Abstract
Background
A modified Fong clinical score (m-Fong CS) that includes the RAS mutation status has recently been proposed and offered an improved survival stratification of patients who undergo surgery and systemic chemotherapy for colorectal liver metastases (CLM). The aim of this study is to assess whether a CS that includes RAS status is influenced by whether patients receive perioperative chemotherapy.
Methods
We created a new CS using multivariate analysis of data of patients who underwent hepatectomy for CLM for the first time between 2010 and 2016 at a single hospital (n = 341, 79% received perioperative chemotherapy). The resulting CS and m-Fong CS were then validated in the patient cohort at three other hospitals (n = 309). Furthermore, the applicability of the two CS in the total cohort (n = 650) was tested according to whether the patients received perioperative chemotherapy.
Results
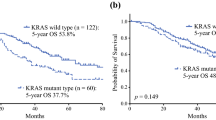
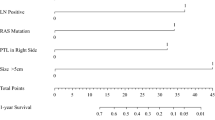
The new CS comprised mutant RAS status, ≥4 CLMs, and a CA19-9 level ≥100 U/mL (1 point per factor). Both the new CS and m-Fong CS failed to stratify the survival of the 309 patients in the validation cohort, including those who did not receive perioperative chemotherapy (29%). Both of the CS accurately stratified the survival of patients who underwent perioperative chemotherapy but not of those who underwent surgery alone.
Conclusion
A CS that includes the RAS mutation status can stratify the survival of patients who undergo hepatectomy combined with perioperative chemotherapy, but it has limited value for patients who undergo surgery alone.



Similar content being viewed by others
References
Nordlinger B, Guiguet M, Vaillant JC et al (1996) Surgical resection of colorectal carcinoma metastases to the liver. a prognostic scoring system to improve case selection, based on 1568 patients. Assoc Francaise de Chirurgie Cancer 77:1254–1262
Passot G, Denbo JW, Yamashita S et al (2017) Is hepatectomy justified for patients with RAS mutant colorectal liver metastases? an analysis of 524 patients undergoing curative liver resection. Surgery 161:332–340
Nordlinger B, Sorbye H, Glimelius B et al (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–1215
Alberts SR, Horvath WL, Sternfeld WC et al (2005) Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer treatment group phase II study. J Clin Oncol off J Am Soc Clin Oncol 23:9243–9249
Pozzo C, Basso M, Cassano A et al (2004) Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Annals Oncol Off J Eur Soc Med Oncol/ESMO 15:933–939
Petrelli F, Barni S (2012) Resectability and outcome with anti-EGFR agents in patients with KRAS wild-type colorectal liver-limited metastases: a meta-analysis. Int J Colorectal Dis 27:997–1004
Fong Y, Fortner J, Sun RL, et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Annals of surgery 230:309–318; discussion 318–321
Kattan MW, Gonen M, Jarnagin WR et al (2008) A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg 247:282–287
Beppu T, Sakamoto Y, Hasegawa K et al (2012) A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a project study for hepatic surgery of the japanese society of hepato-biliary-pancreatic surgery. J Hepato-Biliary-Pancreat Sci 19:72–84
Imai K, Allard MA, Castro Benitez C et al (2016) Nomogram for prediction of prognosis in patients with initially unresectable colorectal liver metastases. Br J Surg 103:590–599
Van Cutsem E, Cervantes A, Adam R et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol Off J Eur SocMed Oncol/ESMO 27:1386–1422
Petrowsky H, Sturm I, Graubitz O et al (2001) Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol J Eur Soc Surg Oncol British Assoc Surg Oncol 27:80–87
Cejas P, Lopez-Gomez M, Aguayo C et al (2009) KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS ONE 4:e8199
Russo A, Migliavacca M, Bazan V et al (1998) Prognostic significance of proliferative activity, DNA-ploidy, p53 and Ki-ras point mutations in colorectal liver metastases. Cell Prolif 31:139–153
Rose JS, Serna DS, Martin LK et al (2012) Influence of KRAS mutation status in metachronous and synchronous metastatic colorectal adenocarcinoma. Cancer 118:6243–6252
