Abstract
Background
An individualized treatment decision is based on the accurate evaluation of clinical risk factors and prognosis for resectable colorectal liver metastases. The current study aimed to develop an effective nomogram to predict progression-free survival (PFS) and to design a treatment schedule preoperatively.
Methods
The study enrolled a primary cohort of 532 patients with resectable colorectal liver metastases (CRLM) who underwent hepatic resection at two institutions and a validation cohort of 237 patients at two additional institutions with resectable CRLM between 1 January 2008 and 31 December 2018. A nomogram was created based on the independent predictors in the multivariable analysis of progression-free survival in the primary cohort. The predictive accuracy and discriminative ability of the nomogram were determined by the concordance index (C-index) and the calibration curve. The score was compared with the current standard Fong score and validated with an external cohort.
Results
The independent risk factors for CRLM patients identified in the multivariable analysis were tumor larger than 5 cm, more than one tumor, RAS mutation, primary lymph node metastasis, and primary tumor located on the right side. All five factors were considered in the nomogram. The C-index of the nomogram for predicting survival was 0.696. With external validation, the C-index of the nomogram for the prediction of the PFS was 0.682, which demonstrated that this model has a good level of discriminative ability. For high-risk patients (score > 16), neoadjuvant chemotherapy improved PFS and overall survival (OS) after hepatic resection.
Conclusion
The current nomogram demonstrated an accurate performance in predicting PFS for resectable CRLM patients with liver-limited disease. Based on the current nomogram, high-risk patients (nomogram score > 16) might benefit from neoadjuvant chemotherapy.



Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Leung U, Gonen M, Allen PJ, et al. Colorectal cancer liver metastases and concurrent extrahepatic disease treated with resection. Ann Surg. 2017;265:158–65.
Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–83.
Abdalla EK. Resection of colorectal liver metastases. J Gastrointest Surg. 2011;15:416–9.
Imai K, Allard MA, Benitez CC, et al. Early recurrence after hepatectomy for colorectal liver metastases: what optimal definition and what predictive factors? Oncologist. 2016;21:887–94.
de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–8.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18 (discussion 318–321).
Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62.
Nielsen K, Rolff HC, Eefsen RL, Vainer B. The morphological growth patterns of colorectal liver metastases are prognostic for overall survival. Mod Pathol. 2014;27:1641–8.
Brudvik KW, Jones RP, Giuliante F, et al. RAS mutation clinical risk score to predict survival after resection of colorectal liver metastases. Ann Surg. 2019;269:120–6.
Reissfelder C, Rahbari NN, Koch M, et al. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:3279–88.
Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15.
Strowitzki MJ, Schmidt T, Keppler U, et al. Influence of neoadjuvant chemotherapy on resection of primary colorectal liver metastases: a propensity score analysis. J Surg Oncol. 2017;116:149–58.
Imai K, Allard MA, Castro Benitez C, et al. Nomogram for prediction of prognosis in patients with initially unresectable colorectal liver metastases. Br J Surg. 2016;103:590–9.
Adams RB, Aloia TA, Loyer E, et al. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB Oxf. 2013;15:91–103.
Iwatsuki S, Dvorchik I, Madariaga JR, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–9.
Sasaki K, Morioka D, Conci S, et al. The tumor burden score: a new “metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg. 2018;267:132–41.
Amano R, Yamada N, Nakata B, et al. Prognostic indicator for the resection of liver metastasis of colorectal cancer. Surg Today. 2014;44:1287–92.
Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: the evolution of determining prognosis. World J Gastrointest Oncol. 2013;5:207–21.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Tosi F, Magni E, Amatu A, et al. Effect of KRAS and BRAF mutations on survival of metastatic colorectal cancer after liver resection: a systematic review and meta-analysis. Clin Colorectal Cancer. 2017;16:e153–63.
Liu W, Wang HW, Wang K, Xing BC. The primary tumor location impacts survival outcome of colorectal liver metastases after hepatic resection: a systematic review and meta-analysis. Eur J Surg Oncol. 2019;45:1349–56.
Wang XY, Zhang R, Wang Z, et al. Meta-analysis of the association between primary tumour location and prognosis after surgical resection of colorectal liver metastases. Br J Surg. 2019;106:1747–60.
Margonis GA, Sasaki K, Gholami S, et al. Genetic and morphological evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg. 2018;105:1210–20.
Zakaria S, Donohue JH, Que FG, et al. Hepatic resection for colorectal metastases: value for risk-scoring systems? Ann Surg. 2007;246:183–91.
Pollock CB, Shirasawa S, Sasazuki T, Kolch W, Dhillon AS. Oncogenic K-RAS is required to maintain changes in cytoskeletal organization, adhesion, and motility in colon cancer cells. Cancer Res. 2005;65:1244–50.
Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70.
Nasti G, Ottaiano A, Berretta M, et al. Preoperative chemotherapy for colorectal cancer liver metastases: an update of recent clinical trials. Cancer Chemother Pharmacol. 2010;66:209–18.
Chua TC, Saxena A, Liauw W, Kokandi A, Morris DL. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann Surg Oncol. 2010;17:492–501.
Ayez N, van der Stok EP, Grunhagen DJ, et al. The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: clinical risk score as possible discriminator. Eur J Surg Oncol. 2015;41:859–67.
Acknowledgment
This study was supported by Grants (No. 81874143 and No. 31971192) from the National Nature Science Foundation of China and Beijing Hospitals Authority Youth Program (code: QMS20201105). No preregistration exists for the studies reported in this article. We acknowledge Kun Wang, who contributed toward the study by making substantial contributions to the acquisition of the data, and Jie-Ying Liang, Lian-Meng Huang, and Hong-Tu Zheng, who made substantial contributions to the analysis and interpretation of the data. All these contributors were involved in drafting the manuscript but did not meet the criteria for authorship.
Funding
The manuscript has not been a podium or poster meeting. We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
There are no conflict of interest.
Ethics Approval and Consent to Participate
The present study is a retrospective study and all subjects have given their written informed consent. The investigation project has been examined and certified by Ethics Committee of Beijing Cancer Hospital. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2021_10143_MOESM1_ESM.docx
Supplementary Table 1. Demographic and clinical characteristics of study patients in NC and upfront resection groups. (DOCX 100 KB)
10434_2021_10143_MOESM7_ESM.jpg
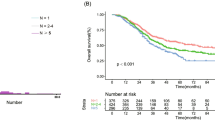
Supplementary Figure 2a,2b. Kaplan-Meier Curve showing OS and PFS of primary cohort (2a) and validation cohort (2b). (PDF 32 KB)
10434_2021_10143_MOESM8_ESM.jpg
Supplementary Figure 2a,2b. Kaplan-Meier Curve showing OS and PFS of primary cohort (2a) and validation cohort (2b). (PDF 31 KB)
10434_2021_10143_MOESM9_ESM.jpg
Supplementary Figure 3a,3b,3c. Kaplan-Meier Curve showing OS and PFS of primary cohort (2a) and validation cohort (2b). (PDF 172 KB)
10434_2021_10143_MOESM10_ESM.jpg
Supplementary Figure 3a,3b,3c. Kaplan-Meier Curve showing OS and PFS of primary cohort (2a) and validation cohort (2b). (PDF 167 KB)
10434_2021_10143_MOESM11_ESM.jpg
Supplementary Figure 3a,3b,3c. Kaplan-Meier Curve showing OS and PFS of primary cohort (2a) and validation cohort (2b). (PDF 167 KB)
10434_2021_10143_MOESM12_ESM.jpg
Supplementary Figure 4. Performance of the nomogram in predicting PFS. Time-dependent AUC values showed the performance of the nomogram and other models in predicting PFS in the primary cohort (4a), validation cohort (4b). (PDF 25 KB)
10434_2021_10143_MOESM13_ESM.jpg
Supplementary Figure 4. Performance of the nomogram in predicting PFS. Time-dependent AUC values showed the performance of the nomogram and other models in predicting PFS in the primary cohort (4a), validation cohort (4b). (PDF 34 KB)
10434_2021_10143_MOESM16_ESM.jpg
Supplementary Figure 6. Kaplan-Meier Curve showing PFS of NC and upfront resection in low-risk(6a) and high-risk (6b) groups. (PDF 31 KB)
10434_2021_10143_MOESM17_ESM.jpg
Supplementary Figure 6. Kaplan-Meier Curve showing PFS of NC and upfront resection in low-risk(6a) and high-risk (6b) groups. (PDF 31 KB)
10434_2021_10143_MOESM18_ESM.jpg
Supplementary Figure 7. Kaplan-Meier Curve showing OS of NC and upfront resection in low-risk(7a) and high-risk (7b) groups. (PDF 31 KB)
10434_2021_10143_MOESM19_ESM.jpg
Supplementary Figure 7. Kaplan-Meier Curve showing OS of NC and upfront resection in low-risk(7a) and high-risk (7b) groups. (PDF 31 KB)
Rights and permissions
About this article
Cite this article
Liu, W., Zhang, W., Xu, Y. et al. A Prognostic Scoring System to Predict Survival Outcome of Resectable Colorectal Liver Metastases in this Modern Era. Ann Surg Oncol 28, 7709–7718 (2021). https://doi.org/10.1245/s10434-021-10143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10143-6