Abstract
For most acute myeloid leukemia (AML) patients, an allogeneic hematopoietic stem cell transplantation (HSCT) offers the highest chance of sustained remissions and long-term survival. At diagnosis, high expression of the AML-associated genes BAALC (brain and acute leukemia, cytoplasmic) and MN1 (meningioma-1) were repeatedly linked to inferior outcomes in patients consolidated with chemotherapy while data for patients receiving HSCT remain limited. Using clinically applicable digital droplet PCR assays, we analyzed the diagnostic BAALC/ABL1 and MN1/ABL1 copy numbers in 302 AML patients. High BAALC/ABL1 and MN1/ABL1 copy numbers associated with common adverse prognostic factors at diagnosis. However, while high diagnostic copy numbers of both genes associated with shorter event free survival (EFS) and overall survival (OS) in patients receiving chemotherapy, there was no prognostic impact in patients undergoing HSCT. Our data suggests that the adverse prognostic impact of high BAALC and MN1 expression are mitigated by allogeneic HSCT. But preHSCT BAALC/ABL1 and MN1/ABL1 assessed in remission prior to HSCT remained prognosticators for EFS and OS independent of the diagnostic expression status. Whether allogeneic HSCT may improve survival for AML patients with high diagnostic BAALC or MN1 expression should be investigated prospectively and may improve informed decisions towards individualized consolidation options in AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous disease for which reliable risk stratifications are needed to individualize treatment strategies [1]. Today, potential consolidation therapies for AML patients in remission after successful induction therapy include intensive chemotherapy cycles alone or an allogeneic hematopoietic stem cell transplantation (HSCT). Through immunological graft-versus-leukemia (GvL) effects, where the donor’s immunocompetent cells are believed to eradicate residual disease [2, 3], allogeneic HSCT remains the treatment option with the highest chance of sustained remissions in most AML patients, albeit the associated morbidity and mortality [1].
The AML-associated genes BAALC (brain and acute leukemia, cytoplasmatic) and MN1 (meningioma-1) have been shown to be physiologically expressed at high levels in myeloid progenitor cells and downregulated during maturation and to promote leukemogenesis through blockage of myeloid differentiation [4,5,6]. While BAALC maps to chromosome band 8q22.3 and was initially identified in AML patients harboring a trisomy 8 [7], MN1 is located on chromosome 22q12.3 and a transcription coactivator firstly described in meningioma pathogenesis [8]. High expression levels of both genes at AML diagnosis have repeatedly been associated with adverse outcomes in both younger [4, 9] and older AML patients [10, 11], especially in the context of a normal karyotype [12,13,14]. Furthermore, the expression levels of both genes have been identified as feasible markers for residual disease in AML patients in complete remission (CR) independent of the applied consolidation therapy [15,16,17,18,19].
However, the majority of the studies investigating the prognostic impact of diagnostic BAALC and MN1 expression levels focused on patients consolidated with standard cytarabine-based chemotherapies or autologous HSCT in which either none or only a small number of the analyzed individuals received allogeneic HSCT for consolidation. Only one recently published manuscript analyzed the data of 71 AML patients from The Cancer Genome Atlas (TCGA) and suggested no prognostic impact of BAALC expression levels at diagnosis in patients receiving allogeneic HSCT [20]. This study was restricted by patient numbers and limited information on the applied treatments (e.g., intensity of conditioning regimens). Here, we analyzed the prognostic significance of the differential diagnostic BAALC and MN1 expression levels in a well-defined cohort of AML patients who were either treated with chemotherapy alone or received an allogeneic HSCT as consolidation therapy at our institution. For better reproducibility, and to develop a feasible clinical routine assay, we adopted a digital droplet PCR (ddPCR) technology for absolute diagnostic BAALC and MN1 quantification [21].
