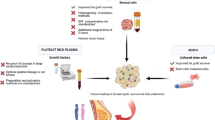
Abstract
Background
Autologous fat grafting has gained increasing popularity used in plastic surgery as a strategy to improve functional and aesthetic outcome. However, variable augmentation results have concerned surgeons in that volume loss of grafted fat reported fluctuates unsteadily.
Aim
An optimal technique that clinically maximizes the long-term survival rate of transplantation is in urgent need to be identified.
Method
The PubMed/MEDLINE database was queried to search for animal and human studies published through March of 2022 with search terms related to adipose grafting encompassing liposuction, adipose graft viability, processing technique, adipose-derived stem cell, SVF and others.
Results
45 in vivo studies met inclusion criteria. The principal of ideal processing technique is effective purification of fat and protection of tissue viability, such as gauze rolling and washing-filtration devices. Cell-assisted lipotransfer including SVF, SVF-gel and ADSCs significantly promotes graft retention via differentiation potential and paracrine manner. ADSCs induce polarization of macrophages to regulate inflammatory response, mediate extracellular matrix remodeling and promote endothelial cell migration and sprouting, and differentiate into adipocytes to replace necrotic cells, providing powerful evidence for the benefits and efficacy of cell-assisted lipotransfer.
Conclusion
Based on the current evidence, the best strategy can not be decided. Cell-assisted lipotransfer has great potential for use in regenerative medicine. But so far mechanically prepared SVF-gel is conducive to clinical promotion. PRP as endogenous growth factor sustained-release material shows great feasibility.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.


Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Vyas KS, Vasconez HC, Morrison S, Mogni B, Linton S, Hockensmith L, Kabir T, Zielins E, Najor A, Bakri K, Mardini S (2020) Fat Graft enrichment strategies: a systematic review. Plast Reconstr Surg 145:827–841
Li J, Gao J, Cha P, Chang Q, Liao Y, Liu C, Li K, Lu F (2013) Supplementing fat grafts with adipose stromal cells for cosmetic facial contouring. Dermatol Surg 39:449–456
Missana MC, Laurent I, Barreau L, Balleyguier C (2007) Autologous fat transfer in reconstructive breast surgery: indications, technique and results. Eur J Surg Oncol (EJSO) 33(6):685–690. https://doi.org/10.1016/j.ejso.2006.12.002
Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E (2017) Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg 20:49–60. https://doi.org/10.1016/j.amsu.2017.06.059
Strong AL, Rubin JP, Kozlow JH, Cederna PS (2019) Fat grafting for the treatment of scleroderma. Plast Reconstr Surg 144:1498–1507
Tabit CJ, Slack GC, Fan K, Wan DC, Bradley JP (2012) Fat grafting versus adipose-derived stem cell therapy: distinguishing indications, techniques, and outcomes. Aesth Plast Surg 36(3):704–713. https://doi.org/10.1007/s00266-011-9835-4
Wang GH, Zhao JF, Xue HY, Li D (2019) Facial aesthetic fat graft retention rates after filtration, centrifugation, or sedimentation processing techniques measured using three-dimensional surface imaging devices. Chin Med J 132(01):69–77
Gontijo-de-Amorim NF, Charles-de-Sá L, Rigotti G (2017) Mechanical supplementation with the stromal vascular fraction yields improved volume retention in facial lipotransfer: a 1-year comparative study. Aesthet Surg J 37:975–985
Zhang K, Liu F, Zhang Y, Huang X, Tang M, Hou Y, Lv Q, Jin D, Li Y, Kong L (2020) Mechanical vibration-extracted stromal vascular fraction improves volume retention after autologous fat grafting. Plast Reconstr Surg 146:1275–1284
Shridharani SM, Broyles JM, Matarasso A (2014) Liposuction devices: technology update. Med Dev (Auckland, NZ). 7:241
Molitor M, Trávníčková M, Měšťák O, Christodoulou P, Sedlář A, Bačáková L, Lucchina S (2021) The influence of low- and high-negative-pressure liposuction and different harvesting sites on the viability and yield of adipocytes and other nucleated cells. Aesthetic Plast Surg 45:2952–2970
Lee JH, Kirkham JC, McCormack MC, Nicholls AM, Randolph MA, Austen WG Jr (2013) The effect of pressure and shear on autologous fat grafting. Plast Reconstr Surg 131(5):1125–1136
Mojallal A, Auxenfans C, Lequeux C, Braye F, Damour O (2008) Influence of negative pressure when harvesting adipose tissue on cell yield of the stromal–vascular fraction. BioMed Mater Eng 18(4–5):193–197
