Abstract
Colorectal cancer (CRC) is the second most common cause of cancer mortality, with mismatch repair proficient (pMMR) and/or microsatellite stable (MSS) CRC making up more than 80% of metastatic CRC. Programmed death-ligand 1 (PD-L1) and programmed death 1 (PD-1) immune checkpoint inhibitors (ICIs) are approved as monotherapy in many cancers including a subset of advanced or metastatic colorectal cancer (CRC) with deficiency in mismatch repair (dMMR) and/or high microsatellite instability (MSI-H). However, proficient mismatch repair and microsatellite stable (pMMR/MSS) cold CRCs have not shown clinical response to ICIs alone. To potentiate the anti-tumor response of PD-L1/PD-1 inhibitors in patients with MSS cold cancer, combination strategies currently being investigated include dual ICI, and PD-L1/PD-1 inhibitors in combination with chemotherapy, radiotherapy, vascular endothelial growth factor (VEGF) /VEGF receptor (VEGFR) inhibitors, mitogen-activated protein kinase (MEK) inhibitors, and signal transducer and activation of transcription 3 (STAT3) inhibitors. This paper will review the mechanisms of PD-1/PD-L1 ICI resistance in pMMR/MSS CRC and potential combination strategies to overcome this resistance, summarize the published clinical experience with different combination therapies, and make recommendations for future avenues of research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of immunotherapeutic drugs has led to significant improvements in overall and progression-free survival for many patients with cancer [1,2,3,4,5,6]. For colorectal cancer (CRC) in particular, immunotherapy has demonstrated significant benefit in the metastatic setting for a subset of patients [3, 4, 6, 7]. Programmed death 1 (PD-1) is an immune checkpoint receptor mainly expressed in T cells, B cells, natural killer cells (NKs), and myeloid-derived suppressor cells (MDSCs) [8,9,10,11,12]. It binds to its ligands, programmed death-ligand 1 and 2 (PD-L1/2), which are expressed on antigen presenting cells and cancerous cells [10,11,12,13]. The interaction between PD-1 and PD-L1/2 induces T cell exhaustion, inhibits T cell activation and cytotoxic activity, and transforms T effector cells to regulatory T cells (Treg) [10,11,12,13]. As such, blockade of the PD-1/PD-L1/2 pathway can enhance T cell anti-tumor activity and thereby immune control and killing abilities against cancerous cells. The introduction of immunotherapy with immune checkpoint inhibitors (ICIs) targeting PD-1 and PD-L1 has revolutionized management of certain cancers, transforming short-term responses into durable clinical benefits [4, 5, 13, 14]. However, tumors that do not elicit an immune response, so called ‘cold’ tumors, exhibit resistance to this strategy [6, 7, 13, 15, 16]. Many CRCs have a cold phenotype [17]. In 2017, the US Food and Drug Administration (FDA) approved PD-1 immune checkpoint inhibitors pembrolizumab and nivolumab for patients with unresectable or metastatic, mismatch repair deficient (dMMR) and microsatellite instability high (MSI-H) solid tumors who have failed first-line therapy [18, 19]. However, patients with dMMR and MSI-H metastatic CRC (mCRC) comprise only 15% of CRC cases, while the more common mismatch repair proficient (pMMR) and microsatellite stable (MSS) CRC do not respond to ICIs [20]. New strategies are urgently needed for cold mCRCs.
To overcome the hyporesponsiveness to PD-1/PD-L1 inhibitors, recent preclinical studies and clinical trials have demonstrated combination strategies to potentiate the effectiveness of anti-PD-1 and anti-PD-L1 immunotherapy in patients with cold CRC. The FDA has approved combination use of PD-1/PD-L1 inhibitors and other therapy/inhibitors for treatment of patients with cold metastatic cancer. For example, combination of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, tremelimumab, and PD-1 inhibitor, durvalumab, was approved for treating patients with unresectable hepatocellular carcinoma in 2022 [21, 22]. This review will discuss hyporesponse mechanisms and challenges of PD-1/PD-L1 inhibitors in pMMR/MSS cold cancer and explore potential combination strategies to overcome hyporesponsiveness. Further, we discuss clinical experience with combination therapy and recommendations for future research using CRC as an example.
Basic mechanisms of hyporesponse in pMMR/MSS cancer
Carcinogenesis of pMMR/ MSS cancer vs. dMMR/MSI-H cancer
Genomic instability is a trademark of tumor cells. There are two different types of genomic instability: (1) chromosomal instability, which is the consequence of the loss or gain of chromosomes or large chromosomal fragments and is associated with the majority of CRCs, and (2) microsatellite instability (MSI) which is observed in a small fraction of CRCs. [23] Microsatellites are repeated DNA sequences widely dispersed throughout the genome. [24] These repetitive regions are generally associated with higher mutation rates, and replication errors are corrected by the mismatch repair (MMR) system. [25] If there is a deficiency of the MMR system, microsatellites are more prone to replication errors, resulting in MSI. [26] Tumors with dMMR are more likely to be MSI-high (dMMR/MSI-H), while tumor with all tested MMR proteins intact are expected to be MSS or MSI-low (pMMR/MSS). Five microsatellite markers BAT-25, BAT-26, D2S123, D5S346, and D17S250 have been identified; [27] the MSI status of a patient is categorized based on the number of microsatellite markers that demonstrate instability: MSI-H if at least two microsatellite markers show instability; MSI-L (low-frequency MSI) if only one marker show instability; and microsatellite stable (MSS) if there is no instability present among the markers [28]. dMMR/MSI-H CRC comprises 15% of all CRC cases [20]. Growing clinical studies have demonstrated that anti-PD-1 and anti-PD-L1 immunotherapy have positive responses in dMMR/MSI-H cancers but no objective responses in cold pMMR/MSS CRC [6, 7, 15]. There remains a substantial need for novel therapeutic approaches and treatment strategies in metastatic pMMR/MSS CRC.
Immunogenic features of MSS vs. MSI-H CRC
dMMR/MSI-H CRCs generally have a higher tumor mutational burden (TMB). TMB directly correlates to tumor’s ability to harbor a plethora of neoantigens [29]. Immunogenic neoantigens, in turn, increase anti-tumor immunity by presenting on major-histocompatibility-complex class I molecules (MHC-1) for T cell recognition. The increased neoantigen in dMMR/MSI-H CRCs results in greater abundance of tumor-infiltrating lymphocytes (TIL) and memory T cells; they are described as hot tumors [30, 31]. By comparison, MSS tumors generally produce self-antigens that fail to activate immune response against tumor cells, and increased activation of oncogenic signaling pathways upsurges immunosuppressive cells and cytokines [32]. The loss of peptides involved in antigen processing further dampens the immunogenicity of MSS tumors [33]. As a result, MSS cancer is associated with absent or inadequate T cell infiltration and an immunosuppressive tumor microenvironment (TME); they are described as cold tumors [34]. An escape from immune surveillance and immune attack leads to the absence of clinical response to PD-1/PD-L1 blockades in pMMR/MSS tumors compared to dMMR/MSI-H tumors.
PD-1 Inhibitors and PD-L1 Inhibitors in clinical application
To date, many anti-PD-1 antibodies (Abs) and anti-PD-L1 Abs have been developed to block PD-1/PD-L1 signaling. Table 1 lists Abs against PD-1 and PD-L1. Anti-PD-1 Abs (nivolumab, pembrolizumab, and cemiplimab) and anti-PD-L1 Abs (atezolizumab, avelumab, and durvalumab) have been approved by FDA for some solid tumor and hematologic cancers. Nivolumab (Opdivo) is the first human IgG4 monoclonal antibody (mAb) against PD-1 approved by the FDA based on the results from CheckMate-037 with advance melanoma patients [35, 36]. Its indications were expanded to squamous non-small-cell lung cancer (NSCLC) and advanced renal cell carcinoma (RCC) in 2015, [36] Hodgkin’s lymphoma [36] and relapsed/refractory metastatic squamous cell cancer of head and neck (SCCHN) in 2016, [36] and small-cell lung cancer (SCLC) patients in 2018 [36]. The FDA approved the anti-PD-1 mAb pembrolizumab and nivolumab as the second-line treatment for patients with dMMR/MSI-H mCRC in 2017 and approved pembrolizumab as the first-line treatment of patients with dMMR/MSI-H mCRC in June 2020 [35, 36].
