Abstract
Background
Improvements to autologous fat grafting for soft tissue augmentation are needed to overcome the unpredictable volume retention. Approaches such as fat harvesting and processing, injection technique, preparation of the recipient site, and supplemental biologics are topics of ongoing research. Here, an energy-based device was investigated as a stimulatory tool for recipient site preparation for improving fat graft retention.
Objective
The objective was to measure the stimulatory responses in fat grafts after 4 weeks when using a helium-based radiofrequency device to pretreat the recipient tissue.
Methods
Using an autologous fat grafting mouse model, the inguinal fat pad was grafted in a small cranial pocket after either a saline injection alone (control) or a saline injection followed by pretreatment (treated). The fat pad was resected after 4 weeks, sectioned and stained with immunofluorescence markers to investigate tissue remodeling.
Results
Pretreatment resulted in higher viability of adipocytes, a higher concentration of viable ASCs in areas of adipose tissue regeneration, and localized macrophages in the areas of regeneration when compared to the control. There was no observable difference in vascularity or angiogenesis. The staining for ASCs was higher in the pretreated group in comparison with the control group (5.0% vs. 3.3%, p=0.36) when using a pixel classifier in QuPath in the viable adipose tissue regions.
Conclusions
The use of a helium-based radiofrequency device as a pretreatment tool appears to increase the viability of the adipose tissue likely due to higher concentration of ASCs. The apparent increase in viable ASCs may be due to enhanced proliferation or paracrine recruitment of these cells in response to the helium-based radiofrequency treatment.
No Level Assigned
This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Bullet List of Important Points:
-
Pretreatment of the fat graft recipient site increases the viability of the adipose tissue after 4 weeks in comparison with the control grafts.
-
The increased viability is likely due to the observed increase in adipose-derived stem cells in the pretreated group.
-
Pretreatment enhanced the adipose tissue remodeling as colocalization of adipose-derived stem cells and macrophages showed an active remodeling, whereas the control group exhibited more necrotic and fibrotic tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
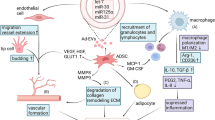
Widespread adoption of autologous fat grafting has led to numerous reconstructive, esthetic, and regenerative applications [1,2,3]. The benefits of autologous fat grafting include its inherent biocompatibility, low cost, accessibility, and low immunogenicity. Due to its myriad uses, surgeons from different medical specialties developed new techniques geared toward increasing fat retention. These developments in fat harvesting, fat processing, preparation for the donor site and the recipient site, and fat grafting techniques have improved patient outcomes. For soft tissue augmentation, these techniques enhance fat retention by increasing fat graft survival. However, there is no standardized method for autologous fat grafting leading to unpredictability and variable rates of fat retention.
Strategies to enhance autologous fat graft retention aim to increase angiogenesis into the graft and the proliferation of progenitor cells [4]. Grafted adipose tissue loses volume as mature adipocytes undergo apoptosis within the first 72 hours due to a lack of oxygen and nutrients [5]. Adipose-derived stem cells (ASCs) are more resistant to these hypoxic conditions and begin replacing the dead adipocytes after these older cells are removed by macrophages. The new blood vessels provide the cells, nutrients, and growth factors to support this remodeling of the fat graft. Adipose tissue that remains without oxygen for too long becomes necrotic leading to tissue fibrosis and cyst formation. Approaches to overcome poor retention for soft tissue augmentation increase tissue oxygen levels, the survival and number of progenitor cells, and growth factors in the recipient site. The main strategies include physical manipulation of the recipient site (e.g., BRAVA®, microneedling, biostimulation of tissue) [6, 7], or injection of either botulinum toxin for facial fat graft [8], growth factors [9,10,11], or progenitor cells during the fat graft, i.e., cell-assisted lipotransfer (CAL) [12]. Supplementation of autologous fat grafts with ASCs [13], platelet-rich plasma (PRP) [14], stromal vascular fraction (SVF) [15], or exosomes [16] either alone or in combination have increased fat graft retention [17]. These techniques lead to increased vascularity within the graft and increased proliferation and number of ASCs.