Subjects and methods
Patients and treatment
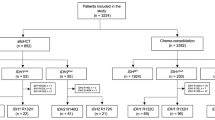
We analyzed the diagnostic bone marrow material of 302 AML patients who were treated at the University of Leipzig between November 2000 and October 2018 for their BAALC/ABL1 and MN1/ABL1 copy numbers. Median age at diagnosis was 62.2 years (range 14.5–87.8 years). All nonAPL karyotypes were included in the analysis. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. For all 302 patients, associations of diagnostic BAALC/ABL1 and MN1/ABL1 copy numbers with baseline clinical and genetic factors were assessed (“association set”). Of the 207 patients who received an allogeneic HSCT for consolidation therapy, 186 patients were transplanted in CR or CR with incomplete peripheral recovery (CRi) and were eligible for outcome analyses. Of the 95 patients who were treated with chemotherapy alone, 77 patients received at least one cycle of intensive chemotherapy and survived 28 days after diagnosis and were also included in the outcome analyses. Thus, outcome was evaluated for 263 AML patients (“outcome set”). For details, please see the flow chart in Supplementary Fig. S1.
All patients in the outcome set received age-dependent standard cytarabine–based chemotherapy protocols (please see Supplementary Information for details). Conditioning regimens in the 186 patients receiving allogeneic HSCT were either myeloablative (n = 47, using 2 × 60 mg/kg body weight cyclophosphamide and 12 Gray [Gy] total body irradiation) or nonmyeloablative (n = 139, using 3 × 30 mg/m2 fludarabine and 2 Gy total body irradiation). Median time from diagnosis to allogeneic HSCT was 139 days. Reasons for the chosen consolidation therapy as well as conditioning regime in case of allogeneic HSCT are given in the Supplementary Information. All transplanted patients received granulocyte colony stimulating factor–stimulated peripheral blood stem cells. Stem cell donors were human leukocyte antigen (HLA) matched related (n = 42, 23%), HLA matched unrelated (n = 108, 58%) or HLA mismatched unrelated (n = 36, 19%). Further patients’ characteristics are shown in Table 1 and Supplementary Table S1 and S2. Median follow-up after diagnosis was 5.0 years for patients alive.
Assessment of BAALC/ABL1 and MN1/ABL1 copy numbers and cutoff point definitions
For all patients, absolute BAALC and MN1 copy numbers at diagnosis were assessed using specific ddPCR assay (BioRad, Hercules, California, USA). ddPCR was performed on a QX100 platform (BioRad), and QuantaSoft software (Biorad) was used for raw data processing as previously described [15]. Both genes were normalized to ABL1 copy numbers as internal control. To evaluate the prognostic impact, the median BAALC/ABL1 (absolute 0.2538) and MN1/ABL1 copy numbers (absolute 0.2424) were used to define patients with high or low BAALC/ABL1 and MN1/ABL1 copy numbers at diagnosis. For validation of the ddPCR results, in 110 patients, qRT-PCR was performed to assess BAALC and MN1 expression levels at diagnosis additionally to ddPCR. For details regarding qRT-PCR analysis, please see Supplementary Information.
We previously reported on the prognostic significance of preHSCT BAALC [15] as well as preHSCT MN1 copy numbers [16]. In the here-presented patient population, preHSCT BAALC/ABL1 and preHSCT MN1/ABL1 copy numbers were available in 77 and 76 patients, respectively. The previously published cutoffs were used to define patients with high or low preHSCT BAALC/ABL1 and preHSCT MN1/ABL1 copy numbers [15, 16].
Cytogenetics, molecular marker, and flow cytometry
Diagnostic cytogenetic analyses were performed centrally using standard techniques of banding and in situ hybridization. Bone marrow mononuclear cells at diagnosis were assessed for surface presence of an institutional standard panel as previously described [22]. The mutation status of the CCAAT/enhancer-binding protein alpha (CEBPA), nucleophosmin 1 (NPM1), and FLT3 tyrosine kinase (FLT3-TKD) gene as well as the presence or absence of internal tandem duplications in the FLT3 gene (FLT3-ITD) were evaluated as previously described [23]. For patients with material available, mutation status of 54 genes included in the TruSight Myeloid Sequencing Panel (Illumina) was evaluated at diagnosis as previously described [22, 24]. Patients were grouped according to the ELN2017 genetic classification [1].
Definition of clinical endpoints and statistical analyses
All statistical analyses were performed using the R statistical software platform (version 3.4.3) [25]. Overall survival (OS) was calculated from diagnosis until death from any cause. Event free survival (EFS) was calculated from diagnosis to event (i.e., nonachievement of a CR or CRi after two cycles of chemotherapy, relapse or death from any cause). Associations with baseline clinical, demographic, and molecular features were compared using the Kruskal–Wallis Test and Fisher’s exact tests for continuous and categorical variables, respectively. Survival estimates were calculated using the Kaplan–Meier method, and groups were compared using the log-rank test. Multivariate analyses methods are described in the Supplementary Information.