Most Abs are genetically engineered for high binding specificity and low off-target adverse effects (AEs) [14, 37, 38]. In general, PD-1/PD-L1 blockades exhibit immune-related AEs including colitis and hepatitis, as well as neutropenia, diarrhea, fatigue, stomatitis, and nausea [6, 15, 38,39,40,41]. ICIs have fewer severe AEs than traditional chemotherapy [16, 39].
So far, anti-PD-1 and anti-PD-L1 mAb therapies confer significant clinic benefit only in specific patient populations. Specifically, there are almost no objective responses to anti-PD-1 and anti-PD-L1 therapies observed for patients with ‘cold’ tumors such as MSS mCRC. Combatting resistance mechanisms or hyporesponse of the anti-PD-1/PD-L1 therapy remains a challenge.
New strategies to overcome hyporesponsiveness: combination treatment
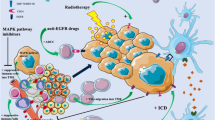
The low immunogenic properties of MSS cancer lead to resistance to PD-L1/PD-1 blockade. To enhance clinical response to the PD-1/PD-L1 inhibitors in pMMR/MSS cancer, one promising strategy is to combine with other anti-tumor agents that target different pathways and increase the immunogenicity of the TME, converting cold tumors to hot tumors. It has been demonstrated that inhibition of CTLA-4, vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR), mitogen-activated protein kinase (MEK), and signal transducer and activator of transcription 3 (STAT3), or treatment with cytotoxic chemotherapy and radiotherapy increases tumor neoantigens, upregulates MHC-1 expression, enhances dendritic cell (DC) antigen presentation and the release of proinflammatory cytokines, increases the activation, infiltration, and killing activities of T cells, and decreases immunosuppressive cells and cytokines. [13, 16, 42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] A cold pMMR/MSS tumor is subsequently converted into a hot tumor, which can then be targeted by PD-1/PD-L1 blockades to confer anti-tumor immunity. This synergistic anti-tumor effect is a promising avenue of study, and clinical trials investigating these approaches are summarized in Table 2.
Combination of CTLA-4 and PD-1/PD-L1 inhibitors
CTLA-4 is an immunoglobulin cell surface receptor constitutively expressed on FoxP3 + Treg as well as conventional T cells following activation by T cell receptor (TCR) signaling [43, 84, 85]. CTLA-4 is a negative T cell regulator structurally similar to the second activation receptor CD28 and exhibits shared binding to B7 ligands on antigen presenting cells (APC) [51]. In the TME, the higher affinity of CTLA-4 for B7 ligands outcompetes the co-stimulatory CD28 receptor and depletes CD28 present in the immune synapse [85, 86]. The loss of the second activation signal (B7-CD28) leads to functionally inactivated and hyporesponsive T cells [87]. Hence, CTLA-4 inhibitors directly reduce the competition between CTLA-4 and CD28 for B7 ligands, promoting naïve T cell priming at the draining lymph nodes [44]. The increased CD28-mediated co-stimulation leads to increased effector T cell proliferation and function [43]. CTLA-4 inhibitors have also been shown to decrease Treg-mediated immunosuppression by selectively depleting Treg in the TME [42]. Ipilimumab and tremelimumab are anti-CTLA-4 Abs approved by the FDA [36, 88].
PD-1 and CTLA-4 function on different subsets of T cells, and on T cells at distinct locations and timing during the cancer-immune response [89, 90]. PD-1 is involved in exhaustion mechanisms in the TME and acts in later stages, while CTLA-4 is primarily involved in the lymph nodes and acts early [51, 91]. As such, the dual ICI treatment with anti-PD-1/anti-PD-L1 and anti-CTLA-4 has shown to reverse the upregulation of other immune checkpoints on T cells, which are induced as a compensatory effect by either drug alone [92]. Furthermore, recent studies have found additional anti-tumor effects specific to the dual combination. The combination prevented CD8 + T cell exhaustion and maintained CD8 + T cells in a responsive state with robust killing abilities against tumor cells [93]. This leads to the terminal differentiation of activated effector CD8 + T cells. The dual inhibition also led to a combination-specific increase in T helper type 1 (Th1) cells [93]. Th1 cells mediated anti-tumor activity through increasing CD8 + T cell infiltration, enhancing antibody responses, and exhibiting Th1 specific cytotoxicity against tumor cells [93, 94]. Inhibitors of CTLA-4 such as ipilimumab and tremelimumab are the first ICIs used for treating cancer patients. Currently, the FDA approved the combination treatment with nivolumab and ipilimumab for dMMR/MSI-H mCRC patients who failed in chemotherapy [95]. Clinical trials of the dual ICI in cold pMMR/MSS CRC are ongoing.
A phase II randomized clinical trial (NCT02870920) studying anti-PD-1 (durvalumab) and anti-CTLA-4 (tremelimumab) combination in patients with pMMR/MSS reported that the dual ICI achieves a prolonged median overall survival (mOS) of 6.6 months in pMMR/MSS mCRC patients, but without objective response (OR) and significant improvement in median progression-free survival (mPFS) [57, 96]. Further subgroup analyses showed that the combination increased overall survival (OS) in patients with TMB higher than 28 months, and patients with consensus molecular subtypes (CMS) 2 had improved OS compared to those with CMS 4 [57, 96,97,98]. The CMS classification system stratifies colorectal cancer into four subtypes based on gene expression profiles: 1) CMS1 is immunogenic, associated with MSI-H; 2) CMS2 is epithelial and canonical; 3) CMS3 is epithelial and metabolic; and 4) CMS4 is mesenchymal [99]. The available clinical study highlights a therapeutic potential for a subset of pMMR/MSS patients and TMB and CMS might be useful stratification biomarkers.
Combination of VEGF/VEGFR and PD-1/PD-L1 inhibitors
Scientific Rationale of VEGF/FEGFR and PD-1/PD-L1 inhibitors
VEGF/VEGFR signaling plays a vital role in forming the immune-suppressive TME in CRC through indirect and direct pathways (Fig. 1). Overexpression of VEGF/VEGFR signal promotes pathologic angiogenesis, forming highly permeable neovasculature in tumors [100]. The resultant abnormal tumor neovasculature increases fluid accumulation and interstitial fluid pressure in the TME, which acts as a direct barrier against cytotoxic T lymphocyte (CTL) infiltration into tumor tissue [101, 102]. New vessels also differentially express important regulatory molecules involved in anti-tumor immunity. Adhesion molecule downregulation impairs the ability of T cells to move through the vessel walls toward the TME [103, 104]. On the other hand, vascular endothelial cells within the tumor vasculature over-express PD-L1 and Fas ligand (FasL), which induce T cell exhaustion/suppression and selectively kill CTLs, resulting in the predominant infiltration of Treg [105,106,107]. Furthermore, angiogenesis-mediated hypoxia in the TME increases the expression of chemokines which enhance Treg recruitment and promotes the polarization of tumor-associated macrophages (TAM) to M2-like immunosuppressive phenotype [108, 109]. Beyond angiogenesis, VEGF/VEGFR signaling induces immune suppression by directly acting on immune cells (Fig. 1). VEGF-VEGFR transduction inhibits differentiation, maturation, and antigen presentation of DCs and increases PD-L1 expression on DCs [45, 46, 110, 111]. This leads to reduced naïve CD8 + T cell priming and decreased maintenance of cytotoxic responses against tumors [112]. VEGF also directly inhibits the differentiation of progenitor cells into conventional T cells, decreasing T cell proliferation and cytotoxicity and prompting PD-L1-driven T cell exhaustion [47, 48]. Moreover, it increases the abundance of suppressive or pro-tumor cells such as Treg, myeloid-derived suppressor cell (MDSC), and M2-like immunosuppressive TAM and drives T cell exhaustion [101, 113,114,115]. Therefore, the inhibition of VEGF/VEGFR signaling could synergistically reduce immune escape to increase the effectiveness of anti-PD-L1/PD-1 inhibitors in patients with cold CRC. Both anti-VEGF therapy and VEGFR tyrosine kinase inhibitors (TKIs) function to inhibit the VEGF signaling pathway. Combination of VEGFR inhibitors (such as regorafenib, lenvatinib, apatinib, and fruquintinib) and PD-1/PD-L1 blockades significantly inhibited angiogenesis and tumor growth in small animal models [116, 117]. The combinations also decreased Treg, shifted macrophages toward M1-like TAM polarization and increased secretion of IFN-γ (an important cytokine involved in tumor.