An uncommon technique for soft tissue augmentation is the use of energy-based devices for recipient site preparation prior to grafting the fat. While ultrasound and laser devices are successfully used for removal of fat [18, 19], energy-based devices have rarely been investigated for their tissue stimulating properties regarding recipient site preparation. Tissue biostimulation using a subdermal fractional CO2 laser was utilized by Jianu et al. to improve patient outcomes from facial fat grafting. The approach uses low-level laser therapy, hypothesized to induce ASCs to release proangiogenic and proliferative factors as observed in vitro [7]. The resulting photobioactivation changes the redox state of the ASCs and likely the surrounding tissue [20].
Renuvion (Apyx Medical Corporation, Clearwater, Florida), a helium-based radiofrequency device that combines radiofrequency (rf) energy and helium plasma, may be another approach to stimulate the tissue by combining their two effects: promotion of angiogenesis through heating the tissue, and the proliferation of ASCs and paracrine recruitment of immune cells in response to plasma treatment [21,22,23,24,25]. Furthermore, plasma treatment in mouse wound models was shown to augment tissue oxygenation [26]. The combination of these effects may prove to be another strategy to promote fat graft retention.
For this study, we hypothesized that pretreatment of the recipient site with a helium-based radiofrequency device stimulates the tissue to enhance fat graft retention. To investigate this, the viability, vascularity, and the presence of macrophages and ASCs in the fat graft were stained in FFPE slides at 4 weeks in pretreated mice in comparison with the controls. Given the anecdotal evidence for plasma-enhanced recruitment of immune cells, enhanced proliferation of ASCs, increased tissue oxygenation, and promotion of angiogenesis, it was hypothesized that pretreatment of the recipient tissue would enhance fat grafting of bolus fat grafts. We observed more viable adipose tissue in the pretreated group in comparison with the control mice. Additionally, the overlapping location of ASCs with macrophages in the pretreated group suggested increased remodeling activity of the dead adipose tissue. The pretreatment did not show any evidence for enhanced angiogenesis or increased secretion of vascular endothelial growth factor (VEGF). Furthermore, increased fibrosis and damaged adipose tissue morphology were observed in the control group.
Materials and Methods
The study was conducted at the Comparative Medicine facility at the University of South Florida (USF) and approved by the USF’s Institutional Animal Care and Use Committee (IACUC). The paraffin embedding, sectioning and immunofluorescence staining were performed by the Molecular Pathology Core at the University of Florida. Whole slide imaging performed by the Analytic Microscopy Core Facility at H. Lee Moffitt Cancer Center.
Mouse Model for Autologous Fat Grafting
The animals used in this study were cared for in accordance with the USF institutional guidelines. Fifteen mice (Eight-week-old female C57BL/6, Jackson Labs) were split into the following three study arms: control, pretreated, and baseline. The control and pretreated mice were anesthetized with 2% isoflurane, whereas the baseline mice were euthanized under CO2 for initial fat pad collections. Following collection, the inguinal fat pads were weighed, and the donor sites were sutured closed in both the control and pretreated groups. The fat pad (85 mg on average) was then inserted into the recipient site which followed the cranial fat graft model as published by Kato et al. [27]. Briefly, a small pocket was made on top of the cranium through a small incision (5 mm long) and expanded using forceps. The inguinal fat pad from the same animal was then inserted into the recipient site after either injection of 0.1 ml of saline (control), or after injection of 0.1 ml of saline followed by pretreatment (helium-based rf device). Mice were euthanized 28 days later, and the grafted fat pad was excised and weighed. The baseline, control, and pretreated group fat pads were fixed in 10% neutral buffered formalin (Fisher Scientific) for 24 hours, then washed twice with 1X PBS and embedded in paraffin for immunofluorescence staining and assessment.
