Abstract
Honey bee foragers specialize on collecting pollen and nectar. Pollen foraging behavior is modulated by at least two stimuli within the nest: the presence of brood pheromone and young larvae and the quantity of stored pollen. Genetic variation in pollen foraging behavior has been demonstrated repeatedly. We used selected high and low pollen-hoarding strains of bees that differ dramatically in the quantity of pollen collected to determine if the observed differences in foraging could be explained by differential responses to brood stimuli. Workers from the high and low pollen-hoarding strains and wild-type bees were co-fostered in colonies with either brood or no brood. As expected based on previous studies, returning high pollen-hoarding foragers collected heavier pollen loads and lighter nectar loads than low pollen-hoarding bees. Effects of brood treatment were also observed; bees exposed to brood collected heavier pollen loads and initiated foraging earlier than those from broodless colonies. More specifically, brood treatment resulted in increased pollen foraging in high pollen-hoarding bees but did not affect pollen foraging in low pollen-hoarding bees, suggesting that high pollen-hoarding bees are more sensitive to the presence of brood. However, response to brood stimuli does not sufficiently explain the differences in foraging behavior between the strains since these differences persisted even in the absence of brood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honey bees demonstrate a pronounced division of labor between bees that work in the nest and those that forage. Worker honey bees usually initiate foraging when they are 2–3 weeks old (Rösch 1925). Most bees collect both pollen and nectar, but individuals may concentrate more on one resource than the other. Pollen specialists tend to carry relatively more pollen and less nectar than nectar specialists (Hunt et al. 1995; Page et al. 2000). Some of the observed variation in foraging behavior is due to genetic variation (Hellmich et al. 1985; Calderone and Page 1988, 1991, 1996; Calderone et al. 1989; Dreller et al. 1995; Robinson and Page 1989; Rothenbuhler and Page 1989; Hunt et al. 1995; Page et al. 1995, 2000; Fewell and Page 1993, 2000; Guzman-Novoa and Gary 1993, Guzmán-Novoa et al. 1994; Rueppell et al. 2004; Chapman et al. 2007); however, foragers are also responsive to stimuli within the nest (see Schmickl and Crailsheim 2004 for review of nest homeostasis). Larvae and their associated pheromones stimulate pollen foraging behavior (Filmer 1932; Free 1967; Cale 1968; Todd and Reed 1970; Al-Tikrity et al. 1972; Fewell and Page 1993; Le Conte et al. 2001; Pankiw and Page 1999, 2001b; Pankiw et al. 1998, 2004a; Pankiw 2007; Schmickl and Crailsheim 2004) while stored pollen reduces pollen foraging activity (Free 1967; Barker 1971; Free and Williams 1971; Moeller 1972; Fewell and Winston 1992; Fewell and Page 1993; Dreller et al. 1999; Dreller and Tarpy 2000; Schmickl and Crailsheim 2004). Recent studies have shown that sensory responses of young worker honey bees (prior to the onset of foraging) correlate with ovary size (number of ovarioles) and transcription levels of vitellogenin, an insect yolk protein precursor (Tsuruda et al. 2008), while foraging behavior correlates with responses to sugar (Pankiw and Page 2000; Pankiw 2003; Pankiw et al. 2004b), suggesting that foraging division of labor and reproductive regulatory networks are linked (Page and Amdam 2007).
