Abstract
Better knowledge of the evapotranspiration and carbon exchange of fruit trees is needed to optimize the trade-off between water use and carbon assimilation and to better understand the role of agriculture in the biogeochemical cycles. In this work, we measured water and carbon fluxes with eddy covariance and transpiration with sap flow in a drip-irrigated peach orchard of 70% ground cover located in southern Spain for 2 years. The empirically measured crop coefficient (Kc) under good watering conditions in the summer ranged from 1 to 1.1. The daytime net ecosystem exchange (NEE) flux of the orchard averaged 30 g CO2 m2 day−1 during the period of maximum activity in July. The daytime ecosystem water use efficiency (WUE) of the orchard reached a minimum in late June, flattened around 4 g CO2 L−1 throughout the summer, and increased in autumn, but was unaffected by fruit removal or post-harvest irrigation reduction imposed by the farm (30% reduction). The response of instantaneous peach ecosystem WUE to VPD was also investigated. Both Kc, NEE, leaf water potential and stomatal conductance decreased sharply after harvest. Transpiration data from some purposely over-irrigated experimental trees demonstrated that the post-harvest alterations we found were not caused by fruit removal, but are result of mild water stress originated by the irrigation reduction. Hence, the often-observed alterations in water relations after harvest in well-watered trees were not observed in this experiment. This work adds insight on peach irrigation efficiency and on the contribution of orchards to agricultural carbon budgets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The peach tree (Prunus persica L.), first domesticated in China, found enthusiastic acceptance and its cultivation spread greatly over time (Bassi and Monet 2008). Today peach farming is a global business: more than 1,700,000 ha are cultivated worldwide, the most part in Asian countries (FAO 2018). In Europe, Spain invests close to 50,000 ha of land in the production of peaches, second only to Italy (FAO 2018).
In many production areas, especially in semi-arid climates, irrigation is needed to achieve commercial competitiveness in both yield quantity and fruit size. Peach irrigation practice is quite water-demanding in Mediterranean climate (Allen et al. 1998; Steduto et al. 2012) and, as peach farming is often concentrated into areas of high commercial quality, the cultivation may locally draw a significant amount of local water resources. As a result, peach farms in Mediterranean climates often compete for water against themselves and other irrigated crops in undersupplied areas, or versus other economy sectors installed in the same watersheds. This fostered the study of the consumptive water use of peach orchards during the last decades, with various measurement approaches. For instance, a number of works monitored tree transpiration (Ep) from measurements of sap-flow using different techniques (Remorini and Massai 2003; Conejero et al. 2007; Zhou et al. 2017), while peach evapotranspiration (ET) was measured by soil water balance (Blaney 1954; Garnier et al. 1986) and either by drainage (Mitchell et al. 1991; Boland et al. 1993; Abrisqueta et al. 2013) or weighing (Goldhamer et al. 1999; Johnson et al. 2000; Ayars et al. 2003) lysimeters. Less frequently, the eddy covariance technique (EC) was also used to determine ET (Paço et al. 2006; Ouyang et al. 2013; Anderson et al. 2017).
Increasing climate change concerns have put the carbon footprint of agricultural systems into the spotlight. Terrestrial vegetation naturally subtracts atmospheric CO2 through photosynthesis, storing it as biomass or in the soil, which contributes to the mitigation of the atmospheric CO2 increment (Zanotelli et al. 2018). However, understanding whether an ecosystem behaves as a source or as a sink of carbon requires considering the net balance between the vegetation system and the atmosphere, including the biological activities of both autotrophic and heterotrophic organisms. Hence, the evaluation of the net ecosystem exchange (NEE), which is defined as the net carbon flux resulting from the imbalance between atmospheric CO2 uptake through photosynthesis and loss by respiration, has gained interest in recent times. The EC technique has been often used for assessing NEE in unmanaged biomes and field crops, but the available information for fruit orchards is far more limited. To our best knowledge, the work by Ouyang et al. (2013) is the only source of EC carbon flux measurements over a peach orchard to date, but the climate (continental, semi-humid monsoon climate) and watering conditions (flood-irrigation twice a year with 1800 mm) in that study are completely different from those of Mediterranean drip-irrigated orchards of southern Europe. Anderson et al. (2017) also measured EC carbon fluxes in their experiment in California, but the results were not reported in their work (personal communication).
Plant water use and carbon uptake are strictly bound together through the trade-off determined by the mechanisms of stomatal regulation. In Mediterranean semi-arid climates, this ever-present structural relationship produces the strongest effects over crop productivity and sustainability, in particular where the irrigation resource is scarce. This trade-off must be optimized by maximizing the crop water use efficiency (WUE), i.e. the ratio between the amounts of carbon assimilated by plants and water lost in the process. WUE is continuously and remarkably altered by the atmospheric water demand, in particular for well-coupled canopies like tree crops (e.g. Testi et al. 2008). Being able to measure ET and the net carbon flux simultaneously and almost continuously, the EC technique is suited for monitoring WUE at the stand level. Likewise, it can also help clarifying if and how much the plant water use or WUE are influenced by subtler drivers as for example changes in the carbon sink–source balance of the plant. In this regard, reductions in photosynthesis, stomatal conductance or transpiration after fruit removal have been frequently observed for several tree crops such as olive (Bustan et al. 2016), orange (Syvertsen et al. 2003) or peach (Duan et al. 2008; Cheng et al. 2009). As a result, it is frequently assumed that carbon assimilation and water use are modulated by the demand for assimilates of the fruits, although the sink effects on photosynthesis and water use are not always observable in all conditions and species (Nebauer et al. 2011).
This work provides concurrent high-grade measurements of sap-flow and EC fluxes (water and carbon) during 2 years in a mature, drip-irrigated peach orchard growing in southern Spain. By collecting this dataset we aim to partially fill some knowledge gaps regarding the carbon exchange of this important tree crop under semiarid Mediterranean climate conditions, simultaneously evaluating the seasonal patterns of water use and WUE at the stand-level and the instantaneous response of the latter to the Vapor Pressure Deficit (VPD). Besides, the experimental data are used to test the hypothesis that fruit removal at harvest induces a reduction on the water use and photosynthesis due to a sink limitation.