Image Acquisition
Fluorescence-labeled slides were scanned at 20X (0.5 mm) with an Akoya Fusion slide scanner equipped with fluorescence LED light source and DAPI, OPAL520, OPAL570, and OPAL690 filters. The same acquisition settings were used among slides within each staining panel. Whole slide images were created in QPTIFF format.
Immunofluorescence Assessment
The fat grafts in this study were resected and compared at 4 weeks post-graft to assess energy-based pretreatment as a recipient tissue preparation technique. Viable adipocytes and ASCs, macrophages, and the vascularity were visualized by immunofluorescence staining for perilipin and CD44, MAC2, and CD31, respectively. After preparing 4-mm-thick sections from the paraffin-embedded sections, the slides were stained with the following primary antibodies at 4°C overnight: guinea pig anti-mouse perilipin (Progen, 1:100 dilution), rat anti-mouse CD44 (Invitrogen, 1:200 dilution), goat anti-mouse CD31 (R&D Systems, 1:25 dilution), rabbit anti-mouse VEGF (Invitrogen 1:100 dilution), and rat anti-mouse MAC2/Galectin 3 (Invitrogen, 1:100 dilution). The following secondary antibodies were used at room temperature for triple fluorescence staining: Alexa Fluor 488-conjugated donkey anti-guinea pig immunoglobulin G (Jackson ImmunoResearch Labs, 1:500 dilution), Alexa Fluor 647-conjugated donkey anti-rat immunoglobulin G (Jackson ImmunoResearch Labs, 1:500 dilution), Cyanine3-conjugated donkey anti-goat immunoglobulin G (Jackson ImmunoResearch Labs, 1:500 dilution), Cyanine3-conjugated donkey anti-rabbit immunoglobulin G (Jackson ImmunoResearch Labs, 1:500 dilution). A slide with the three associated secondary antibody staining was used as a negative control for each triple fluorescence-stained slide. Nuclei were stained with DAPI (Invitrogen, 1:500 dilution). All images were spectrally unmixed in Phenochart. The ASC quantification was done in QuPath using a pixel threshold classifier after annotating the viable adipocyte region in each mouse tissue.
Results
Helium-based Radiofrequency Pretreatment Increased Adipose Viability
At baseline, the adipose tissue is healthy with uniformly sized and distributed adipocytes as shown in Fig. 1. The perilipin positive stain in the cytoplasm denotes viable adipose tissue, and the smaller, strongly perilipin positive cells are the preadipocytes, or ASCs. After four weeks post-procedure, the control and pretreated grafts are in the middle of the remodeling phase; the older non-viable adipocyte tissue is recycled and the preadipocytes mature into new, viable tissue. The control tissue after four weeks exhibits a small region of healthy tissue near the boundaries of the tissue and a larger necrotic region as shown by the perilipin negative center. The center tissue morphology also suggests the formation of fibrotic tissue similar to observations by Choi et al detailing the increase in collagen fibers after grafting through 16 weeks (supplemental Figure 1) [28]. Similar to Kato et al., there is a low presence of ASCs observed in the center of the tissue [27]. In contrast, the pretreated grafts showed a larger amount of surviving adipocytes and remodeling from ASCs. The viability of the adipocytes is observed several hundreds of micron deeper into the tissue than that of the controls, and the remodeling is still ongoing as evident by the uneven morphology. The morphology of the pretreated grafts is more similar to the baseline tissue than the control fat graft, suggesting that the remodeling phase is progressing more rapidly. The decrease in fibrotic tissue in the pretreated grafts in comparison with the control grafts suggests there is less damaged adipose tissue four weeks after the initial graft.