Page and Fondrk (1995) conducted a bidirectional selection program and produced strains of bees that differ dramatically in the amount of surplus pollen they store in combs in the nest (pollen hoarding). High-strain bees also are much more likely to become pollen specialists, collect larger loads than low-strain bees when foraging for pollen, and initiate foraging at an earlier age (Page and Fondrk 1995; Page et al. 1995, 1998, 2000; Hunt et al. 1995; Fewell and Page 2000; Pankiw et al. 2002; Pankiw and Page 2001a; Rueppell et al. 2004). High-strain bees and wild-type pollen foragers are more responsive to sugar solutions than low-strain bees and wild-type nectar foragers, respectively, and bees with high sucrose responsiveness are also more responsive to pollen than bees with low sucrose responsiveness when tested with the proboscis extension response test (Bitterman et al. 1983; Scheiner et al. 2001a,b, 2004; Page et al. 1998; Pankiw and Page 1999, 2000; Pankiw et al. 2001, 2004b; Pankiw 2003; Rueppell et al. 2006). This suggests that differences in foraging behavior between high- and low-strain bees are a result of differential sensitivity to nectar and pollen foraging stimuli. Prior work has shown that high- and low-strain bees respond to changes in the amount of stored pollen by adjusting the foraging effort of individuals, not by changing the number of foragers (Fewell and Page 2000). When in colonies with high pollen stores, the high- and low-strain bees increased nectar foraging rates and decreased pollen foraging rates compared to colonies with low amounts of stored pollen. Brood stimuli also affect foraging choices, which are associated with foraging initiation (Pankiw and Page 2001a). Brood pheromone can accelerate or delay foraging onset depending on dosage and colony conditions (Le Conte et al. 2001; Pankiw et al. 2004a).
Pankiw and Page (2001a) changed both the quantities of brood and stored pollen and tested the responses of high- and low-strain bees under high- and low-pollen foraging stimulus conditions (high brood quantities/low stored pollen and low brood/high stored pollen, respectively). Their study demonstrated a clear effect of the “total” stimulus on foraging behavior of high- and low-strain bees. However, the pollen and brood stimuli were confounded and did not address the question of differential sensitivity of high- and low-strain workers to brood stimulus as a releaser of pollen foraging behavior. They also did not have a brood-free treatment and, therefore, were unable to test for behavioral differences of high- and low-strain bees in the absence of the brood-produced pollen foraging stimuli. This is important with respect to understanding to what degree pollen foraging activity is a response to pollen foraging releasing stimuli or is activated by other mechanisms.
Based on what is known about the multiple effects of brood stimuli, pollen foraging behavior, and differences in the sensory response systems of high- and low-strain bees, and of nectar and pollen foragers, we asked if differences in foraging behavior between high- and low-strain bees are due to differential responses to pollen foraging releasing stimuli provided by brood. We hypothesized that: (1) bees respond to brood stimuli by foraging at younger ages; (2) bees respond to brood stimuli by increasing pollen foraging behavior; (3) high pollen-hoarding bees are more responsive to brood stimuli than bees of the low pollen hoarding strain; and (4) there are no differences between high- and low-strain bees in the absence of brood stimuli.
We held constant the quantity of pollen in colonies and measured the effects of larvae on foraging behavior and foraging age of identified cohorts of bees from the high and low strains. Additional bees from unrelated wild-type colonies (bees not under artificial selection) were also compared.
Materials and methods
Bees
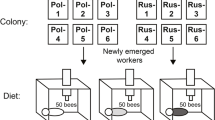
The bees used in this study were derived from the high and low pollen-hoarding strains of Page and Fondrk (1995) and from commercial colonies of wild-type bees that were used as reference points for comparisons between bees of the high and low strains. Workers of known age were obtained by removing combs of developing pupae from colonies about 12 h prior to emergence. The combs were placed in an incubator overnight. Newly emerged adults were paint-marked on the thorax to indicate source strain. Five hundred bees of each genotype (high, low, and wild type) were introduced to each of six unrelated wild-type colonies located in the same apiary in Davis, CA over 2 days beginning 14 April 2004. Honey (two frames), pollen (partial frame), sealed and unsealed brood (two frames), and empty-space areas were equalized between the six colonies. The marked cohorts were allowed to develop under these normal conditions for 8 days, giving all bees similar colony environments during the period they were likely to engage in feeding brood. Unmarked, newly emerged, wild-type bees were added to each colony for 3 days following the introduction of the marked, focal bees.
