Abstract
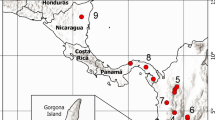
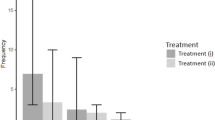
The katydid Neoconocephalus triops exhibits in North America substantial developmental plasticity of male mating calls. The AM rate of the summer calls is significantly faster than that of the winter calls at the same temperature. In the tropics, where N. triops originated, males express only the fast summer-call phenotype. We tested two alternative hypotheses: (1) call plasticity in the population from North America evolved in response to selection by female preference after N. triops colonized North America, or (2) call plasticity evolved before N. triops expanded into North America and its expression in the novel environment led to adaptive change of female preferences. First, we tested whether call plasticity was present in tropical populations of N. triops. Tropical males expressed the winter-call phenotype when reared under winter conditions, indicating that call plasticity did not evolve in response to temperate climates. Second, we compared female preferences among temperate and tropical populations. We found that the temperature dependence of preferred AM rate was significantly steeper in temperate N. triops than in a tropical population of N. triops. Third, we compared temperature dependence of female preference of the N. triops populations to three Neoconocephalus species without call plasticity. Only temperate N. triops had significantly steeper temperature dependence than the other species. This steeper temperature dependence matched female preference to the fast summer call at high temperatures and to the slow winter call at low temperatures in temperate populations. These results support the hypothesis that female preference changed in N. triops in North America to compensate for the plasticity of male calls.





Similar content being viewed by others
References
Andersson M (1994) Sexual selection. Princeton University Press, NJ
Beckers OM, Schul J (2008) Developmental plasticity of mating calls enables acoustic communication in diverse environments. Proc R Soc Lond B 275:1243–1248
Bush SL, Gerhardt HC, Schul J (2002) Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim Behav 63:7–14
Bush SL, Beckers OM, Schul J (2009) A complex mechanism of call recognition in the katydid Neoconocephalus affinis (Orthoptera: Tettigoniidae). J Exp Biol 212:648-655
Büttner UK (2002) Charakterisierung der Gesänge von fünf in Missouri (USA) heimischen Neoconocephalus-Arten (Orthoptera, Tettigoniidae). Diploma thesis, University of Erlangen, Germany
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59(4):433–463
Deily JA, Schul J (2004) Recognition of calls with exceptionally fast pulse rates: female phonotaxis in the genus Neoconocephalus (Orthoptera: Tettigoniidae). J Exp Biol 201:3523–3529
Doherty JA (1985) Temperature coupling and “trade-off” phenomena in the acoustic communication system of the cricket, Gryllus bimaculatus De Geer (Gryllidae). J Exp Biol 144:17–35
Endler JA, Basolo AL (1998) Sensory ecology, receiver biases and sexual selection. Trends Ecol Evol 13(10):415–420
Fonseca PJ, Revez MA (2002) Temperature dependence of cicada song (Homoptera, Cicadoidea). J Comp Physiol A 187:971–976
Gerhardt HC (1991) Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav 42:615–635
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. University of Chicago Press, Chicago
Ghalambor CK, McKay JK, Carroll SP, Reznik DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Grace JL, Shaw KL (2004) Effects of developmental environment on signal preference coupling in a Hawaiian cricket. Evolution 58:1627–1633
Greenfield MD (1990) Evolution of acoustic communication in the genus Neoconocephalus: discontinuous songs, synchrony, and hetereospecific interactions. In: Bailey WJ, Rentz DCF (eds) The Tettigoniidae: biology, systematics and evolution. Springer, Heidelberg, pp 71–97
von Helversen D, von Helversen O (1981) Korrespondenz zwischen Gesang und auslösendem Schema bei Feldheuschrecken. Nova acta Leopold 54(245):449–462
Hill GE, Inouye CY, Montgomerie R (2002) Dietary carotenoids predict plumage coloration in wild house finches. Proc R Soc Lond B 269:1119–1124
Hoikkala A, Isoherranen E (1997) Variation and repeatability of courtship song characters among wild-caught and laboratory-reared Drosophila montana and D. littoralis males (Diptera: Drosophilidae). J Insect Behav 10:193–202
Kenyon SG, Hunter MS (2007) Manipulation of oviposition choice of the parasitoid wasp, Encarsia pergandiella, by the endosymbiontic bacterium Cardinium. J Evol Biol 20:707–716
Lande R (1982) A quantitative genetic theory of life history evolution. Ecology 63:607–615
Laurila A, Karttunen S, Merilä J (2002) Adaptive phenotypic plasticity and genetics of larval histories in two Rana temporaria populations. Evolution 56(3):617–627
Loeschke V (1987) Genetic constraints on adaptive evolution. Springer, Berlin
Martin SD, Gray DA, Cade WH (2000) Fine-scale temperature effects on cricket calling song. Can J Zool 78:706–712
Naskrecki P (2000) Katydids of Costa Rica. Systematics and bioacoustics of the cone- head katydids (Orthoptera: Tettigoniidae: Conecephalinae sensu lato). Orthopterists Society, Philadelphia, Pennsylvania
Newman RA (1994) Genetic variation fro phenotypic plasticity in the larval life history of spadefoot toads (Scaphiopus couchii). Evolution 48(6):1773–1785
Olvido AE, Mousseau TA (1995) Effect of rearing environment on calling song plasticity in the striped ground cricket. Evolution 49(6):1271–1277
Pires A, Hoy RR (1992) Temperature coupling in cricket acoustic communication I. Field and Laboratory studies of temperature effects on calling song production and recognition in Gryllus firmus. J Comp Phyiol A: 69-78
Reinhold R (1983) Houston is at the five plagues and counting. New York Times: 11/13/1983.