Helium-based radiofrequency pretreatment enhances the viability of the adipose tissue in comparison to the control. The sectioned tissue stained with perilipin to assess viability is shown for the baseline (day 0, left), pretreatment (day 28, middle), and control (day 28, right). The top row are whole slide images (scale bar = 800 mm), and the bottom row are 10x sections (scale bar = 100 mm) denoted by the yellow box. Pretreatment enhances the viability at 28 days in comparison to the control. The morphology and presence of ASCs in the pretreated grafts are more similar to the baseline than the control.
The Remodeling Phase is More Robust Following Helium-based Radiofrequency Pretreatment
The remodeling phase of fat graft retention requires the removal of dead adipocytes by macrophages, the proliferation of ASCs and their maturation into mature adipocytes, and the growth of new vascularity into the tissue. The results of these processes after four weeks are visualized in Figure 2. The top row shows the merged comparison between pretreated and control where the adipose viability (green, perilipin, second row), vascularity (red, CD31, third row), and ASCs (purple, CD44, fourth row) are compared in areas undergoing remodeling. The bottom row shows the presence of macrophages (orange, MAC2) stained on a separate section of the same tissue.
Helium-based radiofrequency pretreatment of the recipient tissue enhanced the adipose tissue remodeling 4 weeks after the initial graft. The merged image shows staining for fat viability (perilipin, green), vascularity (CD31, red), and an adipose derived stem cell surface marker (CD44, purple). The individual images for perilipin, CD31, and CD44 are shown below the merged image in row 2, row 3 and row 4 respectively. Macrophages are shown in the bottom row (MAC2, orange) from a different stained section of the same tissue. The fat graft with pretreatment (left column) exhibits stronger staining for viability and ASCs in comparison to the controls (right column). There is no difference in vascularity staining between Renuvion pretreatment and the control. The macrophages in the pretreated group are more localized in the remodeling tissue in contrast to their more uniform distribution in the control group.
The remodeling of the adipose tissue was stimulated by the helium-based rf pretreatment in comparison with the controls. The adipose tissue in the pretreated graft retains the adipose morphology and stains perilipin positive for a few hundred microns into the fat graft, which indicates viable adipose tissue. Beyond these hundred microns, there are large lipid droplets without the surrounding adipocyte tissue, suggesting the accumulation of lipid droplets from dead adipocytes. The main difference between the pretreated and control grafts is the presence of strong perilipin-stained cells surrounding the lipid droplets. These cells colocalize with the staining for CD44, indicating that they are ASCs [29]. CD44 is a transmembrane glycoprotein and cell surface adhesion receptor that is present in many leukocytes and stem cells. CD44 signaling together with the presence of the strong perilipin staining indicates that these cells are viable ASCs [27, 30].
These viable ASCs exhibit stronger staining deeper into the tissue in the pretreated grafts. In combination with the viable mature adipocytes near the edge of the tissue, this observation suggests that the regeneration of the adipose tissue is ongoing deeper into the tissue. In addition, the macrophage staining intensifies deeper into the tissue, showing the active remodeling of tissue in comparison with the healthy adipose tissue in the first few hundred microns from the tissue edge. These macrophages have two functions that rely on their phenotype. The inflammatory M1 macrophages will remove the dead adipocytes and lipid droplets to allow for the ASCs to mature into healthy adipose tissue, while M2 macrophages will develop a cyst wall around the lipid droplets and fail to remodel the tissue [5]. The latter is finalized after the first three months of remodeling; thus, it is too early to diagnose which macrophage function is occurring after four weeks.
The control fat graft does not exhibit similar characteristics to the pretreated fat graft. Viable staining and adipose morphology quickly disappear about 100 microns away from the tissue edge. There is a small fraction of cells that are viable ASCs as depicted by the strong perilipin staining in combination with the CD44 stain; however, it is marginal and indicates that the remodeling phase is not recruiting as many ASCs into the tissue as the pretreated tissue. Furthermore, macrophage staining is present throughout the tissue instead of more localized, which suggests most of the dead adipocytes remain and there is not a large viable adipose tissue zone. The vascularity of the tissue as visualized by CD31 (Fig. 2) and VEGF (Supplemental Figure 2) shows similar staining to the pretreated fat grafts; thus, there is no observed difference in vascularity or signaling for angiogenesis between the samples.