Treatments
On day 9, all colony environments were adjusted to consist of two frames of honey: one frame with 300 cm2 of pollen and empty space and one frame of foundation. In each colony, the queen was confined in a cylindrical wire cage that allowed contact with workers. We tried to make the colonies as similar as possible. The six colonies were then evenly divided into three replicates of paired treatments: (1) brood [approximately one full frame open brood (750 cm2)] and (2) broodless (dummy frame). The dummy frame was made from an empty frame with sheets of wood covering the comb surface. This way, empty space, stored pollen, and honey were equalized between colonies and only the amount of brood differed. We made sure that stored pollen (an inhibitor of pollen foraging) was not different between our brood treatments to be certain that we were only testing the effects of brood on foraging behavior. Every 5 days, the brood combs from the brood treatment colonies were removed, and new frames of young, open larvae were substituted. This allowed us to maintain brood and broodless treatments throughout the experiment. All colonies were reset with 300 cm2 of pollen on day 17 to prevent significantly different amounts of stored pollen due to treatment. The amount of stored pollen was measured at the end of the study and was not found to be significantly different between the treatments (t test, p > 0.05); however, our small sample sizes (three colonies per treatment) should be noted.
Foraging behavior
Sampling began after entrance counts consistently showed bees of all genotypes foraging (day 20). Returning marked foragers were collected each day from a pair of colonies (one with brood and one without brood). We collected all of the observed foragers each day during 10-min sampling periods throughout the hours of 0800 and 1500. Given the limitation of having one person collecting the data, pairs of colonies (replicates) were rotated on a daily basis; for example, colonies 1 and 2 were sampled on days 20, 23, 26, etc. while colonies 3 and 4 were sampled on days 21, 24, 27, etc. and colonies 5 and 6 were sampled on days 22, 25, 28, etc. Six rounds of collections took place over 3 weeks. A mesh screen was placed over the entrance of each hive to facilitate the capture of returning foragers. Individual bees were treated with carbon dioxide gas until immobile to enable the expulsion of crop contents and removal of pollen loads. The weight of the pollen load was attained for each bee by weighing the pollen pellet from the corbicula of one of the hind legs. We recorded the weight of only one pollen load because pollen loads can be accidentally knocked off and lost during collection and processing. Since our scale only measured down to 0.001 g, workers with less than 0.001 g per leg were recorded as having a 0.0005 g per leg (0.001 g total pollen load). Crop contents were expelled into pre-weighed capillary tubes (Kimax-51) that were then re-weighed (Gary and Lorenzen 1976). The sucrose concentrations of the crop contents were then individually measured using a hand-held refractometer (Bausch & Lomb Optical, USA). Processed bees were then frozen to avoid re-sampling. The pollen proportion of the entire foraging load was calculated from the foraging load data by dividing the pollen load weight by the combined weight of the nectar and pollen loads. It also excludes non-foragers from the analyses since the denominator for these individuals is zero. The experiment was terminated when at least 100 foragers of each genotype from each colony were collected (ending on 22 May 2004). At the termination of the experiment, all colonies were frozen, and remaining focal bees were counted to determine the number of non-foraging individuals.
Statistics
Kolmogorov–Smirnov tests of normality were performed to confirm normal distributions for foraging load measurements and pollen proportions (p < 0.0001 for each). Two-way ANOVA was used to test the effects of genotype and treatment on pollen load weights, nectar load weights, pollen proportions, and nectar load concentrations (pooled across replicates). Fisher’s protected least significant difference tests were used to test the significance of post hoc effects. Foraging age was approximated by the age on the day the individual was collected. The collected remaining focal bees that had not foraged by the termination of the experiment were assigned to the “censused” group for statistical analyses. Nonparametric survival analyses with Kaplan–Meier estimations and Mantel–Cox tests were used to analyze foraging age by genotype and treatment. Analyses were performed with StatView software.
