Abstract
Background
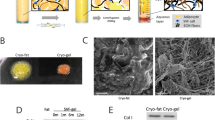
Cryopreserved fat has limited clinical applications due to its rapid absorption, high degree of fibrosis, and risk of complications after grafting. Many studies have verified that Adipose-derived mesenchymal stem cell-derived exosomes (ADSC-Exos) can improve fresh fat graft survival. This study assessed whether ADSC-Exos could improve the survival of cryopreserved fat grafts.
Methods
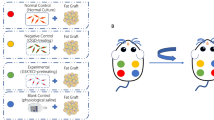
Exosomes were isolated from human ADSCs were subcutaneously engrafted with adipose tissues stored under different conditions (fresh; cryopreserved for 1 month) into the backs of BALB/c nude mice (n = 24), and exosomes or PBS were administered weekly. Grafts were harvested at 1, 2, 4, and 8 weeks, and fat retention rate, histologic, and immunohistochemical analyses were conducted.
Results
At 1, 2, and 4 weeks after the transfer, cryopreserved fat grafts in groups of exosome-treated showed better fat integrity, fewer oil cysts, and reduced fibrosis. Further investigations of macrophage infiltration and neovascularization revealed that those exosomes increased the number of M2 macrophages at 2 and 4 weeks (p<0.05), but had limited impact on vascularization (p>0.05). It's important to note that no significant differences (p>0.05) were observed between the two groups in both histological and immunohistochemical evaluations at 8 weeks post-transplantation.
Conclusions
This study suggests that ADSC-Exos could improve the survival of cryopreserved fat grafts in the short term (within 4 weeks), but the overall improvement was poor (after 8 weeks). This suggests that the utility of using ADSC-Exos to treat cryopreserved adipose tissue grafts is limited.
No Level Assigned
This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.







Similar content being viewed by others
References
Khouri RK, Khouri RK (2017) Current clinical applications of fat grafting. Plast Reconstr Surg 140:466e–486e
Del Vecchio DA, Villanueva NL, Mohan R, Johnson B, Wan D, Venkataram A, Rohrich RJ (2018) Clinical implications of gluteal fat graft migration: a dynamic anatomical study. Plast Reconstr Surg 142:1180–1192
Sinna R, Delay E, Garson S, Delaporte T, Toussoun G (2010) Breast fat grafting (lipomodelling) after extended latissimus dorsi flap breast reconstruction: a preliminary report of 200 consecutive cases. J Plast Reconstr Aesthet Surg 63:1769–1777
Herold C, Ueberreiter K, Busche MN, Vogt PM (2013) Autologous fat transplantation: volumetric tools for estimation of volume survival. A systematic review. Aesthetic Plast Surg 37:380–387
Dong Z, Peng Z, Chang Q, Zhan W, Zeng Z, Zhang S, Lu F (2015) The angiogenic and adipogenic modes of adipose tissue after free fat grafting. Plast Reconstr Surg 135:556e–567e
Mizoguchi T, Kijima Y, Hirata M, Kaneko K, Arima H, Nakajo A, Higashi M, Tabata K, Koriyama C, Arigami T, Uenosono Y, Okumura H, Maemura K, Ishigami S, Yoshinaka H, Shinden Y, Ueno S, Natsugoe S (2015) Histological findings of an autologous dermal fat graft implanted onto the pectoralis major muscle of a rat model. Breast Cancer 22:578–585
Coleman SR (2001) Structural fat grafts: the ideal filler? Clin Plast Surg 28:111–119
Gal S, Pu LLQ (2020) An update on cryopreservation of adipose tissue. Plast Reconstr Surg 145:1089–1097
Li BW, Liao WC, Wu SH, Ma H (2012) Cryopreservation of fat tissue and application in autologous fat graft: in vitro and in vivo study. Aesthetic Plast Surg 36:714–722
Shu Z, Gao D, Pu LL (2015) Update on cryopreservation of adipose tissue and adipose-derived stem cells. Clin Plast Surg 42:209–218
Conti G, Jurga M, Benati D, Bernardi P, Mosconi E, Rigotti G, Buve M, Van Wemmel K, Sbarbati A (2015) Cryopreserved subcutaneous adipose tissue for fat graft. Aesthetic Plast Surg 39:800–817
Ko MS, Jung JY, Shin IS, Choi EW, Kim JH, Kang SK, Ra JC (2011) Effects of expanded human adipose tissue-derived mesenchymal stem cells on the viability of cryopreserved fat grafts in the nude mouse. Int J Med Sci 8:231–238
Lidagoster MI, Cinelli PB, Levee EM, Sian CS (2000) Comparison of autologous fat transfer in fresh, refrigerated, and frozen specimens: an animal model. Ann Plast Surg 44:512–515
Atik B, Ozturk G, Erdogan E, Tan O (2006) Comparison of techniques for long-term storage of fat grafts: an experimental study. Plast Reconstr Surg 118:1533–1537
Pu LL, Cui X, Li J, Fink BF, Cibull ML, Gao D (2006) The fate of cryopreserved adipose aspirates after in vivo transplantation. Aesthet Surg J 26:653–661
Zhang PQ, Tan PC, Gao YM, Zhang XJ, Xie Y, Zheng DN, Zhou SB, Li QF (2022) The effect of glycerol as a cryoprotective agent in the cryopreservation of adipose tissue. Stem Cell Res Ther 13:152
Mashiko T, Yoshimura K (2015) How does fat survive and remodel after grafting? Clin Plast Surg 42:181–190