Results
Comparison of qRT-PCR and ddPCR results
To validate our ddPCR-based expression assays, we compared the results to classical qRT-PCR assays. Results from gene expression analysis by qRT-PCR and copy number analysis by ddPCR correlated well (Spearman correlation coefficient: BAALC r = 0.89 and MN1 r = 0.90, Fig. 1).
Associations of BAALC/ABL1 copy numbers at diagnosis with clinical and genetic characteristics
Patients with high BAALC/ABL1 copy numbers at diagnosis had a lower white blood count at diagnosis (P < .001) and presented with a higher expression of immature surface antigens (i.e., CD34, P < 0.001; CD34+/CD38−, P < 0.001; and CD117, P < 0.001), higher expression of surface antigens indicating T cell differentiation (i.e., CD7, P < 0.001; and CD2, P < 0.001), higher CD13 expression (P = 0.04), but lower expression of other antigens indicating myeloid differentiation (i.e., CD64, P < 0.001; CD11b, P = 0.01; and CD33, P = 0.001) on mononuclear bone marrow cells at diagnosis (Supplementary Table S1). They had a lower frequency of a normal karyotype (P < 0.001) and were more likely to have a core binding factor AML (CBF-AML, P < 0.001) but also to harbor adverse-risk genetics, i.e. del(5)/del(5q) (P = 0.001), del(7)/del(7q) (P = 0.001), a monosomal karyotype (P = 0.02) [26], a complex karyotype (P = 0.02) [1], as well as worse risk according to ELN2017 classification (P < 0.001, Table 1). High BAALC/ABL1 copy numbers also associated with a lower frequency of NPM1 mutations (P < 0.001), FLT3-ITD (P < 0.001), DNMT3A mutations (P = 0.03), by trend TET2 mutations (P = 0.10), and a higher frequency of RUNX1 mutations (P = 0.004), higher MN1/ABL1 copy numbers (P < 0.001), higher GPR56 expression (P < 0.001), and by trend higher EVI1 expression (P = 0.08) at diagnosis.
Associations of MN1/ABL1 copy numbers at diagnosis with clinical and genetic characteristics
Patients with high MN1/ABL1 copy numbers at diagnosis had lower white blood count at diagnosis (P < 0.001) and presented with a higher expression of immature surface antigens (i.e., CD34, P < 0.001; CD34+/CD38−, P < 0.001; and CD117, P < 0.001), higher expression of surface antigens indicating T cell differentiation (i.e., CD2, P < 0.001 and CD7, P < 0.001), higher CD13 (P = 0.007), but lower expression of other antigens indicating myeloid differentiation (i.e., CD33, P < 0.001; CD15, P = 0.05; and CD64, P = 0.001) on mononuclear bone marrow cells at diagnosis (Supplementary Table S1). High MN1/ABL1 copy numbers also associated with a lower frequency of a normal karyotype (P < 0.001) and a higher frequency of CBF-AML (P = 0.001) but also a higher frequency of adverse risk genetics as del(7)/del(7q) (P = 0.001), del(5)/del(5q) (P = 0.01), by trend monosomal karyotype (P = 0.09) and worse risk according to ELN2017 classification (P < 0.001, Table 1). High MN1/ABL1 copy numbers also associated with a lower frequency of NPM1 mutations (P < 0.001), FLT3-ITD (P = 0.004), CEBPA mutations (P = 0.006), by trend TET2 mutations (P = 0.10), as well as a higher frequency of RUNX1 mutations (P = 0.004), higher BAALC/ABL1 copy numbers (P < 0.001), and higher GPR56 expression (P < 0.001) at diagnosis.
Prognostic impact of BAALC/ABL1 and MN1/ABL1 copy numbers at diagnosis
In line with previously published reports, in patients treated with chemotherapy alone, BAALC/ABL1 copy numbers at diagnosis associated with a significantly shorter EFS (P = 0.008, Fig. 2a) as well as shorter OS (P = 0.05, Fig. 2b). In contrast, in patients receiving allogeneic HSCT as consolidation therapy, there was no different EFS (P = 0.60, Fig. 2c) or OS (P = 0.31, Fig. 2d) in patients with high or low BAALC/ABL1 copy numbers at diagnosis.