Cucchiani R, Corrales L (2016) The effects of fat harvesting and preparation, air exposure, obesity, and stem cell enrichment on adipocyte viability prior to graft transplantation. Aesthet Surg J 36:1164–1173
Chen YW, Wang JR, Liao X, Li SH, Xiao LL, Cheng B, Xie GH, Song JX, Liu HW (2017) Effect of suction pressures on cell yield and functionality of the adipose-derived stromal vascular fraction. J Plast Reconstr Aesthetic Surg 70(2):257–66
Vazquez OA, Markowitz MI, Becker H (2020) Fat graft size: relationship between cannula and needle diameters. Cureus 12:e7598
Erdim M, Tezel E, Numanoglu A, Sav A (2009) The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J Plast Reconstr Aesthetic Surg 62(9):1210–1214
Kirkham JC, Lee JH, Medina MA, McCormack MC, Randolph MA, Austen WG (2012) The impact of liposuction cannula size on adipocyte viability. Ann Plast Surg 69(4):479–481. https://doi.org/10.1097/SAP.0b013e31824a459f
Shiffman MA, Mirrafati S (2001) Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg 27(9):819–826
Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno S, Yoshimura K (2012) The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg 129(5):1081–1092. https://doi.org/10.1097/PRS.0b013e31824a2b19
Carpaneda CA, Ribeiro MT (1993) Study of the histologic alterations and viability of the adipose graft in humans. Aesthetic Plast Surg 17(1):43–47. https://doi.org/10.1007/BF00455048
Alharbi Z, Opländer C, Almakadi S, Fritz A, Vogt M, Pallua N (2013) Conventional vs. micro-fat harvesting: how fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J Plast Reconstr Aesthetic Surg 66(9):1271–1278
Flynn TC, Coleman WP, Field LM, Klein JA, Hanke WC (2000) History of liposuction. Dermatol Surg 26(6):515–520. https://doi.org/10.1046/j.1524-4725.2000.00066.x
Lillis PJ (1990) The tumescent technique for liposuction surgery. Dermatol Clin 8(3):439–450. https://doi.org/10.1016/S0733-8635(18)30475-3
Coleman SR (1995) Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 19(5):421–425. https://doi.org/10.1007/BF00453875
Zhu M, Cohen SR, Hicok KC, Shanahan RK, Strem BM, Yu JC, Arm DM, Fraser JK (2013) Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg 131:873–880
Bourne DA, Bliley J, James I, Donnenberg AD, Donnenberg VS (2021) Changing the paradigm of craniofacial reconstruction: a prospective clinical trial of autologous fat transfer for craniofacial deformities. Ann Surg 273:1004–1011
Rongwei W, Yang X, Jin X, Haibin L, Jia Z, Li B, Jiang H, Qi Z (2018) Three-dimensional volumetric analysis of 3 fat-processing techniques for facial fat grafting: a randomized clinical trial. JAMA Facial Plast Surg 20(3):222–229. https://doi.org/10.1001/jamafacial.2017.2002
Sarfati I (2017) A prospective randomized study comparing centrifugation and sedimentation for fat grafting in breast reconstruction. J Plast Reconstr Aesthet Surg 70:1218–1228
Mestak O, Sukop A, Hsueh YS, Molitor M, Mestak J, Matejovska J, Zarubova L (2014) Centrifugation versus puregraft for fatgrafting to the breast after breast-conserving therapy. World J Surg Oncol 12(1):178. https://doi.org/10.1186/1477-7819-12-178
Asilian A, Siadat AH, Iraji R (2014) Comparison of fat maintenance in the face with centrifuge versus filtered and washed fat. J Res Med Sci Official J Isfahan Univ Med Sci 19(6):556–561
Gerth DJ, King B, Rabach L, Glasgold RA, Glasgold MJ (2014) Long-term volumetric retention of autologous fat grafting processed with closed-membrane filtration. Aesthetic Surg J 34(7):985–994. https://doi.org/10.1177/1090820X14542649
Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Curcio CB, Floris M, Fiaschetti V, Floris R, Cervelli V (2012) A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med 1(4):341–351. https://doi.org/10.5966/sctm.2011-0065
Botti G, Pascali M, Botti C, Bodog F, Cervelli V (2011) A Clinical Trial in facial fat grafting: filtered and washed versus centrifuged fat. Plast Reconstr Surg 127:2464–2473
Fan P, Fang M, Li J, Solari MG, Wu D, Tan W, Wang Y, Yang X, Lei S (2021) A novel fat making strategy with adipose-derived progenitor cell-enriched fat improves fat graft survival. Aesthetic Surg J 41(9):1228–1236
Canizares OJ, Thomson JE, Allen RJJ, Davidson EH, Tutela JP, Saadeh PB, Warren SM, Hazen A (2017) The effect of processing technique on fat graft survival. Plast Reconstr Surg 140:933–943
Yin S, Luan J, Fu S, Zhuang Q (2016) Is centrifugation necessary for processing lipoaspirate harvested via water-jet force assisted technique before grafting? Evidence of lipoaspirate concentration with enhanced fat graft survival. Ann Plast Surg 77:477–484