The increase in viable tissue CD44 staining in the pretreated arm compared to the control is shown in Fig. 3. The images were spectrally unmixed, the viable regions were annotated, and a pixel classifier was applied in QuPath for CD44 staining. On average, the pretreated group (5.0%) increased the staining when compared to the control group (3.3%). The variance in the pretreated data was high, with three samples between 6-8% and two samples between 0.5 and 2%. These results suggest a higher concentration of ASCs in the pretreated fat grafts in comparison with the control.
Recipient pretreatment (n=5) increased the presence of ASCs into a bolus fat graft after four weeks in comparison to the control (n=5). The area of viable adipose tissue was measured in QuPath after spectral unmixing with Phenochart. The ASC marker was measured using a pixel classifier to measure ASC signal in areas with viable tissue. On average (middle line), pretreatment increased the signal of the ASC marker, indicating increased presence of ASCs.
Discussion
The use of an energy-based device for stimulation of the recipient tissue was investigated for increased retention of fat grafts. Although new fat grafting techniques and animal models show increased retention using smaller fat grafts over a bolus fat graft model, the latter was used to assess if helium-based rf pretreatment could affect the remodeling of fat with large necrotic tissues in the center [31, 32]. This model allows for the assessment of the depth of effect, which in this study is shown to be improvement of at least a few hundred microns into the tissue at 4 weeks. The cranial fat graft model was selected and modified from Kato et al. because the fat was grafted in a site with minimal native fat and was easily accessible [27]. While typical animal retention studies assess fat grafts between 12 weeks and one year, the mechanisms of adipose tissue regeneration and the effects from energy-based pretreatment occur on shorter times scales. Four weeks were chosen because Kato et al. observed the highest concentration of ASCs at this time point [27]. The results discussed here will need to be proven in larger animal models and clinical studies to project any applicability of recipient site pretreatment using a helium-based rf pretreatment for large volume fat grafting. This results of this study and future animal studies will have limited direct applicability to humans; however, the results will build a foundation for potential mechanisms of action to pursue in clinical trials.
The results suggest that helium-based rf pretreatment leads to an increased viability of the fat grafts through higher concentrations of viable ASCs present in the tissue. These smaller, strongly perilipin positive cells localized near macrophages indicate that the ASCs are migrating to the locations where dead adipocytes and lipids are removed. The apparent increase in viable ASCs may be due to enhanced proliferation or paracrine recruitment of these cells in response to tissue pretreatment.
As shown by several groups, helium plasma treatment of ASCs in vitro resulted in increased proliferation of ASCs [23, 33]. Furthermore, helium plasma treatment stimulated ASCs by upregulating genes for stem cell proliferation while downregulating intrinsic apoptotic pathways and by activating ERK1/2, Akt and NF-kB through nitric oxide production [23, 34]. These activated signaling pathways in response to helium plasma are similar to pathways in ASCs stimulated by PRP [11]. The helium plasma produced by the helium-based rf device has similarities to other helium plasma devices described as cold atmospheric plasma (CAP), namely the production of reactive oxygen and nitrogen species (RONS), ultraviolet light, and electric field gradients [35]. The plasma discharge is created when rf energy delivered to the electrode is transferred to the helium gas, which creates an electrically conductive plasma. As this discharge interacts with medical solutions, such as saline or Ringer’s, the charged molecules in the plasma dissociate the liquid molecules and some recombine into bioactive RONS. Therefore, the enhanced recruitment or proliferation of ASCs in the grafted adipose tissue may be due to the RONS produced by the helium-based rf plasma and may result in similar stimulatory properties to PRP. Future studies are needed to tie these potential mechanisms of action to pretreatment of the recipient site and increased presence of ASCs in pretreated grafts.