Outcome according to BAALC/ABL1 at diagnosis in AML patients (“outcome set”, n = 263). a Event free survival and b overall survival according in patients receiving chemotherapy alone and c event free survival and d overall survival in patients consolidated with an allogeneic stem cell transplantation in CR/CRi
Similarly, high MN1/ABL1 copy numbers associated with shorter EFS (P = 0.009, Fig. 3a), which despite a separation of the curves did not translate into significantly shorter OS (P = 0.20, Fig. 3b). Again, in patients receiving allogeneic HSCT as consolidation therapy, there was no different EFS (P = 0.50, Fig. 3c) or OS (P = 0.30, Fig. 3d) in patients with high or low MN1/ABL1 copy numbers at diagnosis.
Outcome according to MN1/ABL1 at diagnosis in AML patients (“outcome set”, n = 263). a event free survival and b overall survival according in patients receiving chemotherapy alone and c event free survival and d overall survival in patients consolidated with an allogeneic stem cell transplantation in CR/CRi
In multivariate analyses for patients consolidated with chemotherapy, high MN1/ABL1 copy numbers at diagnosis remained a significant factor for shorter EFS after adjustment for age at diagnosis and presence of a monosomal karyotype while high BAALC/ABL1 copy numbers at diagnosis remained a significant factor shorter OS after adjustment for hemoglobin levels at diagnosis and presence of a complex karyotype (Table 2). Also in multivariate analyses neither high BAALC/ABL1 nor high MN1/ABL1 copy numbers at diagnosis were significantly associated with EFS or OS in patients receiving allogeneic HSCT (Table 3).
Similar results were observed when we restricted our analyses to patients with a normal karyotype (Supplementary Figs. S2 and S3) or patients transplanted in first CR (Supplementary Fig. S4). Additionally, we performed a landmark analysis for patients receiving chemotherapy for the first 139 days after diagnosis (median time from diagnosis to HSCT in the HSCT treated cohort) and again observed shorter EFS (P = 0.02) and by trend shorter OS (P = 0.08) for patients with high BAALC/ABL1 copy numbers at diagnosis (Supplementary Fig. S5A, B) as well as shorter EFS (P = 0.05) and by trend shorter OS (P = 0.10) for patients with high MN1/ABL1 copy numbers at diagnosis (Supplementary Fig. S5C, D).
Differences between patients consolidated with chemotherapy and patients receiving allogeneic HSCT are shown in the Supplementary Information and Supplementary Table S3.
Discussion
As a result of the search for better risk stratification in AML patients with normal cytogenetics, high diagnostic expression of the AML-associated genes BAALC and MN1 were shown to have independent adverse prognostic impact on CR achievement, relapse rates, EFS, and OS in younger [4, 9, 12,13,14, 27,28,29] and older [10,11,12] AML patients. Some later investigations also suggested a prognostic impact in AML patients with abnormal cytogenetics [30] or independently from cytogenetic groups [20, 31, 32]. Most of these studies focused on chemotherapy-based consolidation therapies or autologous HSCT with only a very small proportion of patients receiving an allogeneic—and in the majority of cases related donor—HSCT. However, there have already been some indications that the prognostic impact of diagnostic BAALC and MN1 expression may be modulated by the consolidation treatment. Yoon et al. [33] analyzed a cohort of 125 cytogenetically normal AML patients of whom approximately half were consolidated with an allogeneic HSCT and did not observe a prognostic impact of high BAALC expression levels, which might be explained by the mixed consolidation therapies. One recent study suggested comparable EFS and OS for high and low BAALC expressers in the TCGA dataset for patients after allogeneic HSCT, but this analysis was limited by low patient numbers and missing data on the applied chemotherapies and conditioning regimens [20]. In a subanalysis of 48 patients receiving allogeneic HSCT, Baldus et al. [28] observed very low relapse rates irrespective of BAALC expression at diagnosis and suggested that high BAALC expressing patients might benefit from an allogeneic HSCT. With respect to diagnostic MN1 expression, in a donor vs no donor subanalysis by Heuser et al. [4], no benefit of an allogeneic HSCT in high expressers was observed, but also this study was also restricted by low patient numbers (n = 39). Thus, the prognostic significance of BAALC and MN1 expression levels at diagnosis in the context of an allogeneic HSCT remains to be evaluated in a large homogeneously treated and genetically well-defined patient set—which was the main objective of our study.