Materials and methods
Field experiment
The experiment was performed in a commercial mature peach plantation at “Finca La Veguilla” in Cordoba, Spain (37.85º N, 4.8º W, 110 m altitude) during 2007 and 2008. The experimental location has a typical hot-summer Mediterranean-type climate (Csa) in Köppen–Geiger climate classification system (Kottek et al. 2006), featuring completely dry summers with high evaporative demand. Average precipitation is 612 mm/year; average reference evapotranspiration (ET0) calculated by Penman-FAO method (Allen et al. 1998) is 1390 mm/year. The peach cultivar was “BabyGold 6”, a clingstone type, typically fresh-marketed in Spain, grafted on GF677 rootstock. Trees were 15 years old in 2007, vase-trained and drip irrigated; they were producing at full and steady rate for several years. The orchard had a deep (> 2 m) loam soil, classified as Typic Xerofluvent, with moderately high water holding capacity. Plant spacing was 5 × 3.25 m; tree height was approximately 3 m. Ground cover at the start of the experiment was 71%, and 74% at the end, measured by high-resolution nadir images (1 pixel = 20 cm) taken with an UAV (Unmanned Aerial Vehicle). Drip irrigation was applied daily with 4 L/h emitters (3 per tree) during 48 h/week before and 32 h/week after harvest. Irrigation amounts were equivalent to 5.2 and 3.5 mm/day before and after harvest.
The fraction of soil surface wetted area was measured in June 16, July 10 and July 24, 2008, by measuring the width of the wetted surface at 60 points along the tree rows. Harvest was done in three passes: July 4, July 16 and July 23 in 2007, and July 1, July 12 and July 22 in 2008.
Standard meteorological measurements (wind speed and direction at 2-m height, air temperature, relative humidity and global radiation) were taken in an automated weather station at 1000 m distance from the peach plot. These data were used to calculate reference evapotranspiration following Allen et al. (1998).
Eddy covariance
Eddy covariance measurements were taken from 22 June to 6 September 2007 and from 23 May to 25 November 2008; during the second year, the system had also capability for carbon fluxes. The EC tower was equipped with a three-dimensional sonic anemometer (model CSAT3, Campbell Scientific Inc., Logan, Utah, USA) and a krypton hygrometer (Model KH20, Campbell Scientific, Logan, Utah, USA) in 2007 or an open path CO2/H2O analyser (model LI7500, LI-COR Biosciences, Lincoln, Nebraska, USA) in 2008. Fetch was around 200 m in both the east and west directions (main winds) which might be considered adequate as it contributed more than 95% of the measured fluxes according to the footprint analysis of Schuepp et al. (1990). Both sensors were placed 5.5 m above the ground at a horizontal distance of 15 cm. Air temperature and relative humidity were measured at the same height as wind velocity with a combined probe (model CS215, Campbell Scientific Inc., Logan, Utah, USA). All the sensors were connected to a datalogger (model CR1000, Campbell Scientific Inc., Logan, Utah, USA) that registered the measurements with a sampling frequency of 10 Hz. The instruments and related electronics were mounted on an extendable pneumatic mast.
Measurements of energy balance were carried along with those of turbulent fluxes. Net radiation (Rn) was measured at a height of 5.5 m above ground (model Q7.1 net radiometer, REBS—Radiation and Energy Balance Systems, Seattle, WA, USA). Three soil heat flux plates (model HFP3, REBS—Radiation and Energy Balance Systems, Seattle, WA, USA) were installed at 50 mm depth in three positions, namely under the canopy shade in the soil normally wetted by the emitters; under the canopy vertical projection but out of the wetted area; and in the middle of the alley, where the soil receives maximum radiation. Each plate was associated with two soil thermocouples providing soil temperatures at 25- and 75-mm depth; the heat storage term above the plate was calculated from the temperature gradient. The average measured soil heat flux (G) was then obtained by summing the partial fluxes weighted for the respective unshaded, shaded-dry and shaded-wet areas after applying the corrections following Fuchs and Tanner (1968) and Massman (1992).
The fluxes were calculated for periods of 30 min. The Webb–Pearman–Leuning term (Webb et al. 1980) was added to the fluxes, to take into account the fluctuations of air density. Spectral losses were corrected using transfer and gain functions as defined by Moore (1986), based on the theoretical spectral models of Kaimal et al. (1972) and Højstrup (1981). The software TK3 (Mauder and Foken 2015) was used for the flux calculations and corrections. The turbulent fluxes were also corrected for energy balance closure following Twine et al. (2000).
Sap flow and transpiration
During 2008 two trees (M1 and M2) were equipped with 4 sap-flow sensors each, spaced 90º around the trunk. These trees and the neighbours were equipped with an additional dripper line, thus received twice the irrigation water of the farm irrigation schedule to ensure non-limiting water supply throughout the whole season. Measurements started on 21/3/2008 and ended on 25/11/2008. Sap-flow sensors based on the Compensation Heat Pulse (CHP) method plus the Calibrated Average Gradient (CAG) technique (Testi and Villalobos 2009) were used. The probes inserted in the xylem measured the heat pulse velocity at 5, 15, 25 and 35 mm depth from the cambium every 15 min. Wounding errors were corrected (Swanson and Whitfield 1981) assuming a 2.6 mm wound diameter. Sap velocity was integrated first along the trunk radius and then around the azimuth angle (Green et al. 2003).
To obtain unstressed transpiration fluxes, the sap flow data were calibrated against transpiration measured with the water balance. The soil was covered with a black plastic film reaching the neighbour trees to avoid direct soil evaporation and soil water content was measured with gravimetric samples on 9 and 19 June 2008. On each date, soil samples were collected at 32 points regularly spaced around trees M1 and M2. At each point, a Veihmeyer tube was used to sequentially collect 30-cm-deep samples down to 2.1 m, obtaining, 224 soil samples each date. The sap flow measured by the 8 probes was found to always underestimate the transpiration by a fraction ranging between 80 and 20% of the real flux, depending on the probe, stable over time. No relationship was found between the underestimation and the orientation of the probe placement in the trunk.
Applied irrigation water during the calibration period was measured with a flow meter on the M1 and M2 dedicated dripping line. Sixteen intact soil cores (8.9 cm diameter × 12 cm long) were sequentially collected down to 0.6 m depth to measure soil bulk density—which averaged 1.22 t m3—used to convert mass fraction to volumetric fraction.
Auxiliary measurements
Stomatal conductance was measured with a steady-state porometer (model PMR5, PP Systems, Hitchin, Hertfordshire, UK). Twelve daily curves were obtained from June 26 to September 2, 2008. For each curve, measurements were performed in 4 sunlit and 4 shaded leaves per tree in 4 trees, including those with sap flow sensors (trees M1 and M2 with double irrigation) and 2 trees receiving the regular farm supply (A1 and A2). Each curve started 30 min after daybreak and continued until 2 h past solar noon. The measurement interval was 30 min.