Results
Genotype groups (pooled across treatments) differed significantly for pollen load weights, the proportion of their total load weight that was pollen, nectar load weights, and nectar load concentrations (Fig. 1a–d; pollen weight F 2,1425 = 29.423, p < 0.0001, n high = 488, n low = 395, n wild-type = 548; pollen proportion F 2,1089 = 39.076, p < 0.0001, n high = 385, n low = 293, n wild-type = 417; nectar weight F 2,1420 = 13.871, p < 0.0001, n high = 487, n low = 394, n wild-type = 545; nectar concentration F 2,791 = 3.205, p = 0.0411, n high = 225, n low = 256, n wild-type = 316). Post hoc analyses revealed that high-strain bees collected heavier pollen loads, had higher proportions of pollen, lighter nectar loads, and nectar of higher concentration than low-strain bees (Fig. 1a–d; pollen weight p < 0.0001; pollen proportion p < 0.0001; nectar weight p < 0.0001; nectar concentration p = 0.0014). The concentration of nectar from returning foragers decreased over the 3 weeks data were collected (R 2 = 0.015, F 1,795 = 13.155, p = 0.0003, n = 797). Genotype differences in pollen weights and pollen proportions existed within both treatments (Fig. 1a, b; brood: pollen weight F 2,902 = 31.806, p < 0.0001, n = 905, pollen proportion F 2,710 = 37.501, p < 0.0001, n = 713; broodless: pollen weight F 2,523 = 8.264, p = 0.0003, n = 526, pollen proportion F 2,379 = 11.721, p < 0.0001, n = 382). Survival analyses showed that low-strain bees initiated foraging later in life than high-strain bees and wild-type bees (χ 2 = 34.572, df = 2, p < 0.0001).
a Bees from colonies with brood collected heavier pollen loads than bees from broodless colonies. Pollen load weights differed by genotype as well (high > WT > low). High-strain bees are more responsive to the brood treatment than the other genotypes. b The pollen proportions of the entire foraging load [pollen load weight/(pollen load weight + nectar load weight)] for high-strain, low-strain, and wild-type foragers from colonies with and without brood. There are significant differences between the treatments and between genotypes. c Low-strain bees collected heavier nectar loads than high-strain bees. Brood treatment did not affect nectar load weights. d High-strain bees collected nectar loads of higher sugar concentration than low-strain bees. The brood treatment did not affect the nectar load concentration
Treatment effects were seen for pollen load weights and pollen proportions. Bees from the colonies with brood collected heavier pollen loads and higher proportions of pollen than bees from broodless colonies (Fig. 1a, b; pollen weight F 1,1425 = 19.514, p < 0.0001, n brood = 905, n broodless = 526; pollen proportion F 1,1089 = 6.604, p = 0.0103, n brood = 713, n broodless = 382). Nectar load weights and nectar concentrations were not different between the brood and broodless treatments. Genotype × treatment interaction effects on pollen load weights reveal that the high- and low-strain bees responded differently to the presence or absence of brood (Fig. 1a; F 2,1425 = 3.637, p = 0.0266, n = 1431). Multiple comparisons showed that the treatment effect was strong in bees from the high-strain and was not significant in low-strain and wild-type bees (high strain, F 1,486 = 19.129, p < 0.0001, n = 488). There was no significant interaction for pollen proportion. Mantel–Cox survival analyses showed that bees in the brood treatment initiated foraging earlier than bees in the broodless treatment (χ 2 = 156.342, df = 1, p < 0.0001). Brood treatment affected foraging age in all genotypes (Fig. 2; high strain, χ 2 = 31.376, df = 1, p < 0.0001; low strain, χ 2 = 99.626, df = 1, p < 0.0001; wild type: χ 2 = 41.009, df = 1, p < 0.0001).