Qiu L, Su Y, Zhang D, Song Y, Liu B, Yu Z, Guo S, Yi C (2016) Identification of the centrifuged lipoaspirate fractions suitable for postgrafting survival. Plast Reconstr Surg 137:67e–76e
Salinas HM, Broelsch GF, Fernandes JR, McCormack MC, Meppelink AM, Randolph MA, Colwell AS, Austen WGJ (2014) Comparative analysis of processing methods in fat grafting. Plast Reconstr Surg 134:675–683
Ansorge H, Garza JR, McCormack MC, Leamy P, Roesch S, Barere A, Connor J (2014) Autologous fat processing via the revolve system: quality and quantity of fat retention evaluated in an animal model. Aesthetic Surg J 34(3):438–47
Fisher C, Grahovac TL, Schafer ME, Shippert RD, Marra KG, Rubin JP (2013) Comparison of harvest and processing techniques for fat grafting and adipose stem cell isolation. Plast Reconstr Surg 132:351–361
Minn KW, Min KH, Chang H, Kim S, Heo EJ (2010) Effects of fat preparation methods on the viabilities of autologous fat grafts. Aesthetic Plast Surg 34(5):626–631. https://doi.org/10.1007/s00266-010-9525-7
Condé-Green A, de Amorim NF, Pitanguy I (2010) Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg 63(8):1375–1381
Cao Z, Li H, Wang ZH, Liang XQ (2021) High-density fat grafting assisted stromal vascular fraction gel in facial deformities. J Craniofacial Surg 33(1):108–111. https://doi.org/10.1097/SCS.0000000000008038
Allen RJJ, Canizares O, Scharf CL, Paek GK, Nguyen PD, Thanik VD, Warren SM, Saadeh PB, Coleman SR, Hazen A (2009) Spinning into control: centrifugation creates an optimal density for fat grafting. Plast Reconstr Surg 124:35–36
Condé-Green A, Iwen W, Graham I, Chae JJ, Drachenberg CB, Singh DP, Holton L, Slezak S, Elisseeff J (2013) Comparison of 3 techniques of fat grafting and cell-supplemented lipotransfer in athymic rats. Aesthetic Surg J 33(5):713–721. https://doi.org/10.1177/1090820X13487371
Hoareau L, Bencharif K, Girard AC, Gence L, Delarue P, Hulard O, Festy F, Roche R (2013) Effect of centrifugation and washing on adipose graft viability: a new method to improve graft efficiency. J Plast Reconstr Aesthet Surg 66(5):712–9
Ferraro GA, De Francesco F, Tirino V, Cataldo C, Rossano F, Nicoletti G, D’Andrea F (2011) Effects of a new centrifugation method on adipose cell viability for autologous fat grafting. Aesthetic Plast Surg 35(3):341–348
Pfaff M, Wu W, Zellner E, Steinbacher DM (2014) Processing technique for lipofilling influences adipose-derived stem cell concentration and cell viability in lipoaspirate. Aesthetic Plast Surg 38(1):224–229
Gonzalez AM, Lobocki C, Kelly CP, Jackson IT (2007) An alternative method for harvest and processing fat grafts: an in vitro study of cell viability and survival. Plast Reconstr Surg 120(1):285–294
Hu S, Zhang H, Feng Y, Yang Y, Han X, Han X, Zhong Y, Shi J (2007) Introduction of an easy technique for purification and injection of autogenous free fat parcels in correcting of facial contour deformities. Ann Plast Surg 58(6):602–607
Valmadrid AC, Kaoutzanis C, Wormer BA, Farinas AF, Wang L, Al Kassis S, Perdikis G, Braun SA, Higdon KK (2020) Comparison of telfa rolling and a closed washing system for autologous fat processing techniques in postmastectomy breast reconstruction. Plast Reconstr Surg 146:486–497
Fang C, Patel P, Li H, Huang LT, Wan H, Collins S, Connell TL, Xu H (2020) Physical, biochemical, and biologic properties of fat graft processed via different methods. Plast Reconstr Surg Glob Open 8:e3010
Pallua N, Pulsfort AK, Suschek C, Wolter TP (2009) Content of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubation. Plast Reconstr Surg 123:826–833
Smith P, Adams WPJ, Lipschitz AH, Chau B, Sorokin E, Rohrich RJ, Brown SA (2006) Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Reconstr Surg 117:1836–1844
Vezzani B, Shaw I, Lesme H, Yong L, Khan N, Tremolada C, Péault B (2018) Higher pericyte content and secretory activity of microfragmented human adipose tissue compared to enzymatically derived stromal vascular fraction. Stem Cells Transl Med 7:876–886
Vezzani B, Gomez-Salazar M, Casamitjana J, Tremolada C, Péault B (2019) Human adipose tissue micro-fragmentation for cell phenotyping and secretome characterization. JoVE 152:e60117
Ceserani V, Ferri A, Berenzi A, Benetti A, Ciusani E, Pascucci L, Bazzucchi C, Coccè V, Bonomi A, Pessina A, Ghezzi E (2016) Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc Cell 8(1):1–2
Guo B, Sawkulycz X, Heidari N, Rogers R, Liu D, Slevin M (2021) Characterisation of novel angiogenic and potent anti-inflammatory effects of micro-fragmented adipose tissue. Int J Mol Sci 22(6):3271
