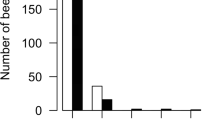
Abstract
Which task a social insect worker engages in is influenced by the worker’s age, genotype and the colony’s needs. In the honeybee, Apis mellifera, genotype influences both the age a worker switches tasks and its propensity of engaging in specialist tasks, such as water collecting, which only some workers will perform. In this study, we used colonies with natural levels of genetic diversity and manipulated colony age demography to drastically increase the stimuli for the generalist tasks of foraging and nursing, which all workers are thought to engage in at some point in their lives. We examined the representation of worker patrilines engaged in nursing and foraging before and after the perturbation. The representation of patrilines among foragers and nurses differed from that of their overall colony’s population. In the case of foraging, over- and underrepresentation of some patrilines was not simply due to differences in rates of development among patrilines. We show that replacement foragers tend to be drawn from patrilines that were overrepresented among foragers before the perturbation, suggesting that there is a genetic component to the tendency to engage in foraging. In contrast, the representation of patrilines in replacement nurses differed from that in the unperturbed nursing population. Our results show that there is a genetic influence on even the generalist tasks of foraging and nursing, and that the way patrilines in genetically diverse colonies respond to increases in task stimuli depends upon the task. The possible significance of this genetic influence on task allocation is discussed.





Similar content being viewed by others
References
Bertram SM, Gorelick R, Gewell JH (2003) Colony response to graded resource changes: an analytical model of the influence of genotype, environment, and dominance. Theor Popul Biol 64:151–162
Breed MD, Robinson GE, Page RE (1990) Division of labor during honey bee colony defense. Behav Ecol Sociobiol 27:398–401
Calderone NW, Page RE (1988) Genotypic variability in age polyethism and task specialization in the honey bee, Apis mellifera (Hymenoptera: Apidae). Behav Ecol Sociobiol 22:17–25
Cohen J (1988) Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum, Hillsdale, NJ
Cox MD, Myerscough MR (2003) A flexible model of foraging by a honey bee colony: the effects of individual behaviour on foraging success. J Theor Biol 223:179–197
Crozier RH, Fjerdingstad EJ (2001) Polyandry in social Hymenoptera—disunity in diversity? Ann Zool Fenn 38:267–285
Crozier RH, Page RE (1985) On being the right size: male contributions and multiple mating in social Hymenoptera. Behav Ecol Sociobiol 18:105–115
Dreller C, Tarpy DR (2000) Perception of the pollen need by foragers in a honeybee colony. Anim Behav 59:91–96
Erdfelder E, Faul F, Buchner A (1996) GPower: a general power analysis program. Behav Res Meth Instrum C 28:1–11
Estoup A, Solignac M, Cornuet JM (1994) Precise assessment of the number of patrilines and of genetic relatedness in honeybee colonies. Proc R Soc Lond B 258:1–7
Fewell JH, Bertram SM (1999) Division of labor in a dynamic environment: response by honeybees (Apis mellifera) to graded changes in colony pollen stores. Behav Ecol Sociobiol 46:171–179
Fewell JH, Page RE (1993) Genotypic variation in foraging responses to environmental stimuli by honey bees, Apis mellifera. Experientia 49:1106–1112
Fewell JH, Page RE (2000) Colony-level selection effects on individual and colony foraging task performance in honeybees, Apis mellifera L. Behav Ecol Sociobiol 48:173–181
Fowler J, Cohen L, Jarvis P (1998) Practical statistics for field biology. Wiley, Brisbane, QLD
Franck P, Coussy H, Le Conte Y, Solignac M, Garnery L, Cornuet JM (1999) Microsatellite analysis of sperm admixture in honeybee. Insect Mol Biol 8:419–421
Franck P, Koeniger N, Lahner G, Crewe RM, Solignac M (2000) Evolution of extreme polyandry: an estimate of mating frequency in two African honeybee subspecies, Apis mellifera monticola and A. m. scutellata. Insectes Soc 47:364–370
Franck P, Solignac M, Vautrin D, Cornuet JM, Koeniger G, Koeniger N (2002) Sperm competition and last-male precedence in the honeybee. Anim Behav 64:503–509
Free JB (1967) Factors determining the collection of pollen by honey bee foragers. Anim Behav 15:134–144
Fuchs S, Moritz RFA (1998) Evolution of extreme polyandry in the honeybee Apis mellifera L. Behav Ecol Sociobiol 9:269–275
Giray T, Robinson GE (1994) Effects of intracolony variability in behavioral development on plasticity of division of labor in honey bee colonies. Behav Ecol Sociobiol 35:13–20
Graham S, Myerscough MR, Jones JC, Oldroyd BP (2006) Modelling the role of intracolonial genetic diversity on regulation of brood temperature in honey bee (Apis mellifera L.) colonies. Insectes Soc 53:226–232
Guzman-Novoa E, Gary NE (1993) Genotypic variability of components of foraging behavior in honey bees (Hymenoptera: Apidae). J Econ Entomol 86:715–721
Guzman-Novoa E, Page RE (1994) Genetic dominance and worker interactions affect honeybee colony defence. Behav Ecol 5:91–97
Guzman-Novoa E, Page RE, Gary NE (1994) Behavioral and life-history components of division of labor in honey bees (Apis mellifera L.). Behav Ecol Sociobiol 34:409–417