Tumor cells increase the release of VEGF, which binds to its receptor (VEGFR) to induce angiogenesis. Angiogenesis in turn increases interstitial pressure and hypoxia at the tumor site, which inhibits cytotoxic T cells (CTL) and promotes regulatory T cell (Treg) infiltration. The neovasculature formed via angiogenesis also has higher expression of immunosuppressive molecules PD-L1 and FasL on the vascular endothelial cells (VECs) and lower expression of adhesion molecules. FasL selectively induces CTL apoptosis and PD-L1 inactivates T cells within the tumor vasculature. VEGF/VEGFR also directly modulates immune cell abundance and function. The binding of VEGF to VEGFR inhibits the differentiation and maturation of DCs, which results in reduced T cell activation in the priming phase. It also promotes the proliferation and activation of Tregs and myeloid-derived suppressor cells (MDSCs) and enhances the polarization of tumor-associated macrophages (TAMs) to an M2 phenotype. These immunoregulatory effects reduce CTL function. VEGF also increases the expression TOX in CTL, which in turn upregulates its PD-1 expression and promotes immune exhaustion. Drugs that inhibit VEGF/VEGFR signaling inhibit VEGF/VEGFR-mediated immunosuppression to increase the abundance and function of CTL at the tumor site. Drugs that inhibit PD-L1/PD-1 signaling would block the binding of PD-L1 on CTL to PD-1 on tumor cells and decrease Treg proliferation and function. In combination, anti-VEGF/VEGFR and anti-PD-L1/PD-1 induces a synergistic anti-tumor response immunosurveillance) and overcame PD-L1-induced T cell suppression [118,119,120,121,122]. In fact, positive therapeutic activity has been observed from dual blocking the VEGF/ VEGFR and PD-1/PD-L1 signaling in multiple tumor types [123,124,125,126,127].
Combination treatment with PD-L1/PD-1 blockade and anti-VEGF agents
The combination of atezolizumab (anti-PD-L1 Ab) and bevacizumab (VEGF inhibitor) was studied in patients with MSI-H mCRC pretreated with chemotherapy (NCT01633970) and resulted in an objective response rate (ORR) of 30% and a disease control rate of 90%. [128] One clinical study also investigated the efficacy of atezolizumab in combination with bevacizumab and FOLFOX (chemotherapy) in patients with mCRC irrespective of microsatellite status [59]. An ORR of 52% was observed in patients receiving FOLFOX plus bevacizumab and atezolizumab with an mPFS of 14.1 months without unexpected safety signals [59]. The combination significantly elevated tumor-infiltrating CD8 + T cells and PD-L1 expression. Unfortunately, a phase 2 trial studying atezolizumab plus bevacizumab in patients with chemotherapy-resistant, MSI-like CRC (NCT02982694) was terminated because the efficacy in the MSS subgroup (MSI like) did not meet the expectation [129]. Subsequently, clinical trials have been focused on triple combination of PD-L1/PD-1 blockade, anti-VEGF agents, and chemotherapy.
AtezoTRIBE (NCT03721653) is a multicenter phase II randomized study for the combination of atezolizumab, bevacizumab, and chemotherapy (FOLFOIXIR) as first-line treatment in patients with unresectable mCRC without prior treatment with chemotherapy [61]. Results show that the combination treatment with atezolizumab did not raise unexpected safety concerns, was well-tolerated and improved PFS. High TMB and high Immunoscore-Immune-Checkpoint (Immunoscore-IC) tumors had better PFS [61].
BACCI (NCT02873195) is a multicenter randomized phase II placebo-controlled clinical trial comparing capecitabine (chemotherapy) and bevacizumab with or without atezolizumab in patients with refractory MSS mCRC [123]. The triple combination resulted in significantly longer mPFS compared to the controlled group in MSS only patients, but did not improve OS (10.55 m v.s 10.61 m in placebo control). The patients without liver metastasis had a higher ORR and greater OS compared with those with liver metastasis, exhibiting synergistic clinical benefits with PD-L1 inhibitor and VEGF inhibition [123].
NCT03396926 is a recent phase II clinical trial evaluating the safety and efficacy of combination capecitabine, bevacizumab and pembrolizumab (anti-PD-1) in locally advanced and metastatic unresectable MSS mCRC patients [60, 130]. To date, the treatment was well-tolerated, and no unexpected safety concerns were reported. About one third of patients had PFS > 6 m, but the ORR was only 5%, not meeting the prespecified target of > = 15% [130]. However, this study did not include a control group; therefore, it is difficult to draw conclusion on the efficacy of the combination.
Overall, anti-PD-1, atezolizumab, or pembrolizumab, in combination with bevacizumab and chemotherapy, has demonstrated promising results across multiple clinical studies. Exploratory analysis within studies demonstrated that besides MMR status, TMB, Immunoscore-IC, and the presence of liver metastasis are important predictors of treatment outcome. AtezoTRIBE demonstrated improved clinical benefit in patients with high TMB and high Immunoscore-IC, both of which are associated with MSI-H tumor [131, 132]. Cold tumors with low TMB and/or low immunoscore-IC remain a challenge. Pre-screening with these biomarkers or features is necessary to predict clinical outcome of the triple combination treatment.
Combination with PD-L1/PD-1 blockade and VEGFR inhibitors
VEGFR inhibitors such as regorafenib, lenvatinib, apatinib, and fruquintinib are studied in combination with PD-L1/PD-1 blockade in patients with pMMR/MSS CRC. The efficacy varied across clinical trials and retrospective studies. Most clinical trials studied the combination of regorafenib and anti-PD-1 mAbs (nivolumab, toripalimab, and pembrolizumab) since regorafenib could enhance T cell activation and increase M1/M2 macrophage ratio compared to inhibitors selective for VEGFR-2 [64, 133].
REGONIVO (NCT03406871, EPOC1603) is a phase Ib/II trial to evaluate regorafenib in combination with anti-PD-1 antibodies nivolumab and toripalimab, respectively, for patients with advanced or metastatic pMMR CRC refractory or intolerant to standard chemotherapy [63]. The results show that 80 mg of regorafenib is optimal in combination with nivolumab, with higher tolerances and fewer toxicities. The study also suggests additional clinical benefits with the combination therapy compared to single agent anti-PD-1, particularly in patients without liver metastasis, CPS < 1, and low TMB [63]. Following the promising findings from the REGONIVO study, the combination of regorafenib and PD-1 inhibitors has been considered as a treatment for refractory pMMR/MSS mCRC patients on a compassionate basis. Two retrospective studies of combination of regorafenib and PD-1 inhibitors (nivolumab or pembrolizumab conducted in the USA, and pembrolizumab, camrelizumab, sintilimab, and toripalimab in China) were conducted in patients with MSS mCRC. No objective responses were reported in patients with the combination therapy, differing from the result of the REGONIVO trial [69, 70]. Consistent with the REGONIVO trial, both retrospective studies suggest that patients with liver metastases do worse despite treatment with regorafenib and anti-PD-1 in pMMR/MSS mCRC. [63, 69, 70] The results of clinical and retrospective studies of regorafenib in combination with anti-PD-1Abs suggest that future investigations of patients with pMMR/MSS mCRC might consider analyzing patients with liver metastases separately, and larger randomized control studies are warranted.