In contrast to previous reports that used qRT-PCR [4, 9, 13, 14, 27, 28] or microarray-based [12, 32] assays for evaluation of BAALC and MN1 expression levels, we adopted a ddPCR technology. This method allows absolute quantification of gene copy numbers at high sensitivity, specificity, and reproducibility without the need of standard curves [21] and enabled us to establish an assay sufficient for a routine clinical assessment of BAALC and MN1 expression. In a subset of 110 patients, we observed a high correlation between qRT-PCR and ddPCR results for both gene expressions (Fig. 1) underlining the feasibility of our ddPCR assays.
The observed associations of diagnostic BAALC and MN1 copy numbers with clinical and genetic parameter stand in line with previously published analyses [4, 9,10,11,12,13,14, 20, 27, 31, 32]. As previously reported [13], high BAALC and MN1 expression correlated with each other, as well as with a high expression of immature markers such as CD34 [4, 9, 10, 31] and CD117 [9]. Additionally, we observed an association of high BAALC/ABL1 and MN1/ABL1 copy numbers with the CD34+/CD38− cell burden, and GPR56, which match the suggestions by Liu et al. [34] that MN1 overexpression might contribute to an expansion of the leukemic stem cell population. High BAALC/ABL1 and MN1/ABL1 copy numbers correlated with a specific immunophenotype, including a lower expression of mature myeloid antigens, e.g., CD11b or CD15, which have already been described for BAALC [27], and higher expression of antigens associated with T cell differentiation. Additionally, both high BAALC/ABL1 and MN1/ABL1 expressing patients showed lower CD33 expression, which might have clinical consequences when considering CD33-targeted treatment approaches [35]. We also observed the previously reported association of high BAALC and MN1 levels with lower white blood counts [9, 11, 14], immature FAB types [12, 14], abnormal cytogenetics [20, 32], NPM1 wild-type [9,10,11,12,13], as well as mutated CEBPA for high MN1 expressers [12]. Within the TCGA data set an association of high BAALC expression levels with mutated RUNX1 was described [20] that we observed for both high BAALC and high MN1 expressing patients. While we did not find an association of high BAALC levels with wild-type PTPN11 [20], there was a not yet reported lower incidence of DNMT3A mutations for high BAALC expressers, as well as a trend for less TET2 mutations in both high BAALC and MN1 expressing patients.
As expected, high BAALC and MN1 copy numbers associated with inferior outcomes in AML patients after chemotherapy-based consolidation. In contrast, within the large group of patients consolidated with an allogeneic HSCT, we observed no prognostic impact of BAALC or MN1 copy numbers at diagnosis, which was also seen in separate analyses for patients with a normal karyotype and patients transplanted in first CR. Noteworthy, also the cumulative incidences of relapse and nonrelapse mortality according to BAALC/ABL1 and MN1/ABL1 copy numbers did not differ after allogeneic HSCT (Supplementary Fig. S6).
This is especially interesting because even though for some prognostic markers allogeneic HSCT has been described to improve outcomes, the prognostic impact of most of these markers retain their prognostic impact in the HSCT context [23, 36, 37]. However, patients with high BAALC or MN1 expression at diagnosis—both markers repeatedly published to confer inferior prognosis in chemotherapy-consolidated AML patients—might benefit from an allogeneic HSCT as consolidation therapy. Noteworthy, genes involved in antigen processing and expression—among those genes encoding for MHC class I and MHC class II molecules—correlate positively with MN1 gene expression signatures [13]. This associated gene expression might support immunologic GvL effects after HSCT to contribute to better outcomes in AML patients with high MN1 expression.