Vinet-Jones H, Darr DK (2020) Clinical use of autologous micro-fragmented fat progressively restores pain and function in shoulder osteoarthritis. Regener Med 15(10):2153–2161
Malanga GA, Chirichella PS, Hogaboom NS, Capella T (2021) Clinical evaluation of micro-fragmented adipose tissue as a treatment option for patients with meniscus tears with osteoarthritis: a prospective pilot study. Int Orthopaed 45(2):473–480
Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K (2020) Cell-Assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 44:1258–1265
Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, Sato K, Inoue K, Suga H, Eto H, Kato H, Harii K (2010) Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J 16:169–175
Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K (2008) Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 34(9):1178–85
Andia I, Maffulli N, Burgos-Alonso N (2019) Stromal vascular fraction technologies and clinical applications. Expert Opinion Biol Ther 19(12):1289–1305. https://doi.org/10.1080/14712598.2019.1671970
Gentile P, Casella D, Palma E, Calabrese C (2019) Engineered Fat graft enhanced with adipose-derived stromal vascular fraction cells for regenerative medicine: clinical, histological and instrumental evaluation in breast reconstruction. J Clin Med 8(4):504. https://doi.org/10.3390/jcm8040504
Jeon HJ, Choi DH, Lee JH, Lee JS, Lee J, Park HY, Yang JD (2021) A prospective study of the efficacy of cell-assisted lipotransfer with stromal vascular fraction to correct contour deformities of the autologous reconstructed breast. Aesthetic Plast Surg 45:853–863
Prantl L, Brix E, Kempa S, Felthaus O, Eigenberger A, Brébant V, Anker A, Strauss C (2021) Facial rejuvenation with concentrated lipograft: a 12 month follow-up study. Cells 10(3):594. https://doi.org/10.3390/cells10030594
Zhu H, Quan Y, Wang J, Jiang SL, Lu F, Cai J, Liao Y (2021) Improving low-density fat by condensing cellular and collagen content through a mechanical process: basic research and Clinical applications. Plast Reconstr Surg. https://doi.org/10.1097/PRS.0000000000008484
Kølle SF, Duscher D, Taudorf M, Fischer-Nielsen A, Svalgaard JD, Munthe-Fog L, Jønsson B, Selvig PB, Mamsen FP, Katz AJ (2020) Ex vivo-expanded autologous adipose tissue-derived stromal cells ensure enhanced fat graft retention in breast augmentation: a randomized controlled clinical trial. Stem Cells Transl Med 9(11):1277–1286
Gentile P, Sterodimas A, Calabrese C, De Angelis B, Trivisonno A, Pizzicannella J, Dionisi L, De Fazio D, Garcovich S (2020) Regenerative application of stromal vascular fraction cells enhanced fat graft maintenance: clinical assessment in face rejuvenation. Expert Opinion Biol Ther 20(12):1503–1513
Jung HK, Kim CH, Song SY (2016) Prospective 1-year follow-up study of breast augmentation by cell-assisted lipotransfer. Aesthet Surg J 36:179–190
Sasaki GH (2015) The safety and efficacy of cell-assisted fat grafting to traditional fat grafting in the anterior mid-face: an indirect assessment by 3D imaging. Aesthetic Plast Surg 39:833–846
Wang L, Luo X, Lu Y, Fan ZH, Hu X (2015) Is the resorption of grafted fat reduced in cell-assisted lipotransfer for breast augmentation? Ann Plast Surg 75(2):128–34
Kølle SFT, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman MLM, Thomsen C, Dickmeiss E, Drzewiecki KT (2013) Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 382(9898):1113–1120. https://doi.org/10.1016/S0140-6736(13)61410-5
Keyhan SO, Hemmat S, Badri AA, Abdeshahzadeh A, Khiabani K (2013) Use of platelet-rich fibrin and platelet-rich plasma in combination with fat graft: which is more effective during facial lipostructure? J Oral Maxillofacial Surg 71(3):610–621. https://doi.org/10.1016/j.joms.2012.06.176
Geeroms M, Fujimura S, Aiba E, Orgun D, Arita K, Kitamura R, Senda D, Mizuno H, Hamdi M, Tanaka R (2021) Quality and quantity-cultured human mononuclear cells improve human fat graft vascularization and survival in an in vivo murine experimental model. Plast Reconstr Surg 147:373–385
Rasmussen BS, Sørensen CL, Kurbegovic S, Ørholt M, Talman M-LM, Herly M, Pipper CB, Kølle S-FT, Rangatchew F, Holmgaard R, Vester-Glowinski PV, Fischer-Nielsen A, Drzewiecki KT (2019) Cell-enriched fat grafting improves graft retention in a porcine model: a dose-response study of adipose-derived stem cells versus stromal vascular fraction. Plast Reconstr Surg 144:397e–408e