Leaf water potential at solar noon was measured using a pressure chamber (Soilmoisture Equipment Corp., Santa Barbara, CA, USA) in sunny and shaded leaves on 9 days from the end of June until the start of September. Four leaves from trees M1 and M2 and four leaves from trees A1 and A2 were used in each measurement.
Calculation of tree transpiration, photosynthesis and canopy conductance
Transpiration (Ep, L h−1) was calculated as a function of sap flow (F, L h−1 after calibration—see Sect. 2.3) by explicit integration of the differential equation:
The value of ks (s), the time constant for water transport in the tree, was calculated for conditions of zero transpiration after rainfall. We searched for rainfall events that started in the daytime (period 9:00–15:00 GMT) and accumulated more than 1 mm in the first 15-min period. We took the sap flux density at that time as starting value (F0) and then used the value 15 min (900 s) later (F15) to calculate the coefficient of the exponential decay of sap flow:
Eleven rainfall events registered during 2008 were used, which resulted in an average ks = 804 s (standard deviation = 296 s).
Canopy conductance was calculated by inversion of the imposed evaporation equation (i.e. assuming negligible aerodynamic resistance). This assumption was already found well suitable for rough, coupled canopies like orchards (Villalobos et al. 2009; Orgaz et al. 2007; Roccuzzo et al. 2014):
where Gc is canopy conductance, (mol m2 s−1), Ep is tree transpiration (mol m2 s−1), Pa is atmospheric pressure (kPa) and VPD is vapor pressure deficit (kPa).
Calculation of net ecosystem exchange and night-time ecosystem respiration
The net ecosystem exchange (NEE) is the net balance of carbon between the vegetation system and the atmosphere, including the biological activities of both autotrophic and heterotrophic organisms in the soil. The NEE of a day is obtained by integrating the carbon fluxes over time; during the night the photosynthesis is null and the only carbon fluxes come from respiration processes in the ecosystem. The stable conditions and lack of turbulence severely degrade the quality of EC measurements (Goulden et al. 1996) making them mostly unreliable during the night hours.
Nevertheless, a limited assessment of night-time ecosystem respiration (Rnight) has been carried out using a subset of night-time data collected under developed turbulence, when the quality of EC measurement is still acceptable. Night-time 30-min EC data were filtered for net radiation < 20 W m−2 and friction velocity (u*) > 0.3 m s−1, a more conservative threshold than that suggested by Reichstein et al. (2002). The application of the gap filling technique of Reichstein et al. (2005) did not yield satisfactory results, as the dependance of ecosystem respiration on temperature—both at ground and EC measurement level—was too feeble and scattered in our dataset to be trusted (data not reported). Therefore, Rnight was estimated only for the nights summing at least two hours of measurements which passed the quality test, and assumed equal to the average of available measurements throughout the nigh duration. In this work, the carbon fluxes are considered positive when entering the ecosystem.
Results
Environmental conditions
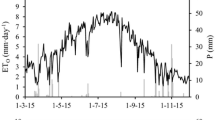
The conditions during the experiment were typical of the Mediterranean-type climate of the area, with hot and almost completely dry summers (Fig. 1). No rainfall occurred from DOY 168 to 254 in 2007 (Fig. 1A) while in 2008 (Fig. 1B) the summer dry period was DOY 153–250. Those dry periods had the same mean maximum temperature of 35.0ºC, while mean ET0 was 6.0 and 6.3 mm day−1 in 2007 and 2008, respectively. In general, the weather conditions during the experimental years were close to the preceding 20-year average (data not shown).
The soil wetted area was 20% before harvest and 18% afterwards.
Evapotranspiration and transpiration
The energy balance closure was checked using the original LE (latent heat) and H (sensible heat) fluxes, after the physical corrections. The sum of the daily turbulent fluxes (LE + H) versus the available energy (Rn–G) showed slopes of the regression line of 0.81 in 2007 and 0.84 in 2008, with negligible intercepts (data not presented).
Maximum ET near or exceeding 8 mm day−1 occurred mainly in the month of July in both years (Fig. 2) when ET0 was in the range 6–8 mm day−1. In 2007 (Fig. 2A), ET decreased after DOY 210 down to 3–4 mm day−1 at the end of August. The plateau was wider in 2008 so the decline was evident after DOY 220 down to very low values (0.5 mm day−1) at the start of December, when leaves were falling and the measurements ended (Fig. 2B). The variation of ET0 was similar to that of ET but with a much wider variability during the autumn.
The measured ratio of ET and ET0, the crop coefficient (Kc), is shown in Fig. 3 for weekly periods. In 2007, the weekly Kc oscillated from 0.8 to 1.15 until DOY 210 and then, starting after harvest and the irrigation reduction, it dropped steadily down to 0.7 by the end of August, when measurements were interrupted. In 2008, maximum values were around 1.1 during July and decreased after harvest down to 0.7 at the end of summer. Some recovery is observed at the start of autumn after 65 mm of rainfall from DOY 265 to 272. After that, the Kc remained between 0.7 and 0.8 until the final drop to 0.4 in the last 2 weeks, when leaves were already senescent and progressively falling off.
Time series of the 7-day averaged water fluxes from the orchard, normalized by ET0. On display: the measured ET/ET0 (Kc) for the years 2007 (circles, dashed line); the same for 2008 (dots, solid line); the tree transpiration coefficient (Ep/ET0, grey line–grey dots), measured in 2008. The vertical dotted lines indicate the dates of the harvests; irrigation was reduced after the last one
The transpiration coefficient (Ep/ET0) of trees M1 and M2 (measured in 2008) increased during the spring until the start of May and then remained almost constant (0.8–0.9) all along the summer (Fig. 3). A small increase to 0.9–1.0 was observed during part of the autumn until a marked decrease during 5 weeks down to 0.17 at the end of the measurements. If we consider the period before harvest from DOY 157 to DOY 221 (irrigation was reduced afterwards), the mean difference between ET and Ep was 1.09 mm day−1, while mean ET and mean ET0 were both equal to 6.5 mm day−1, i.e. soil evaporation was 17% of ET.
Net ecosystem exchange and night-time ecosystem respiration
The daily values of daytime net ecosystem exchange of the peach orchard measured during the year 2008 are presented in Fig. 4. Maximum values of daytime NEE peaked at the end of June (DOY 180). Values exceeding 35 g CO2 m2 day−1 were recorded occasionally in this period, with weekly averages around 30 g CO2 m2 day−1 (Fig. 4). After harvest the weekly average daytime NEE declined to 23 g CO2 m2 day−1, and remained quite stable during August. From then on, NEE decreased at variable paces until the end of November when the daytime NEE practically reached neutrality. Negative daily values of daytime NEE were not detected, i.e. during the measurement period the orchard was always a net sink of carbon during daytime. At the end of the experiment the trees were still only partially defoliated.