Recent clinical studies with similar combination strategies continued to confer variable results. NCT03946917 is a phase 1b/II study that demonstrated promising results in a subset of unselected pMMR/MSS mCRC patients treated with regorafenib and toripalimab (anti-PD-1) who had progressed or were intolerant to at least 2 prior line of chemotherapy [134]. NCT03712943 is a single-arm phase I of regorafenib plus nivolumab in patients with pMMR mCRC [135]. Fatigue and palmar-plantar erythrodysesthesia, which are frequently associated with the use of regorafenib, were the most common adverse events. Dose limiting toxicity (DLT) was observed. There was no correlation between PD-L1 expression and PFS or OS, but low frequency of Tregs resulted in prolonged PFS. In a multicenter phase 2 trial (NCT04126733) studying combination, regorafenib and nivolumab in patients with pMMR/MSS mCRC demonstrated an ORR of 7%. All patients without liver metastasis responded. Better clinical outcomes may be linked with high expression of pre-existing immune sensitivity biomarkers in tumor samples and lower expression of angiogenetic biomarkers in peripheral blood samples [64]. While treatment outcomes from the combination of regorafenib with anti-PD-1 remain inconsistent, potential benefit may exist in subsets of pMMR/MSS CRC patients.
REGOMUNE (NCT03475953) is the first phase II study that evaluated the efficacy and safety of regorafenib in combination with avelumab (anti-PD-L1) in patients with MSS advanced or metastatic CRC refractory to at least one prior standard therapy [65]. The combination treatment was well-tolerated, and no unexpected adverse events were reported. A significant increase in CD8 + T cell infiltration from baseline was reported in the biomarker analysis comparing tumor samples pre- and post-treatment. The patients with increased CD8 + T cell infiltration had significantly better mPFS and median OS [65]. In contrast to the preliminary biomarker analysis reported in the REGONIVO study, no significant differences in mPFS and median OS were observed in patients with varying PD-L1 expression and TMB status. However, regorafenib and avelumab combination has demonstrated promising impacts on the TME of MSS mCRC patients. The study also reported that high-levels of tumor-infiltrating M2 macrophages prior to the treatment was significantly associated with decreased PFS and OS, suggesting the potential use of tumor-infiltrating M2 macrophage as a predictor for the combination therapy [63, 65]. From the results of the preliminary results, the ongoing REGOMUNE study anticipates further investigation of regorafenib plus anti-PD-1/anti-PD-L1 combination in pMMR/MSS mCRC patients selecting for baseline TAM infiltration levels.
LEAP-005 (NCT03797326) is a recent phase II study evaluating the effectiveness of pembrolizumab and lenvatinib (another oral multi-tyrosine kinase inhibitor of VEGFR) in selected refractory solid tumors including the pMMR/non-MSI-H metastatic and/or unresectable CRC cohort [136]. Promising clinical benefits and a manageable safety profile have been observed in patients with previously treated advanced non-MSI-H/pMMR CRC. Currently, the sample size has been expanded to 100 patients, and the results are anticipated to provide a better understanding of the combination’s effect on anti-tumor activity.
NCT03912857 is a phase II trial of the anti-PD-1 mAb, camrelizumab, in combination with apatinib (a selective tyrosine kinase inhibitor for VEGFR-2) for the treatment of advanced or metastatic MSS CRC refractory to two or more prior lines of standard therapy. [68] Objective response was not reported in the study and intolerable toxicity led to treatment interruptions. In contrast, another study of camrelizumab in combination with apatinib in advanced CRC patients unselected for microsatellite status shows that the ORR in the CRC cohort was 30%. Disease was stable in 80%. Grade 3 and above treatment-related adverse events were observed but manageable. [137] This contrast results also highlighted the potential differences in immunogenicity between MSS and MSI-H mCRC.
Despite the glimpse of a new treatment opportunity for pMMR/MSS mCRC patients brought forward by the REGONIVO study, the results were not replicated in other clinical studies. Nonetheless, the studies suggest the potential use of CD8 + T cell infiltration and low-level TAM2 as a positive predictor for treatment efficacy of regorafenib plus avelumab on the TME [65]. The use of regorafenib and anti-PD-1 recently conferred promising effects as a third-line or later treatment of advanced CRC, especially in patients with resected primary lesions [71]. Thus, combination VEGFR TKIs may open the use of PD-L1/PD-1 inhibitors beyond patients with dMMR/MSI-H mCRC.
Combination of MEK and PD-1/PD-L1 inhibitors
Mitogen-activated protein kinase (MAPK) cascades are universally conserved transduction pathways that permit extracellular signals to regulate a range of complex physiological cellular programs including cellular proliferation, development, differentiation, migration, survival, and apoptosis [138]. It is well-established that abnormalities in MAPK signal transduction may dysregulate fundamental cellular processes, resulting in cells that acquire the ability to grow uncontrollably and evade apoptosis, leading to tumorigenesis and the progression of cancer [139]. MEK1/ 2 (MAPK kinases) are the only established direct regulators of extracellular signal-regulated kinase 1 (ERK1) and ERK2 and the most well-characterized MAPKs, therefore, play a central role in the Ras-Raf-MEK-ERK cascade [138]. MEK1/2 inhibitors (MEKi) have received attention as a candidate for clinical use in tumors that depend on the ERK pathway [140]. MEKi may also have effects on the immunogenicity of the TME by acting on both tumor and immune cells [49, 50, 72, 141,142,143]. On tumor cells, MEK can downregulate MHC-I expression [72]. MEKi decrease the secretion of immunosuppressive factors such as VEGF, IL-1, and IL-8, which decreases the recruitment of immunosuppressive cells that inhibit anti-tumor immunity [49]. In addition to tumor cells, MEKi decrease naïve CD8 + T cell priming in the lymph node by preventing MAPK regulation in TCR signaling, while increasing CD8 + T cell infiltration into the TME [50]. MEKi also reduce immunosuppressive cells MDSCs, Tregs, M2-like TAMs, and B-regulatory cells (Breg), which further enhances CD8 + T cell infiltration into the TME [141,142,143]. Given the immunoinhibitory functions associated with MEK signaling, MEK inhibition could potentially increase TME immunogenicity for the subsequent use of anti-PD-1/anti-PD-L1.
NCT01988896 is a phase Ib clinical study that evaluated the efficacy of cobimetinib (a MEK inhibitor) and atezolizumab in patients with solid tumors, 84 of whom have mCRC [72]. The adverse events observed in the combination treatment were consistent with clinical studies of atezolizumab and cobimetinib monotherapies, but many patients experienced intolerance, which resulted in dose reduction or withdrawal. The objective response of the combination failed to exceed the mPFS and mOS reported in anti-PD-1 monotherapy in MSS mCRC patients. The study suggests that CD8 + T cell infiltration could play a role in tumor response, but was insufficient to induce anti-tumor activity.