Harris WM, Plastini M, Kappy N, Ortiz T, Chang S, Brown S, Carpenter JP, Zhang P (2019) Endothelial differentiated adipose-derived stem cells improvement of survival and neovascularization in fat transplantation. Aesthet Surg J 39:220–232
Choi JW, Kim SC, Eun-Jung Park JA (2018) Positive effect of incubated adipose-derived mesenchymal stem cells on microfat graft survival. J Craniofacial Surg 29(1):243–247. https://doi.org/10.1097/SCS.0000000000004071
Cai J, Feng J, Liu K, Zhou S, Lu F (2018) Early macrophage infiltration improves fat graft survival by inducing angiogenesis and hematopoietic stem cell recruitment. Plast Reconstr Surg 141:376–386
Zhang Y, Cai J, Zhou T, Yao Y, Dong Z, Lu F (2018) Improved long-term volume retention of stromal vascular fraction gel grafting with enhanced angiogenesis and adipogenesis. Plast Reconstr Surg 141:676e–686e
Zielins ER, Brett EA, Blackshear CP, Flacco J, Ransom RC, Longaker MT, Wan DC (2017) Purified adipose-derived stromal cells provide superior fat graft retention compared with unenriched stromal vascular fraction. Plast Reconstr Surg 139:911–914
Kakudo N, Morimoto N, Ogawa T, Hihara M, Lai F, Kusumoto K (2017) Adipose-derived stem cell (ASC)-enriched fat grafting: experiments using White rabbits and an automated cell processing apparatus. Med Mol Morphol 50(3):170–177
Li K, Li F, Li J, Wang H, Zheng X, Long J, Guo W, Tian W (2017) Increased survival of human free fat grafts with varying densities of human adipose-derived stem cells and platelet-rich plasma. J Tissue Eng Regen Med 11:209–219
Li F, Guo W, Li K, Mei Y, Tang W, Wang H, Tian W (2015) Improved fat graft survival by different volume fractions of platelet-rich plasma and adipose-derived stem cells. Aesthetic Surg J 35(3):319–333. https://doi.org/10.1093/asj/sju046
Phipps KD, Gebremeskel S, Gillis J, Hong P, Johnston B, Bezuhly M (2015) Alternatively Activated M2 macrophages improve autologous fat graft survival in a mouse model through induction of angiogenesis. Plast Reconstr Surg 135:140–149
Paik KJ, Zielins ER, Atashroo DA, Maan ZN, Duscher D, Luan A, Walmsley GG, Momeni A, Vistnes S, Gurtner GC, Longaker MT, Wan DC (2015) Studies in fat grafting: part V. cell-assisted lipotransfer to enhance fat graft retention is dose dependent. Plast Reconstr Surg 136:67–75
Bae YC, Song JS, Bae SH, Kim JH (2015) Effects of human adipose-derived stem cells and stromal vascular fraction on cryopreserved fat transfer. Dermatol Surg 41(5):605–614. https://doi.org/10.1097/DSS.0000000000000342
Aronowitz JA, Lockhart RA, Hakakian CS, Hicok KC (2015) Clinical safety of stromal vascular fraction separation at the point of care. Ann Plast Surg 75:666–671
Bae YC, Kim KH, Yun HJ, Oh CH, Chang JH, Yi CR, Lee JW, Bae SH (2020) A study on the effective ratio of fat to stromal vascular fraction for cell-assisted lipotransfer. Aesthetic Plast Surg 44:162–167
Li FW, Wang HB, Fang JP, Zeng L, Chen CL, Luo SK (2019) Optimal use ratio of the stromal vascular fraction (SVF): an animal experiment based on micro-ct dynamic detection after large-volume fat grafting. Aesthet Surg J 39(6):NP213–NP24
Yao Y, Dong Z, Liao Y, Zhang P, Ma J, Gao J, Lu F (2017) Adipose extracellular matrix/stromal vascular fraction gel: a novel adipose tissue-derived injectable for stem cell therapy. Plast Reconstr Surg 139:867–879
Prantl L, Eigenberger A, Klein S, Limm K, Oefner PJ, Schratzenstaller T, Felthaus O (2020) Shear force processing of lipoaspirates for stem cell enrichment does not affect secretome of human cells detected by mass spectrometry in vitro. Plast Reconstr Surg 146:749e–758e
Sun M, He Y, Zhou T, Zhang P, Gao J, Lu F (2017) Adipose extracellular matrix/stromal vascular fraction gel secretes angiogenic factors and enhances skin wound healing in a murine model. Biomed Res Int 2017:3105780
Zhu H, Ge J, Chen X, Feng L, Cai J (2019) Mechanical micronization of lipoaspirates for regenerative therapy. J Vis Exp. https://doi.org/10.3791/58765
Zhang P, Feng J, Liao Y, Cai J, Zhou T, Sun M, Gao J, Gao K (2018) Ischemic flap survival improvement by composition-selective fat grafting with novel adipose tissue derived product-stromal vascular fraction gel. Biochem Biophys Res Commun 495(3):2249–2256
Yao Y, Cai J, Zhang P, Liao Y, Yuan Y, Dong Z, Lu F (2018) Adipose stromal vascular fraction gel grafting: a new method for tissue volumization and rejuvenation. Dermatol Surg 44:1278–1286
van Joris A, Dongen AJ, Tuin MS, Jansma J, van der Lei B, Harmsen MC (2018) Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review: intraoperative procedures for stromal vascular fraction isolation. J Tissue Eng Regener Med 12(1):e261–e274. https://doi.org/10.1002/term.2407