Laloze J, Varin A, Bertheuil N, Grolleau JL, Vaysse C, Chaput B (2017) Cell-assisted lipotransfer: current concepts. Ann Chir Plast Esthet 62:609–616
Luan A, Duscher D, Whittam AJ, Paik KJ, Zielins ER, Brett EA, Atashroo DA, Hu MS, Lee GK, Gurtner GC, Longaker MT, Wan DC (2016) Cell-assisted lipotransfer improves volume retention in irradiated recipient sites and rescues radiation-induced skin changes. Stem Cells 34:668–673
Toyserkani NM, Quaade ML, Sorensen JA (2016) Cell-assisted lipotransfer: a systematic review of its efficacy. Aesthetic Plast Surg 40:309–318
Vyas KS, Vasconez HC, Morrison S, Mogni B, Linton S, Hockensmith L, Kabir T, Zielins E, Najor A, Bakri K, Mardini S (2020) Fat graft enrichment strategies: a systematic review. Plast Reconstr Surg 145:827–841
Kolle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML, Thomsen C, Dickmeiss E, Drzewiecki KT (2013) Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 382:1113–1120
Doorn J, Moll G, Le Blanc K, van Blitterswijk C, de Boer J (2012) Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev 18:101–115
Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H (2014) Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 92:387–397
See F, Seki T, Psaltis PJ, Sondermeijer HP, Gronthos S, Zannettino AC, Govaert KM, Schuster MD, Kurlansky PA, Kelly DJ, Krum H, Itescu S (2011) Therapeutic effects of human STRO-3-selected mesenchymal precursor cells and their soluble factors in experimental myocardial ischemia. J Cell Mol Med 15:2117–2129
Aronowitz JA, Lockhart RA, Hakakian CS, Hicok KC (2015) Clinical safety of stromal vascular fraction separation at the point of care. Ann Plast Surg 75:666–671
Poulos J (2018) The limited application of stem cells in medicine: a review. Stem Cell Res Ther 9:1
Banyard DA, Salibian AA, Widgerow AD, Evans GR (2015) Implications for human adipose-derived stem cells in plastic surgery. J Cell Mol Med 19:21–30
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 23:812–823
Colombo M, Raposo G, Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R (2012) Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 64:676–705
Tkach M, Thery C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164:1226–1232
Schorey JS, Bhatnagar S (2008) Exosome function: from tumor immunology to pathogen biology. Traffic 9:871–881
De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC (2014) Extracellular vesicles: potential roles in regenerative medicine. Front Immunol 5:608
Sun B, Peng J, Wang S, Liu X, Zhang K, Zhang Z, Wang C, Jing X, Zhou C, Wang Y (2018) Applications of stem cell-derived exosomes in tissue engineering and neurological diseases. Rev Neurosci 29:531–546
Dougherty JA, Mergaye M, Kumar N, Chen CA, Angelos MG, Khan M (2017) Potential role of exosomes in mending a broken heart: nanoshuttles propelling future clinical therapeutics forward. Stem Cells Int 2017:5785436
Sun Z, Zhao H (2021) Supplementation with extracellular vesicles derived from adipose-derived stem cells increases fat graft survival and browning in mice: a cell-free approach to construct beige fat from white fat grafting. Plast Reconstr Surg 147:882e–883e
Mou S, Li Y, Sun D, Zhou M, Li J, Chen L, Liu S, Yang J, Xiao P, Tong J, Wang Z, Sun J (2022) Delayed supplementation strategy of extracellular vesicles from adipose-derived mesenchymal stromal cells with improved proregenerative efficiency in a fat transplantation model. Stem Cells Int 2022:2799844
Huang H, Feng S, Zhang W, Li W, Xu P, Wang X, Ai A (2017) Bone marrow mesenchymal stem cell-derived extracellular vesicles improve the survival of transplanted fat grafts. Mol Med Rep 16:3069–3078
Chen B, Cai J, Wei Y, Jiang Z, Desjardins HE, Adams AE, Li S, Kao HK, Guo L (2019) Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plast Reconstr Surg 144:816e–827e
Mou S, Zhou M, Li Y, Wang J, Yuan Q, Xiao P, Sun J, Wang Z (2019) Extracellular vesicles from human adipose-derived stem cells for the improvement of angiogenesis and fat-grafting application. Plast Reconstr Surg 144:869–880
Chen K, Xiong J, Xu S, Wu M, Xue C, Wu M, Lv C, Wang Y (2022) Adipose-derived stem cells exosomes improve fat graft survival by promoting prolipogenetic abilities through Wnt/beta-catenin pathway. Stem Cells Int 2022:5014895
Li FW, Wang HB, Fang JP, Zeng L, Chen CL, Luo SK (2019) Optimal use ratio of the Stromal Vascular Fraction (SVF): an animal experiment based on micro-CT dynamic detection after large-volume fat grafting. Aesthet Surg J 39:NP213–NP224
Cui XD, Gao DY, Fink BF, Vasconez HC, Pu LL (2007) Cryopreservation of human adipose tissues. Cryobiology 55:269–278
Hwang SM, Lee JS, Kim HD, Jung YH, Kim HI (2015) Comparison of the viability of cryopreserved fat tissue in accordance with the thawing temperature. Arch Plast Surg 42:143–149
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247:C125–C142