Similarly, another phase Ib clinical study (NCT02876224) of atezolizumab and bevacizumab in combination with cobimetinib (MEKi) was conducted in patients with mCRC refractory to one or more lines of prior chemotherapy [144]. They found an ORR of 8%. In a multicenter phase III randomized controlled trial (IMblaze370, NCT02788279) evaluating atezolizumab with cobimetinib in patients with (predominantly) MSS mCRC refractory to two or more lines of chemotherapy, the results show similar OS between the combination and atezolizumab monotherapy and similar mPFS and ORR across all treatment cohorts [73]. No significant differences were demonstrated in PFS and OS between patients with MSS mCRC with different PD-L1 expression and RAS mutation status. More grade 3–4 treatment-related adverse events were reported compared to atezolizumab monotherapy.
The addition of cobimetinib was insufficient to overcome MSS mCRC resistance to atezolizumab. However, potential synergistic activity between MEKi, anti-VEGF, and anti-PD-L1 therapy was observed in the primary analysis of a clinical study described above. Although it is difficult to draw conclusions as to whether the effects were due to the addition of anti-VEGF and/or MEKi, the lack of therapeutic options available for patients with chemo-refractory pMMR/MSS mCRC suggests that a three agent combination strategy is worth exploring.
In addition to MAPK signaling, PI3K/AKT/mTOR signaling is associated with cell survival, migration, division, and other activities. A phase I/II clinical trial (NCT03711058) is currently studying the combination of nivolumab with copanlisib (PI3K inhibitor) in relapsed/refractory pMSS CRC [74].
Combination of STAT3 and PD-1/PD-L1 inhibitors
STAT3 is an intracellular signaling molecule and transcription factor shown to regulate an array of specific target genes involved in key cellular processes [52]. Sustained activation of STAT3 in tumor cells mediates carcinogenesis through tumor development and growth, angiogenesis, and metastasis [52]. On the other hand, hyperactivation of STAT3 in tumor and immune cells induces immunosuppression and immune evasion [52]. Activation of STAT3 in tumor cells stimulates the release of immunosuppressive factors (e.g., IL-10, VEGF, PD-L1 and indoleamine 2,3-dioxygenase 1) while suppressing proinflammatory cytokines and chemokines [53, 145,146,147]. Released anti-inflammatory factors in turn activate STAT3 in DCs to prevent DC maturation [148]. With the decrease in antigen presentation by DC, cytotoxic T cells and natural killer cell activation is impeded, and tumor-specific T cell responses are reduced. Therefore, STAT3 inhibition may enhance the activity of anti-PD-L1/anti-PD-1 in patients with MSS mCRC.
Napabucasin is a STAT3 inhibitor studied in combination with anti-PD-1 pembrolizumab in a multicenter phase II clinical trial (NCT02851004) in patients with mCRC refractory or intolerant to at least one regimen of standard chemotherapy [75]. Adverse events associated with the combination of napabucasin and pembrolizumab exhibited safety profiles similar to those observed for either drug alone. The greatest objective response was observed in patients with a higher CPS, and objective response was correlated with an increased TMB. Furthermore, the study found that consensus molecular subtype-2 (CMS2) MSS tumors were more likely to be unresponsive to the combination treatment, while right-sided primary colon cancer was associated with greater clinical benefit [75].
Although primary end point was not met in this clinical trial, napabucasin with pembrolizumab showed greater anti-tumor activity compared to both agents alone. Future studies in a targeted population based on related biomarkers should be further investigated to identify the subset of MSS CRC patients that may receive clinical benefits from the combination therapy.
Combination cytotoxic chemotherapy and PD-1/PD-L1 inhibitors
Cytotoxic chemotherapy is a fundamental part of treatment for patients with mCRC [149]. Currently, fluorouracil (5-FU) and folinic acid (FA) in combination with oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) is the standard chemotherapy regimen for patients with mCRC [150]. Trifluridine/tipiracil (FTD/TPI) is another chemotherapy combination of trifluridine (a thymidine analog) and tipiracil which inhibits the enzyme involved in trifluridine degradation to maintain bioavailability of trifluridine [151]. Cytotoxic chemotherapy not only kills cancer cells or arrests cancer proliferation, but can also enhance immunogenic effects [54]. Chemotherapies induce more cell death, which triggers the release of tumor-associated antigens (TAA) that are then presented by APC to induce tumor-specific cytotoxic response [152]. On the other hand, chemotherapies could increase ICI expression, introducing rationale for combination with inhibitor of PD-L1/PD-1 signaling [153]. Table S3 lists the immunogenic effects of relevant cytotoxic chemotherapy agents.
A multicenter phase II study (NCT02860546) was conducted in combination of FTD/TPI and nivolumab in patients with chemotherapy-refractory MSS mCRC [76]. The addition of FTD/TPI failed to demonstrate significant potentiation of nivolumab activity, and no objective response was reported. In contrast, another phase II clinical study (NCT02375672) of pembrolizumab and FOLFOX for patients with advanced CRC unselected for MMR status shows that the combination had a promising ORR (53%) in naïve MSS CRC patients with acceptable toxicity [77]. This study suggested that there may be opportunities for chemotherapy-ICI combinations within the context of treating naïve MSS CRC.
As discussed in Sect. 4.2, clinical trials are investigating the potentiation of anti-PD-L1/anti-PD-1 by combining chemotherapy (e.g., FOLFOX, FOLFIRI, FOLFOXFIRI, capecitabine) and anti-VEGF inhibitor (bevacizumab). Promising results from the triple agent regimen have suggested that chemotherapy and anti-VEGF can synergistically modulate the TME to make PD-L1/PD-1 ICI more effective against cold pMMR/MSS CRC [59, 61, 123]. Therefore, both chemotherapy-ICI and chemotherapy/anti-VEGF/ICI are worth exploring for patients with cold mCRC.
Combination radiotherapy and PD-L1/PD-1 inhibitors
Radiation therapy has been shown to exhibit immune stimulatory effects on the TME via three distinct and overlapping mechanisms: (1) induction of immunogenic cell death (ICD) of tumor cells; (2) upregulation of neoantigen presentation on MHC-1; and (3) direct alteration of the TME at the site of radiation [161]. The ICD induced at the radiation site releases cytokines as well as death-associated molecular patterns (DAMP), which increase the recruitment of DCs and enhance DCs’ ability to phagocytose apoptotic cells and to process and present antigens [55, 161]. This increases T cell priming and infiltration of tumor-specific T cells. Cytokine (Type-I interferons) release further enhances DC stimulation and T cell activation [162]. Radiation also directly upregulates molecules on the surface of tumor cells, which increases the recognition and killing by T cells and NK cells [56]. Beyond the immediate irradiated field, radiotherapy has been shown to induce systemic immunity via abscopal effects [163]. The distinctive immunostimulatory properties of radiotherapy provides a clear rationale for the combination of radiotherapy-anti- PD-1/PD-L1 in patients with MSS mCRC unresponsive to PD-L1/PD-1 blockade alone. Preclinical studies in tumor-bearing mice found that the combination of tumor radiation and anti-PD-L1 synergistically reduced abundance of MDSC within the TME [164].
To date, no significant clinical responses have been observed across four clinical studies in combination with PD-1 inhibitors [79, 80, 82]. There is a phase II study (NCT02437071) evaluating the anti-tumor response at a distant site outside of the irradiated field patients with pMMR mCRC refractory to at least 2 lines of standard therapy treated with pembrolizumab following radiotherapy [165]. Preliminary results reported objective response in 9% of the patients without grade 3 or higher adverse events; therefore, the study continues, and results are anticipated.
One potential approach to improve the efficacy of anti-PD-1 plus radiotherapy in patients with MSS mCRC relies on the use of multiple nonredundant ICIs. In a phase II clinical trial (NCT03104439), MSS mCRC patients refractory to two or more lines of prior therapy received a combination treatment with ipilimumab (anti-CTLA-4) and nivolumab in conjunction with 8 Gy of radiotherapy [83]. The combination of dual ICIs with radiotherapy was feasible and demonstrated durable activity in patients with MSS mCRC. Correlative serial tumor biopsies and updated efficacy results are anticipated. As follow-up, a phase 2 trial of the same regimen is currently enrolling subjects (NCT04575922) [166].