Haberl M, Tautz D (1998) Sperm usage in honey bees. Behav Ecol Sociobiol 42:247–255
Huang ZY, Otis GW (1989) Factors determining hypopharyngeal gland activity of worker honey bees (Apis mellifera L.). Insectes Soc 36:264–276
Huang ZY, Robinson GE (1996) Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol 39:147–158
Huang ZY, Otis GW, Teal PEA (1989) Nature of brood signal activating the protein synthesis of hypopharyngeal gland in honey bees Apis mellifera (Apidae: Hymenoptera). Apidologie 20:455–464
Hunt GJ, Guzman-Novoa E, Uribe-Rubio JL, Prieto-Merlos D (2003) Genotype-enviornment interactions in honeybee guarding behaviour. Anim Behav 66:459–467
Jones JC, Myerscough MR, Graham S, Oldroyd BP (2004) Honey bee nest thermoregulation: diversity promotes stability. Science 305:402–404
Kryger P, Kryger U, Moritz RFA (2000) Genotypical variability for the tasks of water collecting and scenting in a honey bee colony. Ethology 106:769–779
Kolmes SA, Winston ML, Fergusson LA (1989) The division of labor among worker honey bees (Hymenoptera: Apidae): The effects of multiple patrilines. J Kans Entomol Soc 62:80–95
Leoncini I, le Conte Y, Costagliola G, Plettner E, Toth A, Wang MW, Huang Z, Becard JM, Crauser D, Slessor KN, Robinson GE (2004) Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc Natl Acad Sci USA 101:17559–17564
Lindauer M (1971) Communication among social bees. Harvard University Press, Cambridge
Myerscough MR, Oldroyd BP (2004) Simulation models of the role of genetic variability in social insect task allocation. Insectes Soc 51:146–152
Oldroyd BP, Rinderer TE, Buco SM (1992) Intra-colonial foraging specialism by honey bees (Apis mellifera) (Hymenoptera: Apidae). Behav Ecol Sociobiol 30:291–295
Oldroyd BP, Sylvester HA, Wongsiri S, Rinderer TE (1994) Task specialization in a wild bee, Apis florea (Hymenoptera: Apidae), revealed by RFLP banding. Behav Ecol Sociobiol 34:25–30
Page RE, Robinson GE, Britton DS, Fondrk MK (1992) Genotypic variability for rates of behavioral development in worker honeybees (Apis mellifera L.). Behav Ecol 3:173–180
Page RE, Robinson GE, Fondrk MK, Nasr ME (1995) Effects of worker genotypic diversity on honey bee colony development and behavior (Apis mellifera L.). Behav Ecol Sociobiol 36:387–396
Palmer KA, Oldroyd BP (2000) Evolution of multiple mating in the genus Apis. Apidologie 31:235–248
Pankiw T, Page RE, Fondrk MK (1998) Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera). Behav Ecol Sociobiol 44:193–198
Robinson GE (1992) The regulation of division of labour in insect societies. Annu Rev Entomol 37:637–665
Robinson GE (2002) Genomics and integrative analyses of division of labor in Honeybee colonies. Am Nat 160:S160–S172
Robinson GE, Huang ZY (1998) Colony integration in honey bees: genetic, endocrine and social control of division of labor. Apidologie 29:159–170
Robinson GE, Page RE (1988) Genetic determination of guarding and undertaking in honey-bee colonies. Nature 333:356–358
Robinson GE, Page RE (1995) Genetic constraints on plasticity for corpse removal in honey bee colonies. Anim Behav 49:867–876
Robinson GE, Underwood BA, Henderson CE (1984) A highly specialized water-collecting honey bee. Apidologie 15:355–358
Robinson GE, Page RE, Strambi C, Strambi A (1989) Hormonal and genetic control of behavioral integration in honey bee colonies. Science 246:109–112
Robinson GE, Page RE, Strambi C, Strambi A (1992) Colony integration in honey bees—mechanisms of behavioral reversion. Ethology 90:336–348
Rothenbuhler WC (1964) Behavior genetics of nest cleaning in honey bees. IV. Responses of F1 and backcross generations to disease-killed brood. Am Zool 4:111–123
Schluns H, Koeniger G, Koeniger N, Moritz RFA (2004) Sperm utilization pattern in the honeybee (Apis mellifera). Behav Ecol Sociobiol 56:458–463
Seeley TD (1985) Honeybee ecology: a study of adaptation in social life. Princeton University Press, Princeton
Seeley TD, Kolmes SA (1991) Age polyethism for hive duties in honey bees-illusion or reality? Ethology 87:284–297
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. WH Freeman, New York
Winston ML (1987) The biology of the honey bee. Harvard University Press, Cambridge, MA
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, NJ
Acknowledgements
We thank Michael Duncan and Julie Lim for technical assistance and members of the Behaviour and Genetics of Social Insects Laboratory at the University of Sydney for comments on earlier versions of the manuscript. We also thank R. E. Page and three anonymous reviewers for their comments and suggestions that greatly improved the manuscript. Funding was provided by a Marie Curie Outgoing International Fellowship within the 6th European Community Framework Programme (W.O.H.H.) and by the Australian Research Council (B.P.O.). The experiments were performed in accordance with the rules and regulations of Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Page
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM1
Electronic Supplementary Material (Pdf 70.4 kb)
Rights and permissions
About this article
Cite this article
Chapman, N.C., Oldroyd, B.P. & Hughes, W.O.H. Differential responses of honeybee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behav Ecol Sociobiol 61, 1185–1194 (2007). https://doi.org/10.1007/s00265-006-0348-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0348-0