Ichida H, Mise Y, Ito H et al (2019) Optimal indication criteria for neoadjuvant chemotherapy in patients with resectable colorectal liver metastases. World J Surg Oncol 17:100
Matsumura M, Mise Y, Saiura A et al (2016) Parenchymal-sparing hepatectomy does not increase intrahepatic recurrence in patients with advanced colorectal liver metastases. Ann Surg Oncol 23:3718–3726
Vigano L, Capussotti L, Lapointe R et al (2014) Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment: a Liver Met Survey-based study of 6,025 patients. Ann Surg Oncol 21:1276–1286
Rees M, Tekkis PP, Welsh FK et al (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247:125–135
Adam R, Wicherts DA, de Haas RJ et al (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol off J Am Soc Clin Oncol 27:1829–1835
Masi G, Loupakis F, Pollina L et al (2009) Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg 249:420–425
Adam R, Delvart V, Pascal G, et al (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Annals of surgery 240:644–657; discussion 657–648
Sakamoto Y, Miyamoto Y, Beppu T et al (2015) Post-chemotherapeutic CEA and CA19-9 are prognostic factors in patients with colorectal liver metastases treated with hepatic resection after oxaliplatin-based chemotherapy. Anticancer Res 35:2359–2368
Lu Z, Peng J, Wang Z et al (2016) High preoperative serum CA19-9 level is predictive of poor prognosis for patients with colorectal liver oligometastases undergoing hepatic resection. Med OncoL (Northwood, London, England) 33:121
Vauthey JN, Kopetz SE (2013) From multidisciplinary to personalized treatment of colorectal liver metastases: 4 reasons to consider RAS. Cancer 119:4083–4085
Brudvik KW, Kopetz SE, Li L et al (2015) Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg 102:1175–1183
Zimmitti G, Shindoh J, Mise Y et al (2015) RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol 22:834–842
Brudvik KW, Mise Y, Chung MH et al (2016) RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol 23:2635–2643
Tosi F, Magni E, Amatu A et al (2017) Effect of KRAS and BRAF mutations on survival of metastatic colorectal cancer after liver resection: a systematic review and meta-analysis. Clin colorectal cancer 16:e153–e163
Ciliberto D, Prati U, Roveda L et al (2012) Role of systemic chemotherapy in the management of resected or resectable colorectal liver metastases: a systematic review and meta-analysis of randomized controlled trials. Oncol Rep 27:1849–1856
Nigri G, Petrucciani N, Ferla F et al (2015) Neoadjuvant chemotherapy for resectable colorectal liver metastases: what is the evidence? results of a systematic review of comparative studies. Surg J Royal Coll Surg Edinb Irel 13:83–90
Wieser M, Sauerland S, Arnold D et al (2010) Peri-operative chemotherapy for the treatment of resectable liver metastases from colorectal cancer: a systematic review and meta-analysis of randomized trials. BMC Cancer 10:309
Brudvik KW, Jones RP, Giuliante F et al (2019) RAS mutation clinical risk score to predict survival after resection of colorectal liver metastases. Ann Surg 269:120–126
Zakaria S, Donohue JH, Que FG et al (2007) Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg 246:183–191
Iwatsuki S, Dvorchik I, Madariaga JR et al (1999) Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 189:291–299
Shindoh J, Loyer EM, Kopetz S et al (2012) Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol off J Am Soc Clin Oncol 30:4566–4572
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Human and animal rights
The study protocol was approved by Institutional Review Board.
Informed consent
The institutional review board waived the need for informed consent to be obtained from the patients for the use of their medical records, imaging records, or pathology records.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takeda, Y., Mise, Y., Matsumura, M. et al. Accuracy of Modern Clinical Risk Score Including RAS Status Changes Based on Whether Patients Received Perioperative Chemotherapy for Colorectal Liver Metastases. World J Surg 45, 2176–2184 (2021). https://doi.org/10.1007/s00268-021-05976-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-05976-x