Discussion
High-strain bees are more responsive to brood stimuli than low-strain bees, answering our third hypothesis and explaining, in part, differences in pollen foraging behavior between the high and low strains. High-strain bees and wild-type pollen foragers are more responsive and sensitive to other stimuli than low-strain bees and nectar foragers, respectively, when tested under laboratory and field conditions (Scheiner et al. 1999, 2001a,b, 2004; Pankiw and Page 1999, 2000, 2001a; Pankiw et al. 2001, 2002, 2004b; Pankiw 2003; Page et al. 1998; Erber et al. 2006; Tsuruda et al. 2008) suggesting that selection for increased pollen foraging acts on the sensory response system of workers. As hypothesized, high-strain bees respond to brood stimulus by collecting heavier loads of pollen; however, brood stimulus had no measurable effect on nectar loads. Previous studies have shown effects of brood stimuli on the number of pollen foragers but not the number of nectar foragers, suggesting that brood affects the nectar and pollen loading decisions by placing a higher relative value on pollen for any given concentration of nectar (Dreller et al. 1999; Pankiw et al. 1998, Pankiw and Page 2001a,b; Pankiw 2004a,b; Amdam et al. 2008). The decreasing concentration of available nectar during the length of the study provides a plausible explanation why high-strain bees collected nectar of higher concentration than low-strain bees; high-strain bees initiated foraging earlier in life when the more concentrated nectar was available.
The presence of young larvae affected the foraging ages of all genotypes (Fig. 2). Workers in colonies with brood foraged earlier in life than those in broodless colonies, addressing our first hypothesis (Fig. 2). The lack of a differential response between genotypes to the brood treatment suggests that the earlier onset of foraging seen in high-strain bees was not in response to brood stimuli but more likely in response to reduced titers of vitellogenin, a yolk precursor protein associated with reproductive regulatory networks (Page and Amdam 2007). Vitellogenin, as a pacer of behavioral development, inhibits the onset of foraging (Nelson et al. 2007; Page and Amdam 2007; Amdam et al. 2008). Vitellogenin levels are highest in nursed-aged bees, which transfer the protein to colony members, including larvae (Amdam et al. 2003). As the vitellogenin levels decrease (and juvenile hormone levels increase), the transition from in-hive tasks to foraging occurs (Amdam et al. 2003; Fluri et al. 1982). In our colonies where brood is present, nurse bees are able to lose vitellogenin more rapidly (due to feeding larvae) and, therefore, transition to foraging earlier in life than bees in broodless colonies. Le Conte et al. (2001) also showed an earlier onset of foraging in colonies with brood versus colonies without brood as well as dose-dependent effects of brood pheromone on foraging age. Bees in broodless colonies treated with a high dose of brood pheromone foraged later in life than bees from broodless colonies without pheromone treatment. This suggests that brood pheromone and feeding brood have opposing effects on the onset of foraging.
Brood stimuli are not essential for the initiation of foraging or pollen foraging behavior. High, low, and wild-type bees initiated foraging and continued to forage for pollen in the absence of brood. However, in opposition to our last hypothesis, genotype still had an effect on both variables. Under broodless conditions, high-strain bees were more likely to forage earlier in life and collect more pollen than low-strain bees (Figs. 1a and 3). Wild-type bees displayed intermediate phenotypes. We conclude that brood stimuli alone, as opposed to the combined brood and pollen treatments of Pankiw and Page 2001a, affect the pollen foraging behavior of high and low pollen-hoarding strains; however, observed baseline differences in foraging onset and pollen foraging behavior may also be due to intrinsic foraging biases that are modulated by other, as yet undetermined, stimuli.