Time series of daytime net ecosystem exchange (NEE, black dots), measured with eddy covariance; night-time ecosystem respiration (Rnight, black squares, estimated) and total daily NEE (black triangles, estimated) during the year 2008. The 7-day averages are also shown (thick gray lines). Values of NEE > 0 stand here for C moving from atmosphere into the ecosystem. The vertical dotted lines indicate the dates of the harvests; irrigation was reduced just after the last one
The night-time ecosystem respiration—estimated during the nights that allowed quality EC measurements—showed consistently stable values ranging between -6 and -10 g CO2 m2 day−1 (Fig. 4), from June to September. The first rainfall events after the dry summer (7 mm of precipitation on DOY 250 and 50 mm during DOY 265 and 266) are followed by nighs with remarkably high respiration fluxes (-11.6 g CO2 m2 day−1 on DOY 252 and -15.2 and -12.2 g CO2 m2 day−1 on DOY 267 and 268, respectively). These high Rnight fluxes drove the total daily NEE neutral or negative during short periods. The few measurements available at the end of the season (November) with lower temperatures and declining leaf area indicate that the night-time ecosystem respiration decreased around -3 or -4 g CO2 m2 day−1, although enough to drag the total daily NEE slightly negative at the onset of winter (Fig. 4).
Water status and stomatal conductance
Leaf water potential (Fig. 5A) of shaded leaves in trees M1 and M2 (with double irrigation) was almost constant at − 0.7 MPa during the summer but decreased in trees A1 and A2 (normally irrigated) down to − 1.4 MPa just after the reduction in irrigation durations that occurred on DOY 204. Something similar occurred in sunlit leaves which reached − 2.3 and − 2.8 MPa at the beginning of September with double and normal irrigation, respectively. The water potential of sunlit leaves in trees A1 and A2 started showing significative differences from those under double irrigation after DOY 213 (Fig. 5A).
Time courses of leaf water potential at solar noon (A) and stomatal conductance (B)—the last is the average of nine measurements spaced 30-min from 8:00 to 12:00 GMT—in the experimental trees with normal (1X) and double (2X) irrigation. The vertical dotted lines indicate the dates of the harvests; irrigation was reduced just after the last one
The average 8:00–12:00 GMT stomatal conductance in sunlit leaves (Fig. 5B) was slightly higher in trees A1 and A2 until DOY 213 and then it became lower than that of M1 and M2 for the rest of the summer. The remarkably high conductance recorded in both normal and double irrigated trees on 25 Jul 2008 (DOY 206) is probably due to an instrumental problem, as leaf water potential the same day show nothing unusual (Fig. 5A). Mean morning value of stomatal conductance in sunlit leaves was 0.35–0.4 mol m−2 s−1 at the start of summer and 0.15–0.20 mol m−2 s−1 at the end.
Canopy conductance and ecosystem water use efficiency
Estimates of mean daytime canopy conductance obtained by inversion of the imposed evaporation equation in the trees M1 and M2 (overirrigated) are shown in Fig. 6 for the whole growing season of 2008. There was a rapid increase during spring as the trees started growth and a maximum between 0.4 and 0.5 mol m−2 s−1 at the end of May (DOY 135–140). Despite daily values of canopy conductance show similar scatter as in the rest of the season, during the summer dry period it remained relatively stable around 0.2 mol m−2 s−1—on average—during DOY 170–250) with a slight decrease at the end (DOY 225–235) and then recovered quickly (DOY 235–250), keeping average values around 0.3 mol m2 s−1 in the autumn until the final drop at the end of November with leaf senescence and partial fall (Fig. 6).
Seasonal course of mean daytime whole-tree canopy conductance, calculated by inversion of the imposed evaporation equation from transpiration data in trees M1 and M2 (double irrigation). The grey line marks the 7-day averages. The vertical dotted lines indicate the dates of the harvests; irrigation was reduced after the last one
Figure 7 shows examples of diurnal courses of canopy conductance of tree M1 (with double irrigation) during four dates in different seasons. In spring (DOY 122, 1 May) and autumn (DOY 279, 5 October), the conductance of the whole tree reached the maximum values, around 0.55 mol m−2 s−1. In early summer (DOY 183, 1 July), the conductance peaked at 0.4 mol m−2 s−1, and in late summer (DOY 236, 23 Aug), the morning peak was only 0.28 mol m−2 s−1 (Fig. 7; compare also with diurnal averages of Fig. 6). Despite the difference in magnitude, the pattern was very similar for all the curves, all reaching the maximum within 90 min after sunrise, then decreasing almost linearly until dusk.
The daytime ecosystem WUE (ratio of daytime total NEE and orchard ET) was remarkably constant during summer, around 4 g CO2 L−1, and then it became quite variable during autumn (Fig. 8). Despite the much higher variability, the 7-day averages of daytime ecosystem WUE during autumn (7–8 g CO2 L−1) almost doubled the summer values (Fig. 8).
The relationship of peach ecosystem WUE with ambient VPD is shown in Fig. 9. Here the instantaneous WUE of the orchard (values for 30-min fluxes from eddy covariance measurements, filtered for dry soil, net radiation > 30 W m2 and friction velocity > 0.2 m s−1) are plotted vs. VPD. The data presented are taken from two different summer periods: before (DOY 170–190) and after (DOY 235–249) the irrigation reduction. Both periods were rain-free, with dry soil surface so the direct soil contribution to ET was virtually limited to the fraction of soil surface wetted by the emitters, and expected to be fairly constant (irrigation was daily, thus the wetted soil surface was steady). The maximum recorded values of instant WUE were around 16–19 g CO2 L−1, mainly from relatively cool mornings from the later period; during very warm and dry summer afternoons WUE scored close to 2 g CO2 L−1, in both periods.