The response in the recipient tissue to pretreatment may exhibit enhanced immune cell function as observed by cold plasma treatment [36, 37]; however, the pretreatment does not show any evidence for influencing angiogenesis into the tissue. In comparison with the study by Kato et al., the remodeling zone of the control groups looks similar at 4 weeks, and however, the helium-based rf pretreated groups resemble remodeling at 8 weeks [27]. Furthermore, there was less fibrotic tissue observed in the pretreated grafts in comparison with the control grafts. These observations suggest that either the biological effects of this pretreatment result in a hastened remodeling of the tissue, or that there are more immune cells recruited to the tissue that may contribute to the observed healthier tissue. However, immune cell function was not measured, and more studies are needed to prove recruitment of immune cells or enhancement of function contributed to the healthier adipose tissue in pretreated grafts. CD44 has a role in both pro- and anti-inflammatory environments for regulating immune cell migration and recruitment [30, 38, 39] and its higher signal in the pretreated fat grafts may influence the rate of adipose tissue remodeling. Unlike other reports suggesting increased angiogenesis due to rf energy or CAP treatment [22, 40, 41], the helium-based rf pretreatment of the tissue did not stimulate angiogenesis into the fat graft. This may be due to the difference in heat delivery to the tissue (rf energy), RONS generation and delivery to the tissue (plasma), or the differences in CAP devices. This also suggests that treatment using a helium-based rf device may stimulate the tissue differently than other energy-based devices, specifically low-level laser therapy (CO2 fractional lasers) where increased angiogenesis was observed post-treatment in vitro [7].
The mechanism of action behind the increased viability of the fat grafts with pretreated recipient sites will need more detailed investigations to show the role of recipient cells and donor graft cells. An extension of this model using native and green fluorescent protein expressing mice was utilized by Doi et al. to show that mature adipocytes mostly derived from the donor tissue ASCs after 12 weeks of remodeling [42]. For vascularity, the mural cells and nearly half of the vascular endothelial cells originated from the donor and recipient bone marrow, respectively. The recipient immune cells were shown to infiltrate into the areas of necrotic adipose tissue. These results serve as a suitable model to further investigate if the helium-based rf pretreatment affects the immune cell recruitment and ASC replacement into the tissue. The time points to investigate would also need to change from the current study (4 weeks) to measure promotion of angiogenesis and recruitment of immune cells (1-2 weeks) the long-term fat retention and formation of necrotic cysts (12 weeks).
Conclusion
This work is the first step in understanding the impact of helium-based radiofrequency pretreatment on tissue in anticipation of grafting fat, confirming what has been observed clinically. The stimulated tissue and cellular responses are consistent with other enhanced graft survival optimization techniques. The pretreatment increased the viability of the adipose tissue four weeks after the fat graft procedure. The results suggest the increase in viability is due to the higher concentration of ASCs in the tissue. The mechanism may be through either proliferation or recruitment by paracrine signaling. Future work needs to consider the complete impact of helium-based radiofrequency pretreatment for fat graft retention by investigating final retention volume after remodeling is completed, the mechanisms behind proliferation and migration of ASCs, and how this treatment would interact with native adipose tissue in the recipient site.