References
Al-Tikrity WS, Benton AW, Hillman RC, Clarke WW (1972) The relationship between the amount of unsealed brood in honeybee colonies and their pollen collection. J Apic Res 11:9–12
Amdam GV, Norberg K, Hagen A, Omholt SW (2003) Social exploitation of vitellogenin. Proc Natl Acad Sci USA 100:1799–1802. doi:10.1073/pnas.0333979100
Amdam GV, Ihle K, Page RE Jr (2008) Regulation of worker honey bee (Apis mellifera) life histories by vitellogenin. In: Fahrbach S (ed) Hormones, brain and Behavior. Elsevier, San Diego
Barker RJ (1971) The influence of food inside the hive on pollen collection by a honeybee colony. J Apic Res 10:23–26
Bitterman ME, Menzel R, Fietz A, Schäfer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97:107–119
Calderone NW, Page RE (1988) Genotypic variability in age polyethism and task specialization in the honey bee, Apis mellifera (Hymenoptera: Apidae). Behav Ecol Sociobiol 22:17–25. doi:10.1007/BF00395694
Calderone NW, Page RE Jr (1991) Evolutionary genetics of division of labor in colonies of the honey bee (Apis mellifera). Am Nat 138:69–92. doi:10.1086/285205
Calderone NW, Page RE (1996) Temporal polyethism and behavioural canalization in the honey bee, Apis mellifera. Anim Behav 51:631–643. doi:10.1006/anbe.1996.0068
Calderone NW, Robinson GE, Page RE (1989) Genetic structure and division of labor in honeybee societies. Cell Mol Life Sci 45:765–767
Cale GH (1968) Pollen gathering relationship to honey collection and egg laying in honey bees. Am Bee J 108:8–9
Chapman N, Oldroyd B, Hughes W (2007) Differential responses of honeybee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behav Ecol Sociobiol 61:1185–1194. doi:10.1007/s00265-006-0348-0
Dreller C, Tarpy DR (2000) Perception of the pollen need by foragers in a honeybee colony. Anim Behav 59:91–96. doi:10.1006/anbe.1999.1303
Dreller C, Fondrk MK, Page RE (1995) Genetic variability affects the behavior of foragers in a feral honeybee colony. Naturwissenschaften 82:243–245. doi:10.1007/s001140050179
Dreller C, Page RE Jr, Fondrk MK (1999) Regulation of pollen foraging in honeybee colonies: effects of young brood, stored pollen, and empty space. Behav Ecol Sociobiol 45:227–233. doi:10.1007/s002650050557
Erber J, Hoormann J, Scheiner R (2006) Phototactic behaviour correlates with gustatory responsiveness in honey bees (Apis mellifera L.). Behav Brain Res 174:174–180. doi:10.1016/j.bbr.2006.07.023
Fewell JH, Page RE (1993) Genotypic variation in foraging responses to environmental stimuli by honey bees, Apis mellifera. Cell Mol Life Sci 49:1106–1112. doi:10.1007/BF01929923
Fewell JH, Page RE Jr (2000) Colony-level selection effects on individual and colony foraging task performance in honeybees, Apis mellifera L. Behav Ecol Sociobiol 48:173–181. doi:10.1007/s002650000183
Fewell JH, Winston ML (1992) Colony state and regulation of pollen foraging in the honey bee, Apis mellifera L. Behav Ecol Sociobiol 30:387–393. doi:10.1007/BF00176173
Filmer RS (1932) Brood area and colony size as factors in activity of pollination units. J Econ Entomol 25:336–343
Fluri P, Lüscher M, Wille H, Gerig L (1982) Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J Insect Physiol 28:61–68
Free JB (1967) Factors determining the collection of pollen by honeybee foragers. Anim Behav 15:134–144. doi:10.1016/S0003-3472(67)80024-1
Free JB, Williams IH (1971) The effect of giving pollen and pollen supplement to honeybee colonies on the amount of pollen collected. J Apic Res 10:87–90