Relationship between ecosystem WUE (obtained as NEE/ET) and VPD in the peach orchard. Data are calculated from semi-hourly fluxes of water vapour and CO2 from Eddy Covariance (2008) of the periods DOY 170–190 (before the irrigation reduction, black dots) and DOY 235–249 (after the irrigation reduction, circles), filtered for dry soil, net radiation > 30 W m2 and friction velocity > 0.2 m s−1. The solid line is the fitted curve (equation on chart) of all the data pooled together. Also shown is the relationship found in olive by Testi et al. (2008)
Discussion
In this experiment, the empirically measured Kc (the ratio between the measured ET and ET0) exceeded unity most of the time from the end of June to the end of July, in daily values (not shown) as in weekly averages (Fig. 3). We measured an empirical Kc of 0.97 and 1.05 as a monthly average of July 2007 and 2008, respectively (Fig. 3). These results should be compared with previous experiments carried on in similar climates and ground cover. Ayars et al. (2003) measured ET exceeding 8 mm day−1 by means of a lysimeter in a peach orchard in California of similar ground cover. They found maximum empirical daily Kc values ranging between 0.9 and 1.3, which yielded an average value of 1.06 at full cover, very similar to what was found in this experiment. Anderson et al. (2017) also measured—this time by eddy covariance—peak ET during the summer exceeding 8 mm day−1 and Kc in the range 0.8–1.3 in a 80% ground cover peach orchard in San Joaquin valley (California). This orchard was furrow-irrigated every 4–14 days, so their records of ET and Kc show more fluctuations due to the soil wet surface fraction varying over time (and probably being larger than in our experiment, in the days following irrigations). Nevertheless, they reported an average Kc for the summer around 1.1, very close to our findings. Similar peak values of ET close to 8 mm day−1 were also recorded by Ouyang et al. (2013) during the summer, but they did not report the empirical Kc. Abrisqueta et al. (2013), working with drainage lysimeters in a 80% ground cover peach orchard in south-eastern Spain, found average values of empirical Kc between 0.9 and 1.05 in July, in already harvested trees, similar to our results.
The daytime peach NEE measured in 2008 peaked roughly in synchrony with the seasonal course of ET (Figs. 2B and 4). From our data, the daytime NEE of intensive irrigated peach orchards in semi-arid and Mediterranean climates can be sized around 30 g CO2 m2 day−1 at the maximum period of orchard assimilation. Measurements of carbon exchange of peach orchards are extremely scant in literature, which adds value to our dataset but at the same time hinders critical comparisons. To our knowledge, the only NEE fluxes of peach published so far are those of Ouyang et al. (2013). They reported summer NEE of 20–25 g CO2 m2 day−1 in an orchard near Beijing, roughly 30% lower than our findings. Unfortunately, they did not report how they treated nocturnal respiration fluxes and therefore whether their daily values include night-time respiration or not; which might well explain the difference between our datasets. Our night-time ecosystem respiration data during summer (between − 6 and − 10 g CO2 m2 day−1 (Fig. 4) approximately matches the total daily NEE estimated in this experiment (Fig. 4) with that of Ouyang et al. (2013). Differences in NEE between our study and the Chinese one may also arise from the different climate (sub-humid), and soil (coarse sandy); furthermore, Ouyang et al. (2013) do not report the ground cover of their experiment.
The seasonal course of night-time ecosystem respiration (Rnight, Fig. 4) shows a remarkable stability and almost no relation with temperature throughout the whole year, except in the cooler late season (November). On the contrary, Rnight fluxes increased in magnitude with the first significant precipitations at the end of the dry summer period, up to almost doubling the stable summer values during some nights (e.g. DOY 267, after a 50-mm rainfall event, Fig. 4). This behaviour suggests that heterotrophic respiration is limited by soil water availability during summer in this orchard, as already found in other studies in Mediterranean climates (e.g. Inglima et al. 2009). Our data indicate that—in average—the ecosystem respiration during the night returned to the atmosphere 30% (± 4% standard deviation) of the net carbon assimilated the previous day during the dry summer, and 70% (± 20%) after precipitations restart in the fall.
In our experimental orchard the variables measured with eddy covariance—namely ET (2007 and 2008, Fig. 2), the empirical Kc (2007 and 2008, Fig. 3) and the daytime and daily total NEE (2008, Fig. 4)—all decreased after harvest; on the contrary the night-time ecosystem respiration remained unchanged (Fig. 4). Reductions in water use after harvest were found often in fruit trees—e.g. Ayars et al. (2003) for peach, among others – which are mostly interpreted as an effect of the sudden termination of the main carbon sink in the plant (the fruits, Grossman and Dejong 1994) from the tree carbon budget. The excess of photosynthates in the leaves is then re-balanced by a reduction in stomatal conductance, slowing down plant photosynthesis rate along with transpiration (Wibbe and Blanke 1995). But in our experimental orchard the irrigation has been reduced by one third after harvest, so it is important to clarify whether the post-harvest reduction we found in ET, Kc and NEE was due to the removal of the fruits (hypothesis of carbon balance readjustment) or the irrigation reduction that followed (hypothesis of mild water stress).
This question is addressed by looking at the behaviour of the experimental trees instrumented with sap flow probes. They received twice the irrigation of the other trees in the orchard, thus after harvest they still received a larger supply per tree than the rest of the orchard before harvest; this ensured that their water use was always maximum and independent from any irrigation adjustment made by the farm. Nevertheless, they were harvested at the same time and just like any other tree in the orchard; therefore, if the cause of the reduction in water and carbon exchange we measured were carbon balance readjustment and not water stress, their transpiration should follow the orchard’s post-harvest behaviour. The transpiration coefficient Kt (Ep/ET0) of the trees M1 and M2—always well irrigated—showed no changes whatsoever after the last harvest of 22 July, when the irrigation was reduced (Fig. 3). This is enough evidence to affirm that the reduction in Kc (Fig. 3) and in the daytime and total daily NEE (Fig. 4) after harvest was a consequence of moderate water stress, which intensity was sufficient to induce the trees to a partial stomatal closure, while the double irrigated trees remained unaffected.
In general, the values of average stomatal conductance of the double irrigated trees after the harvest passes are practically the same they were before, at least until late in the season, indicating no effect from de-fruiting (Fig. 5). The leaf water potential of normal irrigated trees declined in both shaded and sunlit leaves after the irrigation reduction that followed harvest, indicating that some water stress took place in the orchard (Fig. 5A). Double irrigated trees showed no decrease in water potential until late after, and only in sunlit leaves.
The average daytime full-tree canopy conductance obtained from sap-flow measurements in the double irrigated trees (Fig. 6) remained almost unaffected by the removal of fruit carbon sink; it even increased after the first harvest pass, then decreased after the second but negligibly, providing no support to the sink limitation hypothesis; we can now say that the reduction in conductance after harvest (and, therefore, transpiration and photosynthesis) does not occur always or necessarily. It is reasonable to think that harvest may have a marginal effect on stomatal behaviour if and when other sink compartments are active and able to accept the C surplus, something that may or may not occur depending on the orchard phenology, C status and balance among organs. Care should be taken—therefore—not to switch causes and effects when interpreting pre-/post-harvest gas exchange data in commercial farms trials which often reduce water supply after harvest.