Future directions
Despite the theoretical framework obtained from preclinical studies of pMMR/MSS cold CRC, limited success was observed across clinical studies for the different combination strategies. Small sample sizes and heterogeneity of tumors or TME in each trial could explain this finding. Comparisons between molecular and cellular phenotypes of common mouse syngeneic models and human tumors may increase our understanding of the mismatched results drawn from preclinical and clinical experiences. Better biomarker detection and patient classification prior to treatment is critical to improve outcomes of combination therapies. Furthermore, it is important to note that oncological signaling pathways (VEGF/VEGF, STAT3, MEK1/2, etc.) have broad biological functions that could be difficult to target specifically or selectively in MSS CRC cells. There are other immune-suppressive molecules or pathways in TME; multiple signaling pathways participate in tumor development and progression. New combinations with other signaling inhibitors or reagents such as temozolomide, which can induce mutation in tumor cells, need to be investigated. We recognize the complexity of the TME; therefore, we suggest future studies to focus on identifying better preclinical models that closely mimic the TME of MSS CRC and efficacy biomarkers in the pMMR/MSS CRC population.
Oncologic outcomes are improving with acceptably safe use of aggressive surgical and local therapy for colorectal liver metastases in carefully selected patients. Evaluating the benefit of systemic immunotherapy either in conjunction with those therapies or following them will be an important avenue for future study. An active multicenter early phase II study is currently investigating the effectiveness of local tumor ablation (radiofrequency ablation or stereotactic body radiation therapy) in combination with durvalumab (Anti-PD-1) and tremelimumab (anti-CTLA-4) in ICI naïve patients with unresectable colorectal liver metastases (NCT03101475). 168
Conclusions
Combination strategies with other anti-tumor agents to potentiate the efficacy of anti-PD-L1/anti-PD-1 in patients with pMMR/MSS advanced or metastatic CRC has become a major research interest as it provides new therapeutic opportunities. In general, combination treatment is safe without significant AEs compared with monotherapy. Preliminary analyses of combination anti-PD-1/PD-L1 inhibitors and other anti-cancer therapies revealed potential clinical benefits in certain subgroups of patients with pMMR/MSS mCRC. Focused approaches to studying these combination regimens will improve outcome of PD-1/PD-L1 combination treatment. We believe that combination strategies involving PD-L1/PD-1 blockade remain a priority for future research as it has the potential to elicit benefits that will revolutionize the clinical landscape for patients with pMMR/MSS cold CRC.
Abbreviations
- 5-FU :
-
Fluorouracil
- Ab :
-
Antibody
- AE :
-
Adverse effect
- APC :
-
Antigen presenting cell
- Breg :
-
B-regulatory cell
- CPS :
-
Combined positive score
- CR :
-
Complete response
- CRC :
-
Colorectal Cancer
- CTL :
-
Cytotoxic T lymphocytes
- CTLA-4 :
-
Cytotoxic T lymphocyte antigen 4
- DAMP :
-
Death-associated molecular pattern
- DC :
-
Dendritic cell
- dMMR/MSI-H :
-
Mismatch repair deficient and microsatellite instability high
- FA :
-
Folinic acid
- Fasl :
-
Fas ligand
- FTD :
-
Trifluridine
- ICD :
-
Immunogenic cell death
- ICI :
-
Immune checkpoint inhibitor
- IDO1:
-
Indoleamine 2,3-dioxygenase 1
- MAPK :
-
Mitogen-activated protein kinase
- mCRC :
-
Metastatic colorectal cancer
- MDSC :
-
Myeloid-derived suppressor cell
- MEK :
-
Mitogen-activated protein kinase
- MHC-1 :
-
Major-histocompatibility-complex class I
- mPFS :
-
Median progression-free survival
- OR :
-
Objective response
- ORR :
-
Objective response rate
- OS :
-
Overall survival
- OX :
-
Oxaliplatin
- PD-1 :
-
Programmed death 1
- PD-L1 :
-
Programmed death-ligand 1
- PFS :
-
Progression-free survival
- pMMR/MSS :
-
Mismatch repair proficient and microsatellite stable
- PR :
-
Partial response
- STAT3 :
-
Signal transducer and activator of transcription 3
- TAM :
-
Tumor-associated macrophage
- TCR :
-
T cell receptor
- TIL :
-
Tumor-infiltrating lymphocyte
- TMB :
-
Tumor mutational burden
- TME :
-
Tumor microenvironment
- TPI :
-
Tipiracil
- Treg :
-
Regulatory T cells
- VEGF :
-
Vascular endothelial growth factor
- VEGFR :
-
Vascular endothelial growth factor receptor
References
Lugowska I, Teterycz P, Rutkowski P (2018) Immunotherapy of melanoma. Contemp Oncol (Pozn) 22:61–67
Kanwal B, Biswas S, Seminara RS, Jeet C (2018) Immunotherapy in advanced non-small cell lung cancer patients: ushering chemotherapy through the checkpoint inhibitors? Cureus 10:e3254
Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA (2020) Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 383:2207–2218
Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr (2022) PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med 386:2363–2376
Darvin P, Toor SM, Sasidharan Nair V, Elkord E (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50:1–11
Le DT, Kim TW, Van Cutsem E, Geva R, Jager D, Hara H, Burge M, O’Neil B, Kavan P, Yoshino T, Guimbaud R, Taniguchi H, Elez E, Al-Batran SE, Boland PM, Crocenzi T, Atreya CE, Cui Y, Dai T, Marinello P, Diaz LA Jr, Andre T (2020) Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 38:11–19
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, Andre T (2017) Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 18:1182–1191
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895
Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T (1996) Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8:765–772
Pauken KE, Torchia JA, Chaudhri A, Sharpe AH, Freeman GJ (2021) Emerging concepts in PD-1 checkpoint biology. Semin Immunol 52:101480
Zhao Y, Harrison DL, Song Y, Ji J, Huang J, Hui E (2018) Antigen-presenting cell-intrinsic PD-1 neutralizes PD-L1 in cis to attenuate PD-1 signaling in T cells. Cell Rep 24(379–390):e6
Cai J, Wang D, Zhang G, Guo X (2019) The role of PD-1/PD-L1 axis in treg development and function: implications for cancer immunotherapy. Onco Targets Ther 12:8437–8445
Jiang Y, Chen M, Nie H, Yuan Y (2019) PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother 15:1111–1122
Philips GK, Atkins M (2015) Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 27:39–46
O’Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, Ejadi S, Piha-Paul SA, Stein MN, Abdul Razak AR, Dotti K, Santoro A, Cohen RB, Gould M, Saraf S, Stein K, Han SW (2017) Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS ONE 12:e0189848
Ooki A, Shinozaki E, Yamaguchi K (2021) Immunotherapy in colorectal cancer: current and future strategies. J Anus Rectum Colon 5:11–24
Ma SX, Li L, Cai H, Guo TK, Zhang LS (2023) Therapeutic challenge for immunotherapy targeting cold colorectal cancer: a narrative review. World J Clin Oncol 14:81–88
Kalyan A, Kircher S, Shah H, Mulcahy M, Benson A (2018) Updates on immunotherapy for colorectal cancer. J Gastrointest Oncol 9:160–169