Change history
31 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00266-024-03853-1
References
Hsu VM, Stransky CA, Bucky LP, Percec I (2012) Fat grafting’s past, present, and future: why adipose tissue is emerging as a critical link to the advancement of regenerative medicine. Aesthet Surg J 32:892–899
La Padula S, Ponzo M, Lombardi M et al (2023) Nanofat in plastic reconstructive, regenerative, and aesthetic surgery: a review of advancements in face-focused applications. J Clin Med 12:4351
Wetterau M, Szpalski C, Hazen A, Warren SM (2012) Autologous fat grafting and facial reconstruction. J Craniofac Surg 23:315–318
Shih L, Davis MJ, Winocour SJ (2020) The science of fat grafting. Semin Plast Surg 34(1):5–10
Mashiko T, Yoshimura K (2015) How does fat survive and remodel after grafting? Clin Plast Surg 42:181–190
Oranges CM, Striebel J, Tremp M et al (2019) The preparation of the recipient site in fat grafting: a comprehensive review of the preclinical evidence. Plast Reconstr Surg 143:1099–1107
Constantin A, Dumitrescu M, Mihai MC et al (2017) CO 2 laser increases the regenerative capacity of human adipose-derived stem cells by a mechanism involving the redox state and enhanced secretion of pro-angiogenic molecules. Lasers Med Sci 32:117–127
Wang Z, Cheng R, Du Y et al (2023) The retention-rate improvement of stromal vascular fraction gel in prefrontal filling with botulinum toxin-a injection: a retrospective analysis. Aesthet Surg J. https://doi.org/10.1093/asj/sjac332
Topcu A, Aydin OE, Ünlü M et al (2012) Increasing the viability of fat grafts by vascular endothelial growth factor. Arch Facial Plast Surg 14:270–276
Nakamura S, Ishihara M, Takikawa M et al (2010) Platelet-rich plasma (PRP) promotes survival of fat-grafts in rats. Ann Plast Surg 65:101–106
Lai F, Kakudo N, Morimoto N et al (2018) Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res Ther 9:1–10
Sterodimas A, de Faria J, Nicaretta B et al (2010) Cell-assisted lipotransfer. Aesthet Surg J 30:78–81
Sterodimas A, de Faria J, Nicaretta B, Boriani F (2011) Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: a study. Aesthet Surg J 31:682–693
Gentile P, Kothari A, Casella D, Calabrese C (2020) Fat graft enhanced with adipose-derived stem cells in aesthetic breast augmentation: clinical, histological, and instrumental evaluation. Aesthet Surg J 40:962–977
Tiryaki T, Cohen SR, Canikyan Turkay S et al (2022) Hybrid stromal vascular fraction (Hybrid-SVF): a new paradigm in mechanical regenerative cell processing. Plast Reconstr Surg Glob Open 10:e4702. https://doi.org/10.1097/GOX.0000000000004702
Chen B, Cai J, Wei Y et al (2019) Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plast Reconstr Surg 144:816e–827e
Moustaki M, Papadopoulos O, Verikokos C et al (2017) Application of adipose-derived stromal cells in fat grafting: Basic science and literature review. Exp Ther Med 14:2415–2423
Sterodimas A, Boriani F, Magarakis E, et al (2012) Thirtyfour years of liposuction: past, present and future. Eur Rev Med Pharmacol Sci 16(3):393–406
Goldman A, Schavelzon D, Blugerman G (2001) Laserlipolysis: liposuction using Nd-YAG laser. Rev Bras Cir Plástica 17:17–26
Jianu DM, Filipescu M, Jianu SA et al (2012) The sinergy between lasers and adipose surgery in face and neck rejuvenation: A new approach from personal experience. Laser Ther 21:215–222
Ruff PG IV (2021) Thermal effects of percutaneous application of plasma/radiofrequency energy on porcine dermis and fibroseptal network. J Cosmet Dermatol 20:2125–2131
Dayan E, Chia C, Burns AJ, Theodorou S (2019) Adjustable depth fractional radiofrequency combined with bipolar radiofrequency: a minimally invasive combination treatment for skin laxity. Aesthet Surg J 39:S112
Park J, Lee H, Lee HJ et al (2016) Non-thermal atmospheric pressure plasma efficiently promotes the proliferation of adipose tissue-derived stem cells by activating NO-response pathways. Sci Rep 6:39298