Gary NE, Lorenzen K (1976) A method for collecting the honeysac contents from honeybees. J Apic Res 15:73–79
Guzman-Novoa E, Gary NE (1993) Genotypic variability of components of foraging behavior in honey bees (Hymenoptera: Apidae). J Econ Entomol 86:715–721
Guzmán-Novoa E, Page RE, Gary NE (1994) Behavioral and life-history components of division of labor in honey bees (Apis mellifera L.). Behav Ecol Sociobiol 34:409–417. doi:10.1007/s002650050057
Hellmich RL II, Kulincevic JM, Rothenbuhler WC (1985) Selection for high and low pollenhoarding honey bees. J Hered 76:155–158
Hunt GJ, Page RE Jr, Fondrk MK, Dullum CJ (1995) Major quantitative trait loci affecting honey bee foraging behavior. Genetics 141:1537–1545
Le Conte Y, Mohammedi A, Robinson GE (2001) Primer effects of a brood pheromone on honeybee behavioural development. Proc R Soc Lond B Biol Sci 268:163–8. doi:10.1098/rspb.2000.1345
Moeller FE (1972) Honey bee collection of corn pollen reduced by feeding pollen in the hive. Am Bee J 112:210–212
Nelson CM, Ihle K, Amdam GV, Fondrk MK, Page RE (2007) The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol 5:673–677. doi:10.1371/journal.pbio.0050062
Page RE, Amdam GV (2007) The making of a social insect: developmental architectures of social design. BioEssays 29(4):334–343. doi:10.1002/bies.20549
Page RE, Fondrk MK (1995) The effects of colony-level selection on the social organization of honey bee (Apis mellifera L.) colonies: Colony-level components of pollen hoarding. Behav Ecol Sociobiol 36:135–144. doi:10.1007/BF00170718
Page RE Jr, Robinson GE, Fondrk MK, Nasr ME (1995) Effects of worker genotypic diversity on honey bee colony development and behavior (Apis mellifera L.). Behav Ecol Sociobiol 36:387–396. doi:10.1007/s002650050161
Page RE Jr, Erber J, Fondrk MK (1998) The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 182:489–500. doi:10.1007/s003590050196
Page RE Jr, Fondrk MK, Hunt GJ, Guzman-Novoa E, Humphries MA, Nguyen K, Greene AS (2000) Genetic dissection of honeybee (Apis mellifera L.) foraging behavior. J Hered 91:474–479. doi:10.1093/jhered/91.6.474
Pankiw T (2003) Directional change in a suite of foraging behaviors in tropical and temperate evolved honey bees (Apis mellifera L.). Behav Ecol Sociobiol 54:458–464. doi:10.1007/s00265-003-0640-1
Pankiw T (2004a) Cued in: Honey bee pheromones as information flow and collective decision-making. Apidologie 35:217–226. doi:10.1051/apido:2004009
Pankiw T (2004b) Brood pheromone regulates foraging activity of honey bees (Hymenoptera: Apidae). J Econ Entomol 97:748–51. doi:10.1603/0022-0493(2004)097<0748:BPRFAO>2.0.CO;2
Pankiw T (2007) Brood pheromone modulation of pollen forager turnaround time in the honey bee (Apis mellifera L.). J Insect Behav 20:173–180. doi:10.1007/s10905-007-9071-6
Pankiw T, Page RE Jr (1999) The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 185:207–213. doi:10.1007/s003590050379
Pankiw T, Page RE Jr (2000) Response thresholds to sucrose predict foraging division of labor in honeybees. Behav Ecol Sociobiol 47:265–267. doi:10.1007/s002650050664
Pankiw T, Page RE (2001a) Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behav Ecol Sociobiol 51:87–94. doi:10.1007/s002650100408
Pankiw T, Page RE Jr (2001b) Brood pheromone modulates honeybee (Apis mellifera L.) sucrose response thresholds. Behav Ecol Sociobiol 49:206–213. doi:10.1007/s002650000282
Pankiw T, Page RE Jr, Fondrk MK (1998) Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera). Behav Ecol Sociobiol 44:193–198. doi:10.1007/s002650050531