Vilaboa SDA, Navarro-Palou M, Llull R (2014) Age influence on stromal vascular fraction cell yield obtained from human lipoaspirates. Cytotherapy 16(8):1092–1097. https://doi.org/10.1016/j.jcyt.2014.02.007
Philips BJ, Grahovac TL, Valentin JE, Chung CW, Bliley JM, Pfeifer ME, Roy SB, Dreifuss S, Kelmendi-Doko A, Kling RE, Ravuri SK, Marra KG, Donnenberg VS, Donnenberg AD, Rubin JP (2013) Prevalence of endogenous CD34+ adipose stem cells predicts human fat graft retention in a xenograft model. Plast Reconstr Surg 132:845–858
Mazur S, Zołocińska A, Siennicka K, Janik-Kosacka K, Chrapusta A, Pojda Z (2018) Safety of adipose-derived cell (stromal vascular fraction–SVF) augmentation for surgical breast reconstruction in cancer patients. Adv Clin Exp Med 27(8):1085–1090
Borrelli MR, Patel RA, Blackshear C, Vistnes S, Diaz NM, Deleon SA, Shen AH, Sokol J, Momeni A, Nguyen D, Longaker MT, Wan DC (2020) CD34+CD146+ adipose-derived stromal cells enhance engraftment of transplanted fat. Stem Cells Transl Med 9(11):1389–1400. https://doi.org/10.1002/sctm.19-0195
Deleon NM, Adem S, Lavin CV, Abbas DB, Griffin M, King ME, Borrelli MR, Patel RA, Fahy EJ, Lee D, Shen AH (2021) Angiogenic CD34+ CD146+ adipose-derived stromal cells augment recovery of soft tissue after radiotherapy. J Tissue Eng Regener Med 15(12):1105–1117
Lauvrud AT, Kelk P, Wiberg M, Kingham PJ (2017) Characterization of human adipose tissue-derived stem cells with enhanced angiogenic and adipogenic properties. J Tissue Eng Regener Med 11(9):2490–2502
Peer LA (1956) The neglected free fat graft. Plast Reconstr Surg 18(4):233–250. https://doi.org/10.1097/00006534-195610000-00001
Su F, Luan J, Xin M, Wang Q, Xiao R, Gao Y (2013) Fate of adipose-derived stromal vascular fraction cells after co-implantation with fat grafts: evidence of cell survival and differentiation in ischemic adipose tissue. Plast Reconstr Surg 132(2):363–373. https://doi.org/10.1097/PRS.0b013e31829588b3
Suga H, Eto H, Aoi N, Kato H, Araki J, Doi K, Higashino T, Yoshimura K (2010) Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg 126(6):1911–1923. https://doi.org/10.1097/PRS.0b013e3181f4468b
Mashiko T, Yoshimura K (2015) How does fat survive and remodel after grafting? Clin Plast Surg 42(2):181–190. https://doi.org/10.1016/j.cps.2014.12.008
Yoshimura K, Eto H, Kato H, Doi K, Aoi N (2011) In vivo manipulation of stem cells for adipose tissue repair/reconstruction. Regener Med 6(6s):33–41. https://doi.org/10.2217/rme.11.62
Zhu Y-Z, Zhang J, Hu X, Wang Z, Wu S, Yi Y (2020) Supplementation with extracellular vesicles derived from adipose-derived stem cells increases fat graft survival and browning in mice: a cell-free approach to construct beige fat from white fat grafting. Plast Reconstr Surg 145(8):1183–1195
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233(9):6425–40
Gorgun C, Ceresa D, Lesage R, Villa F, Reverberi D, Balbi C, Santamaria S, Cortese K, Malatesta P, Geris L, Quarto R, Tasso R (2021) Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials 269:120633
Shang Q, Bai Y, Wang G, Song Q, Guo C, Zhang L, Wang Q (2015) Delivery of adipose-derived stem cells attenuates adipose tissue inflammation and insulin resistance in obese mice Through remodeling macrophage phenotypes. Stem Cells Dev 24:2052–2064
Hanson SE, Kim J, Hematti P (2013) Comparative analysis of adipose-derived mesenchymal stem cells isolated from abdominal and breast tissue. Aesthetic Surg J 33(6):888–898. https://doi.org/10.1177/1090820X13496115
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383. https://doi.org/10.1083/jcb.201211138
Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, Cancedda R, Tasso R (2017) Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med 6:1018–1028
Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q (2018) Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing m2 macrophages and beiging in white adipose tissue. Diabetes 67:235–247
Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H, Pardoll D (2013) STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Inv 123(4):1580–9
Monticelli LA, Buck MD, Flamar AL, Saenz SA, Tait ED, Wojno NA, Yudanin LC, Osborne MR, Hepworth SV, Tran HRR, Shah H, Cross JR, Diamond JM, Cantu E, Christie JD, Pearce EL, Artis D (2016) Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol 17(6):656–665. https://doi.org/10.1038/ni.3421
Zhu M, Xue J, Lu S, Yuan Y, Liao Y, Qiu J, Liu C, Liao Q (2019) Anti-inflammatory effect of stromal vascular fraction cells in fat transplantation. Exp Therap Med 17(2):1435–9