Abou-Alfa GK, Lau George, Kudo Masatoshi, Chan Stephen L, Kelley Robin Kate, Furuse Junji, Sukeepaisarnjaroen Wattana, Yoon-Koo Kang Tu, Dao Van, De Toni Enrico N, Rimassa Lorenza, Breder Valeriy, Vasilyev Alexander, Heurgué Alexandra, Tam Vincent C, Mody Kabir, Thungappa Satheesh Chiradoni, Ostapenko Yuriy, Yau Thomas, Azevedo Sergio, Varela María, Cheng Ann-Lii, Qin Shukui, Galle Peter R, Ali Sajid, Marcovitz Michelle, Makowsky Mallory, He Philip, Kurland John F, Negro Alejandra, Sangro Bruno (2022) Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 1:12
Grady WM (2004) Genomic instability and colon cancer. Cancer Metastasis Rev 23:11–27
de la Chapelle A, Hampel H (2010) Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol 28:3380–3387
Li YC, Korol AB, Fahima T, Beiles A, Nevo E (2002) Microsatellites: genomic distribution, putative functions and mutational mechanisms: a review. Mol Ecol 11:2453–2465
Jiricny J (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7:335–346
Losso GM, Moraes Rda S, Gentili AC, Messias-Reason IT (2012) Microsatellite instability–MSI markers (BAT26, BAT25, D2S123, D5S346, D17S250) in rectal cancer. Arq Bras Cir Dig 25:240–244
Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S (1998) A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5:43–51
Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, Sukawa Y, Stewart C, Rosenberg M, Mima K, Inamura K, Nosho K, Nowak JA, Lawrence MS, Giovannucci EL, Chan AT, Ng K, Meyerhardt JA, Van Allen EM, Getz G, Gabriel SB, Lander ES, Wu CJ, Fuchs CS, Ogino S, Garraway LA (2016) Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep 15:857–865
Wu M, Zheng D, Zhang D, Yu P, Peng L, Chen F, Lin Z, Cai Z, Li J, Wei Z, Lin X, Liu J, Liu X (2020) Converting immune cold into hot by biosynthetic functional vesicles to boost systematic antitumor immunity. Science. 23:101341
Loddenkemper C, Nagorsen D, Zeitz M (2008) Foxp3 and microsatellite stability phenotype in colorectal cancer. Gut 57:725–726
Grasso CS, Giannakis M, Wells DK, Hamada T, Mu XJ, Quist M, Nowak JA, Nishihara R, Qian ZR, Inamura K, Morikawa T, Nosho K, Abril-Rodriguez G, Connolly C, Escuin-Ordinas H, Geybels MS, Grady WM, Hsu L, Hu-Lieskovan S, Huyghe JR, Kim YJ, Krystofinski P, Leiserson MDM, Montoya DJ, Nadel BB, Pellegrini M, Pritchard CC, Puig-Saus C, Quist EH, Raphael BJ, Salipante SJ, Shin DS, Shinbrot E, Shirts B, Shukla S, Stanford JL, Sun W, Tsoi J, Upfill-Brown A, Wheeler DA, Wu CJ, Yu M, Zaidi SH, Zaretsky JM, Gabriel SB, Lander ES, Garraway LA, Hudson TJ, Fuchs CS, Ribas A, Ogino S, Peters U (2018) Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov 8:730–749
Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C, Depil S (2019) Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol 10:168
https://www.fda.gov/drugs/resources-information-approved-drugs/.
Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB (2020) Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 12(3):738
Castelli MS, McGonigle P, Hornby PJ (2019) The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacol Res Perspect 7:e00535
Akinleye A, Rasool Z (2019) Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol 12:92
Rajan A, Kim C, Heery CR, Guha U, Gulley JL (2016) Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum Vaccin Immunother 12:2219–2231
Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D (2018) Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 360:k793
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26:2375–2391
Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ (2013) Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 1:32–42
Syed Khaja AS, Toor SM, El Salhat H, Ali BR, Elkord E (2017) Intratumoral FoxP3(+)Helios(+) regulatory T cells upregulating immunosuppressive molecules are expanded in human colorectal cancer. Front Immunol 8:619
Sotomayor EM, Borrello I, Tubb E, Allison JP, Levitsky HI (1999) In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A 96:11476–11481
Alfaro C, Suarez N, Gonzalez A, Solano S, Erro L, Dubrot J, Palazon A, Hervas-Stubbs S, Gurpide A, Lopez-Picazo JM, Grande-Pulido E, Melero I, Perez-Gracia JL (2009) Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer 100:1111–1119
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W (2003) Blockade of B7–H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9:562–567
Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP (2003) VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101:4878–4886
Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M (2015) VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 212:139–148
Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, Yang J, Seestaller-Wehr L, Zhang SY, Hopson C, Tsvetkov L, Jing J, Zhang S, Smothers J, Hoos A (2015) The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res 21:1639–1651
Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, Belvin M, Mellman I (2016) MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44:609–621
Brunner-Weinzierl MC, Hoff H, Burmester GR (2004) Multiple functions for CD28 and cytotoxic T lymphocyte antigen-4 during different phases of T cell responses: implications for arthritis and autoimmune diseases. Arthritis Res Ther 6:45–54
Ferguson SD, Srinivasan VM, Heimberger AB (2015) The role of STAT3 in tumor-mediated immune suppression. J Neurooncol 123:385–394
Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, Dalpke AH, Heeg K (2011) PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol 41:413–424
Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, Arnold JN (2019) Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer. Front Immunol 10:1654
Krombach J, Hennel R, Brix N, Orth M, Schoetz U, Ernst A, Schuster J, Zuchtriegel G, Reichel CA, Bierschenk S, Sperandio M, Vogl T, Unkel S, Belka C, Lauber K (2019) Priming anti-tumor immunity by radiotherapy: dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology 8:e1523097
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ (2006) Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203:1259–1271
Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, Ahmad CE, Goffin JR, Kavan P, Harb M, Colwell B, Samimi S, Samson B, Abbas T, Aucoin N, Aubin F, Koski SL, Wei AC, Magoski NM, Tu D, O’Callaghan CJ (2020) Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the canadian cancer trials group CO.26 study. JAMA Oncol. 6:831–838
Hochster S, Murphy Janet, Leveque Vincent, Cha Edward, Funke Roel, Waterkamp Daniel, Hegde Priti, Bende Johanna (2016) Clinical activity and immune correlates from a phase Ib study evaluating atezolizumab (anti-PDL1) in combination with FOLFOX and bevacizumab (anti-VEGF) in metastatic colorectal carcinoma. Cancer Res 76:2651
Bendell JCPJ, Lieu CH, Eckhardt SG, Hurwitz H, Hochster HS, Murphy JE, Funke RP, Rossi C, Wallin J, Waterkamp D, Pishvaian MJ (2015) Safety and efficacy of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) and/or FOLFOX in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 33:704
Bocobo AG, Wang R, Behr S et al (2021) Phase II study of pembrolizumab plus capecitabine and bevacizumab in microsatellite stable (MSS) metastatic colorectal cancer (mCRC): Interim analysis. J Clin Oncol 39(3):77–77
