Abstract
Background/purpose
The aim of this study was to create a nomogram to predict the disease-free survival of patients with colorectal liver metastases treated with hepatic resection.
Methods
Perioperative factors were assessed in 727 hepatectomized patients with colorectal liver metastases between 2000 and 2004 at the 11 institutions of the “Project Committee of the Liver” in the Japanese Society of Hepato-Biliary-Pancreatic Surgery. A nomogram was developed as a graphical representation of a stepwise Cox proportional hazards regression model.
Results
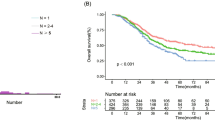
Perioperative mortality was 0.55%. Disease-free and overall survival rates were 31.2 and 63.8% at 3 years, 27.2 and 47.7% at 5 years, and 24.7 and 38.5% at 10 years, respectively. Six preoperative factors were selected to create the nomogram for disease-free survival: synchronous metastases, 3 points; primary lymph node positive, 3 points; number of tumors 2–4, 4 points and ≥5, 9 points; largest tumor diameter >5 cm, 2 points; extrahepatic metastasis at hepatectomy, 4 points, and preoperative carbohydrate antigen 19-9 level >100, 4 points. The estimated median disease-free survival time was easily calculated by the nomogram: >8.4 years for patients with 0 points, 1.9 years for 5 points, 1.0 years for 10 points, and the rates were lower than 0.6 years for patients with more than 10 points.
Conclusions
This nomogram can easily calculate the median and yearly disease-free survival rates from only 6 preoperative variables. This is a very useful tool to determine the likelihood of early recurrence and the necessity for perioperative chemotherapy in patients with colorectal liver metastases after hepatic resection.



Similar content being viewed by others
Abbreviations
- CRLM:
-
Colorectal liver metastases
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- FOLFOX:
-
Chemotherapy with oxaliplatin plus fluorouracil and leucovorin
- FOLFIRI:
-
Chemotherapy with irinotecan plus fluorouracil and leucovorin
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- BMI:
-
Body mass index
- LN:
-
Lymph node
- CEA:
-
Carcinoembryonic antigen
- CA19-9:
-
Carbohydrate antigen 19-9
- Hr1:
-
One sectionectomy
- RCC:
-
Red cell concentrate
References
Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–10.
Jaeck D, Bachellier P, Guiguet M, Boudjema K, Vaillant JC, Balladur P, et al. Long-term survival following resection of colorectal hepatic metastases: Association Francaise de Chirurgie. Br J Surg. 1997;84:977–80.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18.
Adam R, Pascal G, Azoulay D, Tanaka K, Castaing D, Bismuth H. Liver resection for colorectal metastases: the third hepatectomy. Ann Surg. 2003;238:871–83.
Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–72.
de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–8.
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–57.
Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–9.
Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–20.
Figueras J, Valls C, Rafecas A, Fabregat J, Ramos E, Jaurrieta E. Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg. 2001;88:980–5.
Beppu T, Hayashi N, Masuda T, Komori H, Horino K, Hayashi H, et al. FOLFOX enable resectability and excellent prognosis for initially unresectable colorectal liver metastases. Anticancer Res. 2010;30:1015–20.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16.
Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62.
Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–9.
Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–91.
Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–14.
Kattan MW, Gönen M, Jarnagin WR, DeMatteo R, D’Angelica M, Weiser M, et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:282–7.
Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–35.
Kanemitsu Y, Kato T. Prognostic models for predicting death after hepatectomy in individuals with hepatic metastases from colorectal cancer. World J Surg. 2008;32:1097–107.
Gregoire E, Hoti E, Gorden DL, de la Serna S, Pascal G, Azoulay D. Utility or futility of prognostic scoring systems for colorectal liver metastases in an era of advanced multimodal therapy. Eur J Surg Oncol. 2010;36:568–74.
Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, et al. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;1612:3279–88.
Beppu T, Horino K, Komori H, Sugiyama S, Masuda T, Hayashi H, et al. Thermal ablation for colorectal liver metastases. Thermal Med. 2008;24:83–9.
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI Followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37.
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44.
Van Cutsem E, Nowacki M, Lang I, Cascinu S, Shchepotin I, Maurel J, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): the CRYSTAL trial. J Clin Oncol. 2007;25(18S suppl):A4000.
Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–82.
Park IJ, Choi GS, Jun SH. Prognostic value of serum tumor antigen CA19-9 after curative resection of colorectal cancer. Anticancer Res. 2009;29:4303–8.
Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525–31.
Nakagoe T, Sawai T, Tsuji T, Jibiki MA, Nanashima A, Yamaguchi H, et al. Difference in prognostic value between sialyl Lewis(a) and sialyl Lewis(x) antigen levels in the preoperative serum of gastric cancer patients. J Clin Gastroenterol. 2002;34:408–15.
Acknowledgments
The authors give special thanks to Dr. Hirohisa Okabe for his tremendous contribution to analysis of the database.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary Figure: Instructions for physician: Click the bottom of “status” in “Preoperative Score” and choose the individual status of 6 parameters. You can instantly find not only the patient’s estimated disease-free survival curve but also the median and the rates of disease-free survival at 3 and 5 years.
About this article
Cite this article
Beppu, T., Sakamoto, Y., Hasegawa, K. et al. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 19, 72–84 (2012). https://doi.org/10.1007/s00534-011-0460-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-011-0460-z