We previously described the prognostic utility of BAALC/ABL1 and MN1/ABL1 copy numbers for risk stratification in remission prior to an allogeneic HSCT—which are likely to reflect residual disease burden at this time point [15, 16]. In the here-presented patient set, we also observed a strong impact on EFS and OS after HSCT according to preHSCT BAALC/ABL1 (Supplementary Fig. S7A, B) and MN1/ABL1 copy numbers (Supplementary Fig. S8A, B). Noteworthy, there was no correlation between BAALC/ABL1 and MN1/ABL1 copy numbers at diagnosis and in peripheral blood remission samples prior to HSCT (Supplementary Fig. S9). The prognostic impact of preHSCT BAALC/ABL1 and MN1/ABL1 copy numbers was independent of the diagnostic BAALC/ABL1 (Supplementary Fig. S7C–F) or MN1/ABL1 copy numbers (Supplementary Fig. S8C–F). PreHSCT BAALC/ABL1 and MN1/ABL1 copy numbers may have the highest prognostic value in patients with low copy numbers at diagnosis as this may result in higher assay sensitivity (indicated in Supplementary Figs. S7C–F and S8C–F), but larger analyses are needed to confirm this assumption. In contrast, also in patients with high or low preHSCT BAALC/ABL1 or MN1/ABL1 copy numbers, diagnostic BAALC/ABL1 or MN1/ABL1 copy numbers did not impact outcome (Supplementary Fig. S10).
Taken together, these data indicate that in the context of an allogeneic HSCT, the diagnostic BAALC or MN1 expression levels do not impact prognosis. However, independent of the diagnostic BAALC or MN1 expression levels, the assessment of both gene copy numbers in remission prior to allogeneic HSCT allow for relevant risk stratification. This further confirms previous data showing that outcomes of AML patients undergoing allogeneic HSCT remain the most favorable if patients are measurable residual disease negative prior to start of conditioning regimens [15, 16, 38,39,40,41].
In conclusion, we show that the adverse prognostic impact of high BAALC and MN1 expression levels at diagnosis is mitigated in AML patients undergoing allogeneic HSCT. In contrast, in patients receiving chemotherapy alone, we could confirm the described inferior outcomes for individuals with high BAALC or MN1 expression at diagnosis. Our data indicate that patients with high BAALC or MN1 expression at diagnosis might benefit from an allogeneic HSCT which would help to individualize treatment of these patients. Prospective analyses would be helpful to further confirm this observation.
References
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424–447
McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG et al (2001) Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus- tumor effects. Blood 97:3390–3400
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al (1990) Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75:555–562
Heuser M, Beutel G, Krauter J, Döhner K, von Neuhoff N, Schlegelberger B et al (2006) High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood 108:3898–3905
Heuser M, Berg T, Kuchenbauer F, Lai CK, Park G, Fung S et al (2012) Functional role of BAALC in leukemogenesis. Leukemia 26:532–536
Baldus CD, Tanner SM, Kusewittb DF, Liyanarachchi S, Choi C, Caligiuri MA et al (2003) BAALC, a novel marker of human hematopoietic progenitor cells. Exp Hematol 31:1051–1056
Tanner SM, Austin JL, Leone G, Rush LJ, Plass C, Heinonen K et al (2001) BAALC, the human member of a novel mammalian neuroectoderm gene lineage, is implicated in hematopoiesis and acute leukemia. Proc Natl Acad Sci U S A 98:13901–13906
Lekanne Deprez RH, Groen NA, van Biezen NA, Hagemeijer A, van Drunen E, Koper JW et al (1991) A t(4;22) in a meningioma points to the localization of a putative tumor-suppressor gene. Am J Hum Genet 48:783–790
Langer C, Radmacher MD, Ruppert AS, Whitman SP, Paschka P, Mrózek K et al (2008) High BAALC expression associates with other molecular prognostic markers, poor outcome, and a distinct gene expression signature in cytogenetically normal patients younger than 60 years with acute myeloid leukemia: a Cancer and Leukemia Group B (CALGB) study. Blood 111:5371–5379
Schwind S, Marcucci G, Maharry K, Radmacher MD, Mrózek K, Holland KB et al (2010) BAALC and ERG expression levels are associated with outcome and distinct gene and microRNA expression profiles in older patients with de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood 116:5660–5669