Worth to highlight in Fig. 6 is instead the strong seasonal pattern of the whole-tree conductance, with consistently larger values in spring (+ 50%) and autumn (+ 33%) with respect to summer, even in trees (M1 and M2) that unquestionably were free of water stress. This behaviour has been found in other species, usually more adapted to dry climates than peach (Tognetti et al. 2004; Testi et al. 2006; Espadafor et al. 2018), and has been associated to stomatal control even in well-irrigated trees, when water uptake from the roots cannot match the canopy demand under high atmospheric VPD due (also) to the limited volume of wetted soil in drip-irrigated trees (Garcia-Tejera et al. 2017; Espadafor et al. 2018). The striking increase in daytime conductance from DOY 239 to 249 (Fig. 6) corresponds to a steady and steep reduction in VPD—unusual in this area and period—from 2.9 to 1.1 kPa.
The canopy conductance of the double irrigated peach trees (Fig. 7) shows diurnal curves with analogous patterns but very different amplitude in different seasons. Again, we are confident that water stress was not involved in the difference of conductance between dates, as these trees were always over-irrigated, and in good water status (Fig. 5A). The canopy conductance of the whole peach tree, either instantaneous (Fig. 7) or diurnal average values (Fig. 6) is clearly and significantly changing throughout the season, even under optimal water supply. In the case of these peach trees, the patterns of diurnal conductance scale in amplitude between dates (roughly inversely with VPD) although they did not seem otherwise altered in shape (Fig. 7). These data suggest that VPD is an unavoidable input for correctly modelling canopy conductance in peach.
The ecosystem water use efficiency, calculated as NEE/ET during the daytime hours (thus including respiration and evaporation fluxes from the soil) resulted unresponsive of both the harvest actions and the irrigation reduction applied thereafter (Fig. 8). In our experiment, the WUE remained stable (around 4 g CO2 L−1) during all the summer, then increased during the autumn doubling the summer values. The much larger fluctuations of autumn data are most likely caused by the decreased signal/noise ratio of the EC technique as the carbon and water fluxes get lower. A very similar seasonal pattern of the peach WUE (in average trend as well as in variability) was found by Ouyang et al. (2013), although with consistently lower WUE records; which may be due to a different accumulation period (our WUE from EC data are daytime only) and/or to a different soil biological activity or management. Larger WUE in spring and autumn than in summer are found frequently (e.g. Testi et al. 2008 in olive) and are due to the marked, non-linear theoretical relationship of WUE to VPD (Tanner and Sinclair 1983).
This response of instantaneous WUE to VPD of the peach orchard is clear in Fig. 9, where orchard WUE is calculated as NEE/ET. Despite containing soil and plant respiration and soil evaporation in the terms, the relationship followed the theoretical pattern. This pattern was hardly changing for the periods before or after the irrigation reduction (respectively dots and circles, Fig. 9): the fitted regression lines for the two periods are not shown (they resulted undistinguishable to the eye from the pooled fit). The invariance of WUE through null to moderate water stress has a theoretical basis (Tanner and Sinclair 1983) and satisfactorily went through many empirical verifications in field crops (e.g. Steduto et al. 2007) and orchards (Moriana et al. 2002; Testi et al. 2008; Roccuzzo et al. 2014). The relationship found by Testi et al. (2008) in olive is shown because it was obtained from EC data measured and processed the same way, with a relatively similar soil surface wetted by the emitters, and offer the chance of directly compare the WUE models for the two species. The higher efficiency shown by the olive grove may be a symptom of some adaptation to dry climates (e.g. a stronger or quicker stomatal response to VPD), but this hypothesis must be taken with caution, as the living biomass (heterotrophic and autotrophic) may have been different in the two experiments, generating different respiration fluxes. This caveat is an example of the difficulties in measuring and comparing the carbon fluxes of different orchard types, and of the need of quality carbon and water flux data to empower the knowledge and modelling of these heterogeneous agricultural systems.
Conclusions
The present study evaluates the water and carbon fluxes over an intensive drip-irrigated peach orchard (70–75% ground cover) growing in Southern Spain based on the integration of eddy covariance (EC) measurements and other methodologies. For irrigation purposes, the crop coefficient ranged between 1 and 1.1 at midsummer, when the soil surface was dry except for the fraction wetted by the emitters (in our case 20%), while the transpiration coefficient (Ep/ET0) ranged in the interval 0.8 to 0.9. The direct evaporation from the soil during the months of June and July without irrigation restrictions was 17% of ET, and averaged 1.06 mm day−1.
Regarding the EC carbon fluxes, the orchard captured on average 30 g CO2 m−2 day−1 during the daytime hours in early summer, with peaks of 36–38 g CO2 m−2 day−1. In this period, NEE at net of night-time ecosystem respiration (i.e. whole-day) were around 20 g CO2 m−2 day−1. After that, the net ecosystem exchange (NEE) rates decreased gradually until reaching negligible values in late autumn. The canopy conductance of peach was lower in summer (high VPD) than in spring/autumn (low VPD), both as instantaneous and daily values, even under non-limited water supply. The ecosystem water use efficiency (WUE) of this peach orchard was 4 g CO2 L−1 (daytime value) during the dry summer and roughly the double during autumn. Instantaneous WUE of peach scales with VPD following the theoretical model, also at orchard level and was unaffected by water stress or fruit removal. ET, Kc, NEE and whole-tree canopy conductance decreased after harvest, but such responses were a consequence of a 33% irrigation reduction rather than to the disappearance of the fruit carbon sinks.