Kim H, Lee BK (2020) Anti-inflammatory effect of adipose-derived stromal vascular fraction on osteoarthritic temporomandibular joint synoviocytes. Tissue Eng Regen Med 17:351–362
Bai Y, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y (2018) Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun 497(1):305–312
Sacilotto N, Chouliaras KM, Nikitenko LL, Lu YW, Fritzsche M, Wallace MD, Nornes S, García-Moreno F, Payne S, Bridges E, Liu K (2016) MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev 30(20):2297–2309
Huang B, Huang LF, Zhao L, Zeng Z, Wang X, Cao D, Yang L, Ye Z, Chen X, Liu B, He TC (2020) Microvesicles (MIVs) secreted from adipose-derived stem cells (ADSCs) contain multiple microRNAs and promote the migration and invasion of endothelial cells. Genes Dis 7(2):225–234
Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, Bond VC, Chen YE, Liu D (2016) Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl Med 5(4):440–450
Liang X, Zhang L, Wang S, Han Q, Zhao RC (2016) Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci 129(11):2182–2189. https://doi.org/10.1242/jcs.170373
Bridge G, Monteiro R, Henderson S, Emuss V, Lagos D, Georgopoulou D, Patient R, Boshoff C (2012) The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood J Am Soc Hematol 120(25):5063–5072
Zhu Y, Zhang J, Hu X, Wang Z, Wu S, Yi Y (2020) Extracellular vesicles derived from human adipose-derived stem cells promote the exogenous angiogenesis of fat grafts via the let-7/AGO1/VEGF signalling pathway. Sci Rep 10(1):1–4
Lai RC, Yeo RW, Lim SK (2015) Mesenchymal stem cell exosomes. Semin Cell Dev Biol 40:82–88
Serocki M, Bartoszewska S, Janaszak-Jasiecka A, Ochocka RJ, Collawn JF, Bartoszewski R (2018) miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis 21(2):183–202. https://doi.org/10.1007/s10456-018-9600-2
Suga H, Glotzbach JP, Sorkin M, Longaker MT, Gurtner GC (2014) Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann Plast Surg 72(2):234–241. https://doi.org/10.1097/SAP.0b013e318264fd6a
Hao X, Guo Y, Wang R, Xueyuan Y, He L, Shu M (2021) Exosomes from adipose-derived mesenchymal stem cells promote survival of fat grafts by regulating macrophage polarization via let-7c. Acta Biochim et Biophys Sinica 53(4):501–510. https://doi.org/10.1093/abbs/gmab018
Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M (2015) Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 589(11):1257–1265. https://doi.org/10.1016/j.febslet.2015.03.031
Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomón C, Aguayo C (2016) Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol 7:24
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW (2013) Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 8(12):e84256. https://doi.org/10.1371/journal.pone.0084256
Qorri B, Kalaydina RV, Velickovic A, Kaplya Y, Decarlo A, Szewczuk MR (2018) Agonist-biased signaling via matrix metalloproteinase-9 promotes extracellular matrix remodeling. Cells 7(9):117
Wu W, Peng S, Shi Y, Li L, Song Z, Lin S (2021) NPY promotes macrophage migration by upregulating matrix metalloproteinase-8 expression. J Cell Physiol 236(3):1903–1912
Chen B, Cai J, Wei Y, Jiang Z, Desjardins HE, Adams AE, Li S, Kao H-K, Guo L (2019) Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plast Reconstr Surg 144:816e–827e
Mou S, Zhou M, Li Y, Wang J, Yuan Q, Xiao P, Sun J, Wang Z (2019) Extracellular vesicles from human adipose-derived stem cells for the improvement of angiogenesis and fat-grafting application. Plast Reconstr Surg 144:869–880
Billings E, May JW (1989) Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg 83(2):368–831
Doi K, Ogata F, Eto H, Kato H, Kuno S, Kinoshita K, Kanayama K, Feng J, Manabe I, Yoshimura K (2015) Differential contributions of graft-derived and host-derived cells in tissue regeneration/remodeling after fat grafting. Plast Reconstr Surg 135:1607–1617
Kato H, Mineda K, Eto H, Doi K, Kuno S, Kinoshita K, Kanayama K, Yoshimura K (2014) Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg 133:303e–313e
Liao HT, James IB, Marra KG, Rubin JP (2015) The effects of platelet-rich plasma on cell proliferation and adipogenic potential of adipose-derived stem cells. Tissue Eng Part A 21:2714–2722
Lai F, Kakudo N, Morimoto N, Taketani S, Hara T, Ogawa T, Kusumoto K (2018) Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways. Stem Cell Res Ther. https://doi.org/10.1186/s13287-018-0851-z