Schwind S, Marcucci G, Kohlschmidt J, Radmacher MD, Mrózek K, Maharry K et al (2011) Low expression of MN1 associates with better treatment response in older patients with de novo cytogenetically normal acute myeloid leukemia. Blood 118:4188–4198
Metzeler KH, Dufour A, Benthaus T, Hummel M, Sauerland MC, Heinecke A et al (2009) ERG expression is an independent prognostic factor and allows refined risk stratification in cytogenetically normal acute myeloid leukemia: a comprehensive analysis of ERG, MN1, and BAALC transcript levels using oligonucleotide microarrays. J Clin Oncol 27:5031–5038
Langer C, Marcucci G, Holland KB, Radmacher MD, Maharry K, Paschka P et al (2009) Prognostic importance of MN1 transcript levels, and biologic insights from MN1-associated gene and microRNA expression signatures in cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B Study. J Clin Oncol 27:3198–3204
Baldus CD, Tanner SM, Ruppert AS, Whitman SP, Archer KJ, Marcucci G et al (2003) BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: a Cancer and Leukemia Group B study. Blood 102:1613–1618
Jentzsch M, Bill M, Grimm J, Schulz J, Goldmann K, Beinicke S et al (2017) High BAALC copy numbers in peripheral blood prior to allogeneic transplantation predict early relapse in acute myeloid leukemia patients. Oncotarget. 8:87944–87954
Jentzsch M, Bill M, Grimm J, Schulz J, Beinicke S, Häntschel J et al (2019) Prognostic impact of blood MN1 copy numbers before allogeneic stem cell transplantation in patients with acute myeloid leukemia. HemaSphere 3:e167
Weber S, Alpermann T, Dicker F, Jeromin S, Nadarajah N, Eder C et al (2014) BAALC expression: a suitable marker for prognostic risk stratification and detection of residual disease in cytogenetically normal acute myeloid leukemia. Blood Cancer J 4:e173
Najima Y, Ohashi K, Kawamura M, Onozuka Y, Yamaguchi T, Akiyama H et al (2010) Molecular monitoring of BAALC expression in patients with CD34-positive acute leukemia. J Hematol 91:636–645
Carturan S, Petiti J, Rosso V, Calabrese C, Signorino E, Bot-Sartor G et al (2016) Variable but consistent pattern of meningioma 1 gene (MN1) expression in different genetic subsets of acute myelogenous leukaemia and its potential use as a marker for minimal residual disease detection. Oncotarget 7:74082–74096
Zhang J, Shi J, Zhang G, Zhang X, Yang X, Yang S et al (2018) BAALC and ERG expression levels at diagnosis have no prognosis impact on acute myeloid leukemia patients undergoing allogeneic hematopoietic stem cell transplantation. Ann Hematol 97(8):1391–1397
Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ et al (2013) Absolute quantification by droplet digital PCR versus analog real- time PCR. Nat Methods 10:1003–1005
Jentzsch M, Bill M, Grimm J, Schulz J, Schuhmann L, Brauer D et al (2020) High expression of the stem cell marker GPR56 at diagnosis identifies acute myeloid leukemia patients at higher relapse risk after allogeneic stem cell transplantation in context with the CD34+/CD38- population. Haematologica. https://doi.org/10.3324/haematol.2019.229260
Bill M, Jentzsch M, Grimm J, Schubert K, Lange T, Cross M et al (2017) Prognostic impact of the European LeukemiaNet standardized reporting system in older AML patients receiving stem cell transplantation after non-myeloablative conditioning. Bone Marrow Transplant 52:932–935
Grimm J, Bill M, Jentzsch M, Beinicke S, Häntschel J, Goldmann K et al (2019) Clinical impact of clonal hematopoiesis in acute myeloid leukemia patients receiving allogeneic transplantation. Bone Marrow Transplant 54:1189–1197
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Breems DA, van Putten WLJ, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH et al (2008) Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol 26:4791–4797
Bienz M, Ludwig M, Leibundgut EO, Mueller BU, Ratschiller D, Solenthaler M et al (2005) Risk assessment in patients with acute myeloid leukemia and a normal karyotype. Clin Cancer Res 11:1416–1424
Baldus CD, Thiede C, Soucek S, Bloomfield CD, Thiel E, Ehninger G (2006) BAALC expression and FLT3 internal tandem duplication mutations in acute myeloid leukemia patients with normal cytogenetics: prognostic implications. J Clin Oncol 24:790–797