Rodriguez RL, Ramaswamy K, Cocroft RB (2006) Evidence that female preferences have shaped male signal evolution in a clade of specialized plant-feeding insects. Proc R Soc Lond B 273:2585–2593
Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK (2006) Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu Rev Entomol 51:331–357
Schlichting CD (1986) The evolution of phenotypic plasticity in plants. Ann Rev Ecolog Syst 17:667–693
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer Associates, Sunderland
Schul J (1998) Song recognition by temporal cues in a group of closely related bushcricket species (Genus Tettigonia). J Comp Physiol A 183:401–410
Schul J, Patterson AC (2003) What determines the tuning of hearing organs and the frequency of calls? A comparative study in the katydid genus Neoconocephalus (Orthoptera; Tettigoniidae). J Exp Biol 206:141–152
Schul J, von Helversen D, Weber T (1998) Selective phonotaxis in Tettigonia cantans and T. viridssima in song recognition and discrimination. J Comp Physiol A 182:687–694
Snyder RL, Frederick-Hudson KH, Schul J (2009) Molecular phylogenetics of the genus Neoconocephalus (Orthoptera, Tettigoniidae) and the evolution of temperate life histories. PLoS ONE 4(9):e7203
Sokal RR, Rohlf FJ (1997) Biometry. W. H. Freeman And Company, New York
Speaks CE (1999) Introduction to sound. Singular Publishing Group Inc., San Diego
Stearns SC (1989) The evolutionary significance of phenotypic plasticity. Bioscience 39:436–445
Trussel GC, Smith LD (2000) Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc Natl Acad Sci USA 97:2123–2127
Van Tienderen PH (1991) Evolution of generalists and specialists in spatially heterogeneous environments. Evolution 45(6):1327–1331
Wagner WE, Hoback WW (1999) Nutritional effects on male calling behaviour in the variable field cricket. Anim Behav 57:89–95
Walker TJ (1957) Specificity in the response of female tree crickets (Orhtoptera, Gryllidae, Oecanthinae) to calling songs of the males. Ann Entomol Soc Am 50:626–636
Walker TJ (1975) Effects of temperature on rates in poikilotherm nervous systems: evidence from the calling songs of meadow katydids (Orthoptera: Tettigoniidae: Orchelimum) and reanalysis of published data. J Comp Physiol 101:57–69
Walker TJ (2000) Pulse rates in the songs of trilling field crickets (Orthoptera: Gryllidae: Gryllus). Entom Soc Am 93(3):565–572
Walker TJ, Greenfield MD (1983) Songs and systematics of Caribbean Neoconocephalus (Orthoptera: Tettigoniidae). Trans Am Entomol Soc 109:357–389
Walker TJ, Whitesell JJ, Alexander RD (1973) The robust conehead: two widespread sibling species (Orthoptera: Tettigoniidae: Neoconocephalus ‘robustus’). Ohio J Sci 73(6):321–330
Weber T, Thorson J, Huber F (1981) Auditory behaviour of the cricket. I. Dynamics of compensated walking and discrimination paradigms on the Kramer treadmill. J Comp Physiol 141:215–232
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
West-Eberhard MJ (2005) Developmental plasticity and the origin of species differences. Proc Natl Acad Sci USA 102:6543–6549
Whitesell JJ (1974) Geographic variation and dimorphisms in song, development, and color in a katydid: field and laboratory studies (Tettigoniidae, Orthoptera). Ph D dissertation. University of Florida, Gainesville
Whitesell JJ, Walker TJ (1978) Photoperiodically determined dimorphic calling songs in a katydid. Nature 274:887–888
Whitman DW, Agrawal AA (2009) What is phenotypic plasticity and why is it important? In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity in insects: mechanisms and consequences. Science publishers, Enfield, pp 1–63
Yeh PJ, Price TD (2004) Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am Nat 164:531–542
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River
Acknowledgments
This manuscript is dedicated to the memory of Otto von Helversen (1943–2009), who was an exceptional mentor to both of us. We thank Sarah Bush, Carl Gerhardt, and William Wagner Jr. and three anonymous reviewers for their critical comments on earlier versions of the manuscript. We thank Thomas Walker for his support during our fieldwork in Gainesville, FL and Chad Brassil for help with the statistics. We thank the Organization of Tropical Research and Ministerio de Ambiental y Energia for their support of our fieldwork in Costa Rica. This research was supported by a grant from the National Science Foundation (IOB-0445286) to J.S. and a grant of Sigma Xi to O.M.B. who received a Life Sciences Fellowship of the University of Missouri. The experiments comply with the current laws of the U.S.A. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Gwynne
Rights and permissions
About this article
Cite this article
Beckers, O.M., Schul, J. Female adaptation to developmental plasticity in male calling behavior. Behav Ecol Sociobiol 64, 1279–1290 (2010). https://doi.org/10.1007/s00265-010-0942-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0942-z