliflozin was essentially flat, with a slope that was not significantly different from zero. As ΔΔQTcX was independent of dapagliflozin plasma concentration, no further concentration/QT modeling was explored. However, the concentration-QT modeling was consistent with the earlier phase 1 studies, which incorporated greater doses of dapagliflozin than the supratherapeutic dose used in this study. As some have noted,16 if the concentration-QT modeling in early clinical studies suggests that the TQT will be negative, the standard therapeutic dose can be omitted, allowing one arm of the study to be dropped. Although the anticipated maximum therapeutic dose was included, it could have been omitted without altering the conclusions on cardiac repolarization by dapagliflozin.
The sensitivity of the assay to detect small increases in QTc interval was established with the active control, moxifloxacin. Although assay sensitivity was originally defined as a significant increase in QTc interval by the positive control that was consistent with its known effect on the QT interval,2 more recent requirements have proposed a prospective, more quantitative assessment that takes into account what is known about the response and time course of the positive control.15,17 For moxifloxacin, the lower bound of the 90% CI for one time point for ΔΔQTcX should be >5 ms. The time course for the QTc effect of moxifloxacin is known, and the peak effect occurs around the time of Cmax (ie, no hysteresis).18 Because moxifloxacin pharmacokinetic samples were not analyzed in this study, an average approach for Cmax was chosen because the mean tmax was unknown. It was prospectively decided to average the ΔΔQTcX for moxifloxacin over the 1–4-hour time points that encompass the tmax range for moxifloxacin. The ΔΔQTcX for the 1–4-hour average was 7.7 ms, with a lower bound of the 90% CI of 6.2 ms. The maximum baseline-adjusted, placebo-subtracted QTcX for moxifloxacin was 9.7 ms, and the shape of the ΔΔQTcX versus time curve for moxifloxacin was consistent with previously published data.18 Therefore, assay sensitivity was confirmed in a rigorous way, validating the interpretability of the results for dapagliflozin.
Although the discontinuation rate of subjects in this study was greater than expected, it did not impact the results. A four-period crossover trial with a relatively long washout contributed to the dropout rate, as half of the discontinued subjects were dropouts and three additional subjects failed to comply with the protocol requirements. Both the dapagliflozin pharmacokinetic parameters and safety profile are consistent with previous results in healthy volunteers.5 The supratherapeutic dose of dapagliflozin appeared to be well tolerated. The percentage of subjects who experienced an AE was similar across the four groups, and all AEs were mild.
The inclusion of only healthy subjects is a limitation of this study. The typical TQT study is conducted in healthy volunteers, hence the results may not equate to potential proarrhythmic liabilities when a drug is used chronically in a high-risk cardiovascular population. This is pointed out in ICH-E14, as the TQT study is not intended to identify drugs as being proarrhythmic.19
Conclusion
Dapagliflozin at doses up to 150 mg was not associated with QT interval prolongation in healthy male subjects. There were no QTc thresholds above 450 ms or QTc interval increases >30 ms, and the QTc interval changes were independent of dapagliflozin concentrations. Based on these data, dapagliflozin at the proposed therapeutic dose of 10 mg/day is not expected to affect cardiac repolarization in patients with diabetes.
References
International Diabetes Federation diabetes atlas. International Diabetes Federation. Available at: http://www.diabetesatlas.org/. Accessed February 16, 2010.
US Food and Drug Administration, Department of Health and Human Services. International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Federal Register. 2005;70:61134–61135.
Han S, Hagan DL, Taylor JR, et al. Dapagliflozin, a selective SGLT2 inhibitor, improves glucose homeostasis in normal and diabetic rats. Diabetes. 2008;57:1723–1729.
Meng W, Ellsworth BA, Nirschl AA, et al. Discovery of dapagliflozin: a potent, selective renal sodiumdependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem.2008;51:1145–1149.
Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–526.
Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–519.
List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657.
Wilding JPH, Norwood P, T’joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers. Applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–1662.
Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233.
Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase III trial. Diabetes Care. 2010;33:2217–2224.
Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581–2586.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471.
Dota C, Skallefell B, Edvardsson N, Fager G. Computer-based analysis of dynamic QT changes: toward high precision and individual rate correction. Ann Noninvasive Electrocardiol. 2002;7:289–301.
Butler K, Wei C, Teng R. Single-dose ticagrelor does not prolong the QT interval in healthy subjects. Int J Clin Pharmacol Ther. 2010;48:643–651.
Garnett CE, Beasley N, Bhattaram VA, et al. Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol. 2008;48:13–18.
Malik M, Garnett CE, Zhang J. Thorough QT studies: questions and quandaries. Drug Saf. 2010;33:1–14.
European Medicines Agency. ICH Topic E 14. The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Step 5. Questions and Answers. European Medicines Agency. Available at: www.emea.europa.eu/pdfs/human/ich/31013308en.pdf. Accessed October 12, 2008.
Bloomfield DM, Kost JT, Ghosh K, et al. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther. 2008;84:475–480.
International Conference on Harmonization of Technical requirements for registration of Pharmaceuticals for Hymna Use. The Clinical Evaluiation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs E-14. Available at: http://www.ich.org/products/guidelines/efficacy/article/efficacyguidelines. Accessed June 3, 2011.
Acknowledgments
Editorial services and graphics support were provided by W. Watkins of PPSI (a PAREXEL company) and were funded by AstraZeneca and Bristol-Myers Squibb. Glenn F. Carlson is the guarantor for this article, and takes responsibility for the ingegrity of the work as a whole.
All authors are employees of AstraZeneca LP, which in partnership with Bristol-Myers Squibb, is developing dapagliflozin as a potential treatment for T2DM.
Clinical trials registration number NCT00688493.
Open Access. This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
To view enhanced content go to www.diabetestherapy-open.com
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Carlson, G.F., Tou, C.K.P., Parikh, S. et al. Evaluation of the effect of dapagliflozin on cardiac repolarization: a thorough QT/QTc study. Diabetes Ther 2, 123–132 (2011). https://doi.org/10.1007/s13300-011-0003-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-011-0003-2