Choi BBR, Choi JH, Ji J et al (2018) Increment of growth factors in mouse skin treated with non-thermal plasma. Int J Med Sci 15:1203–1209. https://doi.org/10.7150/ijms.26342
Chatraie M, Torkaman G, Khani M et al (2018) In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci Rep 8:1–11
Schmidt A, Nießner F, von Woedtke T, Bekeschus S (2020) Hyperspectral imaging of wounds reveals augmented tissue oxygenation following cold physical plasma treatment in vivo. IEEE Trans Radiat Plasma Med Sci 5:412–419
Kato H, Mineda K, Eto H et al (2014) Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg 133:303e–313e
Chen X, Deng Z, Feng J et al (2021) Necroptosis in macrophage foam cells promotes fat graft fibrosis in mice. Front Cell Dev Biol 9:651360
Mitchell JB, McIntosh K, Zvonic S et al (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. STEM CELLS 24:376–385. https://doi.org/10.1634/stemcells.2005-0234
Ponta H, Sherman L, Herrlich PA (2003) CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4:33–45
Zhang Z, Qin Z, Tang J, et al (2023) Particles of different sizes affect the retention pattern of the fat grafts in a mouse model. Aesthetic Plast Surg 47(5):2106–2116
Yang X, Egro FM, Jones T et al (2020) Comparison of adipose particle size on autologous fat graft retention in a rodent model. Plast Aesthet Res 7:8
Shojaei E, Zare S, Shirkavand A et al (2022) Biophysical evaluation of treating adipose tissue-derived stem cells using non-thermal atmospheric pressure plasma. Sci Rep 12:11127
Park J, Suh D, Tang T et al (2020) Non-thermal atmospheric pressure plasma induces epigenetic modifications that activate the expression of various cytokines and growth factors in human mesoderm-derived stem cells. Free Radic Biol Med 148:108–122
Weltmann K-D, von Woedtke T (2016) Plasma medicine—current state of research and medical application. Plasma Phys Control Fusion 59:014031. https://doi.org/10.1088/0741-3335/59/1/014031
Miller V, Lin A, Fridman G et al (2014) Plasma stimulation of migration of macrophages. Plasma Process Polym 11:1193–1197. https://doi.org/10.1002/ppap.201400168
Smolková B, Frtús A, Uzhytchak M et al (2020) Critical analysis of non-thermal plasma-driven modulation of immune cells from clinical perspective. Int J Mol Sci 21:6226
Johnson P, Ruffell B (2009) CD44 and its role in inflammation and inflammatory diseases. Former Curr Drug Targets - Inflamm Allergy 8:208–220. https://doi.org/10.2174/187152809788680994
Shi X, Leng L, Wang T et al (2006) CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 25:595–606
Arndt S, Unger P, Berneburg M et al (2018) Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J Dermatol Sci 89:181–190
Arjunan KP, Friedman G, Fridman A, Clyne AM (2012) Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J R Soc Interface 9:147–157
Doi K, Ogata F, Eto H et al (2015) Differential contributions of graft-derived and host-derived cells in tissue regeneration/remodeling after fat grafting. Plast Reconstr Surg 135:1607–1617
Acknowledgements
The authors thank Margi Baldwin and the Department of Comparative Medicine for coordinating and conducting the animal study, Ann Fu and the Molecular Pathology Core staff for the tissue processing and immunofluorescence staining, and Joseph Johnson and the Analytical Microscopy Core staff for image acquisition and discussions on image processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Both authors are consultants for Apyx Medical (Clearwater, FL). Drs. Ruff and Sterodimas are members of the medical advisory board for Apyx Medical. This study was funded by Apyx Medical Corporation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruff, P.G., Sterodimas, A. Enhanced Fat Graft Viability and Remodeling Using a Helium-based Radiofrequency Device to Prepare the Recipient Site. Aesth Plast Surg 48, 612–620 (2024). https://doi.org/10.1007/s00266-023-03749-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-023-03749-6