Brand J, van Vliet MH, de Best L, Valk PJ, Viëtor HE, Löwenberg B et al (2013) A standardized microarray assay for the independent gene expression markers in AML: EVI1 and BAALC. Exp Hematol Oncol 2:7
Santamaría C, Chillón MC, García-Sanz R, Pérez C, Caballero MD, Mateos MV et al (2010) BAALC is an important predictor of refractoriness to chemotherapy and poor survival in intermediate-risk acute myeloid leukemia (AML). Ann Hematol 89:453–458
Damiani D, Tiribelli M, Franzoni A, Michelutti A, Fabbro D, Cavallin M et al (2013) BAALC overexpression retains its negative prognostic role across all cytogenetic risk groups in acute myeloid leukemia patients. Am J Hematol 88:848–852
Haferlach C, Kern W, Schindela S, Kohlmann A, Alpermann T, Schnittger S et al (2012) Gene expression of BAALC, CDKNIB, ERG and MN1 adds independent prognostic information to cytogenetics and molecular mutations in adult acute myeloid leukemia. Genes Chromosom Cancer 51:257–265
Yoon JH, Kim HJ, Shin SH, Yahng SA, Lee SE, Cho BS et al (2014) Implication of higher BAALC expression in combination with other gene mutations in adult cytogenetically normal acute myeloid leukemia. Leuk Lymphoma 55:110–120
Liu T, Jankovic D, Brault L, Ehret S, Baty F, Stavropoulou V et al (2010) Functional characterization of high levels of meningioma 1 as collaborating oncogene in acute leukemia. Leukemia 24:601–512
Hills RK, Castaigne S, Appelbaum FR et al (2014) Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol 15(9):986–996
Ho AD, Schetelig J, Bochtler T, Schaich M, Schäfer-Eckart K, Hänel M et al (2016) Allogeneic stem cell transplantation improves survival in patients with acute myeloid leukemia characterized by a high allelic ratio of mutant FLT3-ITD. Biol Blood Marrow Transplant 22:462–469
Grimm J, Jentzsch M, Bill M et al (2020) Prognostic Impact of the European LeukemiaNet 2017 risk classification in acute myeloid leukemia patients receiving allogeneic transplantation. Blood Adv In Press
Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y et al (2016) Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease–based definition of complete remission? J Clin Oncol 34:329–336
Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB et al (2016) Pre- and post-transplant quantification of measurable (‘minimal’) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia 30:1456–1464
Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C et al (2018) Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 132:1703–1713
Bill M, Grimm J, Jentzsch M, Kloss L, Goldmann K, Schulz J et al (2018) Digital droplet PCR-based absolute quantification of pre-transplant NPM1 mutation burden predicts relapse in acute myeloid leukemia patients. Ann Hematol 97:1757–1765
Acknowledgments
The authors thank Christel Müller, Daniela Bretschneider, Evelin Hennig, Sabine Leiblein, Martina Pleß, Ulrike Bergmann, Janet Bogardt, Annette Jilo, and Dagmar Cron for their help in determining cytogenetic, morphologic, and immunological analyses, and Christine Günther, Scarlett Schwabe, Ines Kovacs, and Kathrin Wildenberger for their help in sample processing.
Funding
Open Access funding provided by Projekt DEAL. This study was supported by the Verein Zusammen gegen den Krebs e.V, (SSsch), the Deutsche Jose-Carreras-Stiftung (04R/2016 [SSsch] and PS15/05 [JG]) and a Novartis Research Grant (HRYD-030 [SSsch]).
Author information
Authors and Affiliations
Contributions
MJ and SSch contributed to the design and analysis of this study and the writing of the manuscript, and all authors agreed on the final version. MJ, JG, MB, DBr, DBa, KG, and JS, carried out the laboratory-based research; MJ and SSch performed statistical analyses; and UP, DN, and SSch provided administrative support.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3399 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jentzsch, M., Bill, M., Grimm, J. et al. Allogeneic stem cell transplantation mitigates the adverse prognostic impact of high diagnostic BAALC and MN1 expression in AML. Ann Hematol 99, 2417–2427 (2020). https://doi.org/10.1007/s00277-020-04235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04235-8