Pankiw T, Waddington KD, Page RE (2001) Modulation of sucrose response thresholds in honey bees (Apis mellifera L.): influence of genotype, feeding, and foraging experience. J Comp Physiol A 187:293–301. doi:10.1007/s003590100201
Pankiw T, Tarpy DR, Page RE (2002) Genotype and rearing environment affect honeybee perception and foraging behaviour. Anim Behav 64:663–672. doi:10.1006/anbe.2002.3096
Pankiw T, Roman R, Sagili RR, Zhu-Salzman K (2004a) Pheromone-modulated behavioral suites influence colony growth in the honey bee (Apis mellifera). Naturwissenschaften 91:575–578. doi:10.1007/s00114-004-0568-y
Pankiw T, Nelson M, Page RE, Fondrk MK (2004b) The communal crop: modulation of sucrose response thresholds of pre-foraging honey bees with incoming nectar quality. Behav Ecol Sociobiol 55:286–292. doi:10.1007/s00265-003-0714-0
Robinson GE, Page RE (1989) Genetic determination of nectar foraging, pollen foraging, and nest-site scouting in honey bee colonies. Behav Ecol Sociobiol 24:317–323. doi:10.1007/BF00290908
Rösch GA (1925) Untersuchungen über die Arbeitsteilung im Bienenstat, 1. Teil: Die tätigkeiten im normalen Bienenstaat und ihre Beziehungen zum Alter der Arbeitsbienen. Z verg Physiol 2:571–631. doi:10.1007/BF00337915
Rothenbuhler WC, Page RE Jr (1989) Genetic variability for temporal polyethism in colonies consisting of similarly-aged worker honey bees. Apidologie 29:433–437
Rueppell O, Pankiw T, Page RE Jr (2004) Pleiotropy, epistasis and new QTL: the genetic architecture of honey bee foraging behavior. J Hered 95:481–491. doi:10.1093/jhered/esh072
Rueppell O, Chandra SBC, Pankiw T, Fondrk MK, Beye M, Hunt G, Page RE (2006) The genetic architecture of sucrose responsiveness in the honeybee (Apis mellifera L.). Genetics 172:243–251. doi:10.1534/genetics.105.046490
Scheiner R, Erber J, Page RE Jr (1999) Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.). J Comp Physiol A 185:1–10. doi:10.1007/s003590050360
Scheiner R, Page RE, Erber J (2001a) The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.). Neurobiol Learn Mem 76:138–150. doi:10.1006/nlme.2000.3996
Scheiner R, Page RE, Erber J (2001b) Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav Brain Res 120:67–73. doi:10.1016/S0166-4328(00)00359-4
Scheiner R, Page RE, Erber J (2004) Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 35:133–142. doi:10.1051/apido:2004001
Schmickl T, Crailsheim K (2004) Inner nest homeostasis in a changing environment with special emphasis on honey bee brood nursing and pollen supply. Apidologie 35:249–263. doi:10.1051/apido:2004019
Todd FE, Reed CB (1970) Brood measurement as a valid index to the value of honey bees as pollinators. J Econ Entomol 63:148–149
Tsuruda JM, Amdam GV, Page RE (2008) Sensory response system of social behavior tied to female reproductive traits. PLoS ONE 3:e3397. doi:10.1371/journal.pone.0003397
Acknowledgements
We thank A. Hedrick and W. Leal for helpful comments on the manuscript and M. K. Fondrk for technical assistance. Research was funded by the US Department of Agriculture (NRI-CSREES 2003-01620) and the National Institute of Aging (NIA P01 AG22500). This study complies with current US laws regarding animal care and use.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Moritz
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tsuruda, J.M., Page, R.E. The effects of young brood on the foraging behavior of two strains of honey bees (Apis mellifera). Behav Ecol Sociobiol 64, 161–167 (2009). https://doi.org/10.1007/s00265-009-0833-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0833-3