References
Abrisqueta I, Abrisqueta JM, Tapia LM, Mungía JP, Conejero W, Vera J, Ruiz-Sánchez MC (2013) Basal crop coefficients for early-season peach trees. Agric Water Manage 121:158–163. https://doi.org/10.1016/j.agwat.2013.02.001
Allen RG, Pereira JS, Raes D, Smith M (1998) Crop evapotranspiration : guidelines for computing crop water requirements. FAO irrigation and drainage paper, 56. Food and Agriculture Organization of the United Nations, Rome, p 300
Anderson RG, Alfieri JG, Tirado-Corbalá R, Gartung J, McKee LG, Prueger JH, Wang D, Ayars JE, Kustas WP (2017) Assessing FAO-56 dual crop coefficients using eddy covariance flux partitioning. Agric Water Manage 179:92–102. https://doi.org/10.1016/j.agwat.2016.07.027
Ayars JE, Johnson RS, Phene CJ, Trout TJ, Clark DA, Mead RM (2003) Water use by drip-irrigated late-season peaches. Irrig Sci 22(3–4):187–194. https://doi.org/10.1007/s00271-003-0084-4
Bassi D, Monet R (2008) Botany and taxonomy. In: Layne DR, Bassi D (eds) The peach: botany, production and uses. CABI, UK, pp 1–36
Blaney H (1954) Evapo-transpiration measurements in western United States. General Assembly of the International Union of Geodesy and Geophysics, Rome, Italy
Boland AM, Mitchell PD, Jerie PH, Goodwin I (1993) The effect of regulated deficit irrigation on tree water-use and growth of peach. J Hortic Sci 68(2):261–274. https://doi.org/10.1080/00221589.1993.11516351
Bustan A, Dag A, Yermiyahu U, Erel R, Presnov E, Agam N, Kool D, Iwema J, Zipori I, Ben-Gal A (2016) Fruit load governs transpiration of olive trees. Tree Physiol 36(3):380–391. https://doi.org/10.1093/treephys/tpv138
Cheng J, Fan P, Liang Z, Wang Y, Niu N, Li W, Li S (2009) Accumulation of end products in source leaves affects photosynthetic rate in peach via alteration of stomatal conductance and photosynthetic efficiency. J Am Soc Hortic Sci 134(6):667–676
Conejero W, Alarcón JJ, García-Orellana Y, Nicolás E, Torrecillas A (2007) Evaluation of sap flow and trunk diameter sensors for irrigation scheduling in early maturing peach trees. Tree Physiol 27(12):1753–1759. https://doi.org/10.1093/treephys/27.12.1753
Duan W, Fan PG, Wang LJ, Li WD, Yan ST, Li SH (2008) Photosynthetic response to low sink demand after fruit removal in relation to photoinhibition and photoprotection in peach trees. Tree Physiol 28(1):123–132. https://doi.org/10.1093/treephys/28.1.123
Espadafor M, Orgaz F, Testi L, Lorite IJ, García-Tejera O, Villalobos FJ, Fereres E (2018) Almond tree response to a change in wetted soil volume under drip irrigation. Agric Water Manage 202:57–65. https://doi.org/10.1016/j.agwat.2018.01.026
FAO (2018) FAOSTAT statistical databases. FAO, Food and Agriculture Organization of the United Nations, Rome
Fuchs M, Tanner CB (1968) Calibration and field test of soil heat flux plates. Soil Sci Soc Am Proc 32(3):326–328. https://doi.org/10.2136/sssaj1968.03615995003200030021x
Garcia-Tejera O, López-Bernal Á, Orgaz F, Testi L, Villalobos FJ (2017) Analysing the combined effect of wetted area and irrigation volume on olive tree transpiration using a SPAC model with a multi-compartment soil solution. Irrig Sci 35(5):409–423. https://doi.org/10.1007/s00271-017-0549-5
Garnier E, Berger A, Rambal S (1986) Water balance and pattern of soil water uptake in a peach orchard. Agric Water Manage 11(2):145–158. https://doi.org/10.1016/0378-3774(86)90027-2
Goldhamer DA, Fereres E, Mata M, Girona J, Cohen M (1999) Sensitivity of continuous and discrete plant and soil water status monitoring in peach trees subjected to deficit irrigation. J Am Soc Hortic Sci 124(4):437–444
Goulden ML, Munger JW, Fan SM, Daube BC, Wofsy SC (1996) Measurements of carbon sequestration by long-term eddy covariance: methods and a critical evaluation of accuracy. Glob Change Biol 2(3):169–182. https://doi.org/10.1111/j.1365-2486.1996.tb00070.x
Green S, Clothier B, Jardine B (2003) Theory and practical application of heat pulse to measure sap flow. Agron J 95(6):1371–1379. https://doi.org/10.2134/agronj2003.1371
Grossman YL, Dejong TM (1994) Peach - a simulation-model of reproductive and vegetative growth in peach-trees. Tree Physiol 14(4):329–345. https://doi.org/10.1093/treephys/14.4.329
Højstrup J (1981) A simple model for the adjustment of velocity spectra in unstable conditions downstream of an abrupt change in roughness and heat flux. Bound-Layer Meteorol 21(3):341–356. https://doi.org/10.1007/BF00119278
Inglima I, Alberti G, Bertolini T, Vaccari F, Gioli B, Miglietta F et al (2009) Precipitation pulses enhance respiration of Mediterranean ecosystems: the balance between organic and inorganic components of increased soil CO2 efflux. Glob Chang Biol 15(5):1289–1301. https://doi.org/10.1111/j.1365-2486.2008.01793.x
Johnson RS, Ayars J, Trout T, Mead R, Phene C (2000) Crop coefficients for mature peach trees are well correlated with midday canopy light interception. Acta Hortic 537:455–460
Kaimal J, Izumi Y, Wyngaard J, Cote R (1972) Spectral characteristics of surface layer turbulence. Q J R Meteorol Soc 98(417):563–589. https://doi.org/10.1002/qj.49709841707
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Koppen-Geiger climate classification updated. Meteorol Z 15(3):259–263. https://doi.org/10.1127/0941-2948/2006/0130
Massman WJ (1992) Correcting errors associated with soil heat-flux measurements and estimating soil thermal-properties from soil-temperature and heat-flux plate data. Agric for Meteorol 59(3–4):249–266. https://doi.org/10.1016/0168-1923(92)90096-M
Mauder M, Foken T (2015) Eddy-Covariance Software TK3. Documentation and Instruction Manual of the Eddy-Covariance Software Package TK3 (update). Universität Bayreuth, Bayreuth
Mitchell PD, Boland AM, Irvine JL, Jerie PH (1991) Growth and water use of young, closely planted peach trees. Sci Hortic 47(3):283–293. https://doi.org/10.1016/0304-4238(91)90011-M
Moore CJ (1986) Frequency-response corrections for Eddy-correlation systems. Bound-Layer Meteorol 37(1–2):17–35. https://doi.org/10.1007/BF00122754