Elliott GD, Wang S, Fuller BJ (2017) Cryoprotectants: a review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 76:74–91
Iop L, Paolin A, Aguiari P, Trojan D, Cogliati E, Gerosa G (2017) Decellularized cryopreserved allografts as off-the-shelf allogeneic alternative for heart valve replacement: in vitro assessment before clinical translation. J Cardiovasc Transl Res 10:93–103
Erol OO, Agaoglu G (2013) Facial rejuvenation with staged injections of cryopreserved fat and tissue cocktail: clinical outcomes in the past 10 years. Aesthet Surg J 33:639–653
Gonda K, Shigeura T, Sato T, Matsumoto D, Suga H, Inoue K, Aoi N, Kato H, Sato K, Murase S, Koshima I, Yoshimura K (2008) Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg 121:401–410
Liew A, O’Brien T (2012) Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther 3:28
He C, Zheng S, Luo Y, Wang B (2018) Exosome theranostics: biology and translational medicine. Theranostics 8:237–255
Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK (2012) Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics 2012:971907
Mashiko T, Wu SH, Kanayama K, Asahi R, Shirado T, Mori M, Sunaga A, Sarukawa S, Uda H, Yoshimura K (2018) Biological properties and therapeutic value of cryopreserved fat tissue. Plast Reconstr Surg 141:104–115
Han YD, Bai Y, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y (2018) Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun 497:305–312
Zhu Y, Zhang J, Hu X, Wang Z, Wu S, Yi Y (2020) Extracellular vesicles derived from human adipose-derived stem cells promote the exogenous angiogenesis of fat grafts via the let-7/AGO1/VEGF signalling pathway. Sci Rep 10:5313
Horie K, Kawakami K, Fujita Y, Sugaya M, Kameyama K, Mizutani K, Deguchi T, Ito M (2017) Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res Commun 492:356–361
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, Li HM, Zhang WS, Chen CY, Xie H (2018) Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 8:169–184
Das S, Halushka MK (2015) Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol 24:199–206
Zhang Y, Yu M, Dai M, Chen C, Tang Q, Jing W, Wang H, Tian W (2017) miR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci 130:1158–1168
Ma XH, Shi Y, Hou Y, Liu Y, Zhang L, Fan WX, Ge D, Liu TQ, Cui ZF (2010) Slow-freezing cryopreservation of neural stem cell spheres with different diameters. Cryobiology 60:184–191
Cai J, Feng J, Liu K, Zhou S, Lu F (2018) Early macrophage infiltration improves fat graft survival by inducing angiogenesis and hematopoietic stem cell recruitment. Plast Reconstr Surg 141:376–386
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737
Phipps KD, Gebremeskel S, Gillis J, Hong P, Johnston B, Bezuhly M (2015) Alternatively activated M2 macrophages improve autologous fat graft survival in a mouse model through induction of angiogenesis. Plast Reconstr Surg 135:140–149
Mok H, Feng J, Hu W, Wang J, Cai J, Lu F (2018) Decreased serum estrogen improves fat graft retention by enhancing early macrophage infiltration and inducing adipocyte hypertrophy. Biochem Biophys Res Commun 501:266–272
Acknowledgments
None
Funding
This work was supported by Science and Technology Program of Guangzhou (202102080336, 202201020568)
Author information
Authors and Affiliations
Contributions
Dr. XYJ is a resident of plastic and reconstructive surgery who completed experimental design and implementation performed the statistical analysis and prepared the manuscript. Dr. FL is an attending physician of plastic and reconstructive surgery who emerged the idea, performed the data analysis, prepared and revised the manuscript. Dr. YC is a resident of plastic and reconstructive surgery and a medical student who participated in the in vitro experiment. Dr. JF is a resident of plastic and reconstructive surgery who helped revise the manuscript and the data analysis. Prof. SKL is a professor and consulting physician of plastic and reconstructive surgery who supervised this project, critically reviewed the manuscript, and was responsible for execution of the study. Prof. HW is a professor and consulting physician of plastic and reconstructive surgery who conceived the research idea, supervised this project, critically reviewed the manuscript, and was responsible for execution of the study. All authors have seen and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
All participants give their informed consent in writing prior to inclusion in the study.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and Animal Rights Statement
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Xy., Li, Fw., Chen, Yq. et al. Exosomes Derived from Human Adipose-Derived Stem Cells Cannot Distinctively Promote Graft Survival in Cryopreservation Fat Grafting. Aesth Plast Surg 47, 2117–2129 (2023). https://doi.org/10.1007/s00266-023-03457-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-023-03457-1