Loibl M, Lang S, Hanke A, Herrmann M, Huber M, Brockhoff G, Klein S, Nerlich M, Angele P, Prantl L, Gehmert S (2016) Leukocyte-reduced platelet-rich plasma alters protein expression of adipose tissue-derived mesenchymal stem cells. Plast Reconstr Surg 138:397–408
Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G (2014) Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal 12:26
Mammoto T, Jiang A, Jiang E, Mammoto A (2013) Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc Res 89:15–24. https://doi.org/10.1016/j.mvr.2013.04.008
Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, Landes C, Robert Sader CJ, Kirkpatrick JC (2014) Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol 40(6):679–689. https://doi.org/10.1563/aaid-joi-D-14-00138
Masuki H, Okudera T, Watanebe T, Suzuki M, Nishiyama K, Okudera H, Nakata K, Uematsu K, Su CY, Kawase T (2016) Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dent 2:19
Aizawa H, Tsujino T, Watanabe T, Isobe K, Kitamura Y, Sato A, Yamaguchi S, Okudera H, Okuda K, Kawase T (2020) Quantitative Near-infrared imaging of platelets in platelet-rich fibrin (PRF) matrices: comparative analysis of bio-prf, leukocyte-rich prf, advanced-prf and concentrated growth factors. Int J Mol Sci 21(12):4426
Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ (2016) Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Inves 20(9):2353–2360
Calabriso N, Stanca E, Rochira A, Damiano F, Giannotti L, Di Chiara SB, Massaro M, Scoditti E, Demitri C, Nitti P, Palermo A (2021) Angiogenic properties of concentrated growth factors (CGFs): The role of soluble factors and cellular components. Pharmaceutics 13(5):635
Del Vecchio DA, Del Vecchio SJ (2014) The graft-to-capacity ratio: volumetric planning in large-volume fat transplantation. Plast Reconstr Surg. 133:561–569
Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H (2013) Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 132:1017–1026
Rose JG, Lucarelli MJ, Lemke BN, Dortzbach RK, Boxrud CA, Obagi S, Patel S (2006) Histologic comparison of autologous fat processing methods. Ophthalmic Plast Reconstr Surg 22(3):195–200
Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C (2013) A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transpl 22:2063–2077
Zhu M, Dong Z, Gao J, Liao Y, Xue J, Yuan Y, Liu L, Chang Q, Lu F (2015) Adipocyte regeneration after free fat transplantation: promotion by stromal vascular fraction cells. Cell Transpl 24:49–62
Jung DW, Kim YH, Kim TG, Lee JH, Chung KJ, Lim JO, Choi JY (2015) Improvement of fat transplantation: fat graft with adipose-derived stem cells and oxygen-generating microspheres. Ann Plast Surg 75:463–470
Hong KY, Yim S, Kim HJ, Jin US, Lim S, Eo S, Chang H, Minn KW (2018) The fate of the adipose-derived stromal cells during angiogenesis and adipogenesis after cell-assisted lipotransfer. Plast Reconstr Surg 141:365–375
Guillaume VGJ, Ruhl T, Boos AM, Beier JP (2022) The crosstalk between adipose-derived stem or stromal cells (ASC) and cancer cells and asc-mediated effects on cancer formation and progression—ASCs: safety hazard or harmless source of tropism? Stem Cells Transl Med 11(4):394–406. https://doi.org/10.1093/stcltm/szac002
Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, Egger D (2019) Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2019.00292
Seyhan N, Alhan D, Ural AU, Gunal A, Avunduk MC, Savaci N (2015) The effect of combined use of platelet-rich plasma and adipose-derived stem cells on fat graft survival. Ann Plast Surg 74(5):615–620. https://doi.org/10.1097/SAP.0000000000000480
Liang Z, Huang D, Nong W, Mo J, Zhu D, Wang M, Chen M, Wei C, Li H (2021) Advanced-platelet-rich fibrin extract promotes adipogenic and osteogenic differentiation of human adipose-derived stem cells in a dose-dependent manner in vitro. Tissue Cell 71:101506. https://doi.org/10.1016/j.tice.2021.101506
An Y, Panayi AC, Mi B, Fu S, Orgill DP (2020) Comparative analysis of two automated fat-processing systems. Plast Reconstr Surg Glob Open 8:e2587
Acknowledgements
This work was supported by the Scientific Research Staring Foundation for the Returned Overseas Chinese Scholars, Peking University Third Hospital (No. BYSYLXHG2019001) and Innovation Transformation Fund, Peking University Third Hospital (No. BYSYZHKC2020101)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, M., Shang, Y., Liu, N. et al. Strategies to Improve AFT Volume Retention After Fat Grafting. Aesth Plast Surg 47, 808–824 (2023). https://doi.org/10.1007/s00266-022-03088-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-022-03088-y