Moriana A, Villalobos FJ, Fereres E (2002) Stomatal and photosynthetic responses of olive (Olea europaea L.) leaves to water deficits. Plant Cell Environ 25(3):395–405. https://doi.org/10.1046/j.0016-8025.2001.00822.x
Nebauer S, Renau-Morata B, Guardiola J, Molina R (2011) Photosynthesis down-regulation precedes carbohydrate accumulation under sink limitation in Citrus. Tree Physiol 31(2):169–177. https://doi.org/10.1093/treephys/tpq103
Orgaz F, Villalobos FJ, Testi L, Fereres E (2007) A model of daily mean canopy conductance for calculating transpiration of olive canopies. Funct Plant Biol 34(3):178–188. https://doi.org/10.1071/FP06306
Ouyang Z, Mei X, Li Y, Guo J (2013) Measurements of water dissipation and water use efficiency at the canopy level in a peach orchard. Agric Water Manage 129:80–86. https://doi.org/10.1016/j.agwat.2013.07.016
Paço T, Ferreira M, Conceicao N (2006) Peach orchard evapotranspiration in a sandy soil: comparison between eddy covariance measurements and estimates by the FAO 56 approach. Agric Water Manage 85(3):305–313. https://doi.org/10.1016/j.agwat.2006.05.014
Remorini D, Massai R (2003) Comparison of water status indicators for young peach trees. Irrig Sci 22:39–46. https://doi.org/10.1007/s00271-003-0068-4
Roccuzzo G, Villalobos FJ, Testi L, Fereres E (2014) Effects of water deficits on whole tree water use efficiency of orange. Agric Water Manage 140:61–68. https://doi.org/10.1016/j.agwat.2014.03.019
Schuepp PH, Leclerc MY, Macpherson JI, Desjardins RL (1990) Footprint prediction of scalar fluxes from analytical solutions of the diffusion equation. Bound-Layer Meteorol 50(1–4):353–373. https://doi.org/10.1007/BF00120530
Steduto P, Hsiao T, Fereres E, Raes D (2012) Crop yield response to water. FAO Irrigation and Drainage Paper no. 66. Food and Agriculture Organization (FAO), Rome
Steduto P, Hsiao TC, Fereres E (2007) On the conservative behavior of biomass water productivity. Irrig Sci 25(3):189–207. https://doi.org/10.1007/s00271-007-0064-1
Swanson RH, Whitfield DWA (1981) A numerical-analysis of heat pulse velocity theory and practice. J Exp Bot 32(126):221–239. https://doi.org/10.1093/jxb/32.1.221
Syvertsen J, Goñi C, Otero A (2003) Fruit load and canopy shading affect leaf characteristics and net gas exchange of ‘Spring’ navel orange trees. Tree Physiol 23(13):899–906. https://doi.org/10.1093/treephys/23.13.899
Reichstein M, Falge E, Baldocchi D, Papale D, Aubinet M, Berbigier P et al (2005) On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob Chang Biol 11(9):1424–1439. https://doi.org/10.1111/j.1365-2486.2005.001002.x
Reichstein M, Tenhunen J, Roupsard O, Ourcival J, Rambal S, Dore S et al (2002) Ecosystem respiration in two Mediterranean evergreen Holm Oak forests: drought effects and decomposition dynamics. Funct Ecol 16(1):27–39. https://doi.org/10.1046/j.0269-8463.2001.00597.x
Tanner CB, Sinclair TR (1983) Efficient water use in crop production: research or re-search? In: Taylor HM, Jordan WR, Sinclair TR (eds) Limitations to efficient water use in crop production. American Society of Agronomy, pp 1–27
Testi L, Orgaz F, Villalobos FJ (2008) Carbon exchange and water use efficiency of a growing, irrigated olive orchard. Environ Exp Bot 63(1–3):168–177. https://doi.org/10.1016/j.envexpbot.2007.11.006
Testi L, Orgaz F, Villalobos FJ (2006) Variations in bulk canopy conductance of an irrigated olive (Olea europaea L.) orchard. Environ Exp Bot 55(1–2):15–28. https://doi.org/10.1016/j.envexpbot.2004.09.008
Testi L, Villalobos FJ (2009) New approach for measuring low sap velocities in trees. Agric for Meteorol 149(3–4):730–734. https://doi.org/10.1016/j.agrformet.2008.10.015
Tognetti R, d’Andria R, Morelli G, Calandrelli D, Fragnito F (2004) Irrigation effects on daily and seasonal variations of trunk sap flow and leaf water relations in olive trees. Plant Soil 263(1–2):249–264. https://doi.org/10.1023/B:PLSO.0000047738.96931.91
Twine T, Kustas WP, Norman JM, Cook DR, Houser PR, Meyers TP, Prueger JH, Starks PJ, Wesely ML (2000) Correcting eddy-covariance flux underestimates over a grassland. Agric for Meteorol 103(3):279–300. https://doi.org/10.1016/S0168-1923(00)00123-4
Villalobos FJ, Testi L, Moreno-Perez MF (2009) Evaporation and canopy conductance of citrus orchards. Agric Water Manage 96(4):565–573. https://doi.org/10.1016/j.agwat.2008.09.016
Webb EK, Pearman GI, Leuning R (1980) Correction of flux measurements for density effects due to heat and water-vapor transfer. Q J R Meteorol Soc 106(447):85–100. https://doi.org/10.1002/qj.49710644707
Wibbe M, Blanke M (1995) Effects of defruiting on source-sink relationship, carbon budget, leaf carbohydrate content and water-use efficiency of apple-trees. Physiol Plant 94(3):529–533. https://doi.org/10.1111/j.1399-3054.1995.tb00964.x
Zanotelli D, Vendrame N, López-Bernal Á, Caruso G (2018) Carbon sequestration in orchards and vineyards. Italus Hortus 25(3):13–28. https://doi.org/10.26353/j.itahort/2018.3.1328
Zhou H, Zhang F, Roger K, Wu L, Gong D, Zhao N (2017) Peach yield and fruit quality is maintained under mild deficit irrigation in semi-arid China. J Integr Agric 16(5):1173–1183. https://doi.org/10.1016/S2095-3119(16)61571-X
Acknowledgements
The authors wish to acknowledge Javier Hurtado, Ignacio Calatrava, Rafael del Río and José Luís Vazquez for their excellent technical help in performing measurements and installing and maintaining all the continuous measurement systems and devices. This work would not have been possible without the sincere and enthusiastic collaboration of Francisco Natera, manager of “La Veguilla” farm and his staff.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by the project CONSOLIDER-RIDECO [grant number CSD2006-00067], of the Spanish Ministry of Science and Education through European Union ERDF funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Testi, L., Orgaz, F., López-Bernal, Á. et al. Pre- and post-harvest evapotranspiration, carbon exchange and water use efficiency of a mature peach orchard in semi-arid climate. Irrig Sci 40, 407–422 (2022). https://doi.org/10.1007/s00271-022-00797-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00271-022-00797-9