Antoniotti C, Rossini D, Pietrantonio F, Catteau A, Salvatore L, Lonardi S, Boquet I, Tamberi S, Marmorino F, Moretto R, Ambrosini M, Tamburini E, Tortora G, Passardi A, Bergamo F, Kassambara A, Sbarrato T, Morano F, Ritorto G, Borelli B, Boccaccino A, Conca V, Giordano M, Ugolini C, Fieschi J, Papadopulos A, Massoue C, Aprile G, Antonuzzo L, Gelsomino F, Martinelli E, Pella N, Masi G, Fontanini G, Boni L, Galon J, Cremolini C, Investigators GF (2022) Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 23:876–887
Mettu NB, Ou F-S, Halfdanarson TR, Lenz HJ, Breakstone R, Boland PM, Crysler O, Wu C, Grothey A, Nixon AB, Bolch E, Niedzwiecki D, Fruth B, Schweitzer B, Elsing A, Hurwitz H, Fakih MG, Bekaii-Saab T (2019) BACCI: a phase II randomized, double-blind, multicenter, placebo-controlled study of capecitabine (C) bevacizumab (B) plus atezolizumab (A) or placebo (P) in refractory metastatic colorectal cancer (mCRC)––an ACCRU network study. Ann Oncol 2019(30):203
Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K (2020) Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol 38:2053–2061
Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, Cosgrove D, Fiorillo JA, Tam R, D’Adamo D, Sharma N, Brennan BJ, Wang YA, Coppieters S, Zebger-Gong H, Weispfenning A, Seidel H, Ploeger BA, Mueller U, Oliveira CSV, Paulson AS (2023) Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClin Med 58:101917
Cousin S, Cantarel C, Guegan JP, Gomez-Roca C, Metges JP, Adenis A, Pernot S, Bellera C, Kind M, Auzanneau C, Le Loarer F, Soubeyran I, Bessede A, Italiano A (2021) Regorafenib-avelumab combination in patients with microsatellite stable colorectal cancer (REGOMUNE): A single-arm, open-label. Phase II Trial Clin Cancer Res 27:2139–2147
Wang FHM, Yao Y, Wang Z, Jin Y, Wang FH, Qiu MZ, Lv ZD, Wang DS, Luo HY, Li YH, Zhang DS, Xu R (2020) A phase Ib/II clinical trial of tolerability, safety and efficacy of regorafenib in combination with toripalimab (a PD-1 antibody) in patients with relapsed or metastatic colorectal cancer, 2020. Ann Oncol 31:S425
Gomez-Roca Carlos, Eduardo Y, Im Seock-Ah, Alvarez Eduardo Castanon, Senellart Helene, Doherty Mark, García-Corbacho Javier, Lopez Juanita Suzanne, Basu Bristi, Maurice-Dror Corinne, Gill Sanjeev Singh, Ghori Razi, Kubiak Peter, Jin Fan, Norwood Kevin Glen, Chung Hyun Cheol (2021) LEAP-005: a phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—Results from the colorectal cancer cohort. J Clin Oncol 39:94
Ren C, Mai ZJ, Jin Y, He MM, Wang ZQ, Luo HY, Zhang DS, Wu CY, Wang F, Xu RH (2020) Anti-PD-1 antibody SHR-1210 plus apatinib for metastatic colorectal cancer: a prospective, single-arm, open-label, phase II trial. Am J Cancer Res 10:2946–2954
Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M (2020) Regorafenib and nivolumab or pembrolizumab combination and circulating tumor DNA response assessment in refractory microsatellite stable colorectal cancer. Oncol 25:e1188–e1194
Li J, Cong L, Liu J, Peng L, Wang J, Feng A, Yue J, Li L, Wang X, Wang X (2020) The efficacy and safety of regorafenib in combination with anti-PD-1 antibody in refractory microsatellite stable metastatic colorectal cancer: a retrospective study. Front Oncol 10:594125
Yu W, Tao Q, Zhang Y, Yi F, Feng L (2021) Efficacy and safety of regorafenib combined with toripalimab in the third-line and beyond treatment of advanced colorectal cancer. J Oncol 2021:9959946
Hellmann MD, Kim TW, Lee CB, Goh BC, Miller WH Jr, Oh DY, Jamal R, Chee CE, Chow LQM, Gainor JF, Desai J, Solomon BJ, Das Thakur M, Pitcher B, Foster P, Hernandez G, Wongchenko MJ, Cha E, Bang YJ, Siu LL, Bendell J (2019) Phase Ib study of atezolizumab combined with cobimetinib in patients with solid tumors. Ann Oncol 30:1134–1142
Eng C, Kim TW, Bendell J, Argiles G, Tebbutt NC, Di Bartolomeo M, Falcone A, Fakih M, Kozloff M, Segal NH, Sobrero A, Yan Y, Chang I, Uyei A, Roberts L, Ciardiello F, Investigators IM (2019) Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 20:849–861
Jakubowski CCN, Sugar EA et al (2020) A phase I/II study of PI3Kinase inhibition with copanlisib combined with the anti-PD-1 antibody nivolumab in relapsed/refractory solid tumors with expansions in MSS colorectal cancer. JCO 38:TPS4114
Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, Yuki S, Wakabayashi M, Nomura S, Sato A, Kuwata T, Kawazu M, Mano H, Togashi Y, Nishikawa H, Yoshino T (2020) Multicenter phase I/II trial of napabucasin and pembrolizumab in patients with metastatic colorectal cancer (EPOC1503/SCOOP Trial). Clin Cancer Res 26:5887–5894
Patel MR, Falchook GS, Hamada K, Makris L, Bendell JC (2021) A phase 2 trial of trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable metastatic colorectal cancer. Cancer Med 10:1183–1190
Safi Shahda AMN, Bekaii-Saab Tanios S, O’Neil Bert H, Sehdev Amikar, Shaib Walid Labib, Helft Paul R, Loehrer Patrick J, Tong Yan, Liu Ziyue, El-Rayes Bassel F (2017) A phase II study of pembrolizumab in combination with mFOLFOX6 for patients with advanced colorectal cancer. J Clin Oncol 35:3541
Neil Howard Segal NEK, Cercek Andrea, Reidy Diane Lauren, Raasch Pamela Joan, Warren Peter, Hrabovsky Anastasia E, Campbell Naomi, Shia Jinru, Goodman Karyn A, Erinjeri Joseph Patrick, Solomon Stephen Barnett, Yamada Yoshiya, Saltz Leonard (2016) Non-randomized phase II study to assess the efficacy of pembrolizumab (Pem) plus radiotherapy (RT) or ablation in mismatch repair proficient (pMMR) metastatic colorectal cancer (mCRC) patients. J Clin Oncol 34:3539
Wang C, Park J, Ouyang C, Longmate JA, Tajon M, Chao J, Lim D, Sandhu J, Yin HH, Pillai R, Gozo MC, Avalos C, Egelston CA, Lee PP, Fakih M (2020) A pilot feasibility study of yttrium-90 liver radioembolization followed by durvalumab and tremelimumab in patients with microsatellite stable colorectal cancer liver metastases. Oncologist 25:382-e776
Monjazeb AG-HA, Lako A, Tesfaye AA, Stroiney A, Gentzler RD, Jabbour S, Alese OB, Rahma OE, Cleary JM, Sharon E, Raben D, Mamon HJ, Streicher H, Chen HX, Ahmed M, Gjini E, Rodig S, Hodi FS, Schoenfeld JD (2019) Analysis of colorectal cancer patients treated on ETCTN 10021: A multicenter randomized trial of combined PD-L1 and CTLA-4 inhibition with targeted low-dose or hypofractionated radiation]. J Clin Oncol 37:49
Monjazeb AM, Giobbie-Hurder A, Lako A, Thrash EM, Brennick RC, Kao KZ, Manuszak C, Gentzler RD, Tesfaye A, Jabbour SK, Alese OB, Rahma OE, Cleary JM, Sharon E, Mamon HJ, Cho M, Streicher H, Chen HX, Ahmed MM, Marino-Enriquez A, Kim-Schulze S, Gnjatic S, Maverakis E, Marusina AI, Merleev AA, Severgnini M, Pfaff KL, Lindsay J, Weirather JL, Ranasinghe S, Spektor A, Rodig SJ, Hodi SF, Schoenfeld JD (2021) A randomized trial of combined PD-L1 and CTLA-4 inhibition with targeted low-dose or hypofractionated radiation for patients with metastatic colorectal cancer. Clin Cancer Res 27:2470–2480
Floudas CS, Brar G, Mabry-Hrones D, Duffy AG, Wood B, Levy E, Krishnasamy V, Fioravanti S, Bonilla CM, Walker M, Morelli MP, Kleiner DE, Steinberg SM, Figg WD, Greten TF, Xie C (2019) A pilot study of the PD-1 targeting agent AMP-224 used with low-dose cyclophosphamide and stereotactic body radiation therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer 18:e349–e360
Toor SM, Murshed K, Al-Dhaheri M, Khawar M, Abu Nada M, Elkord E (2019) Immune checkpoints in circulating and tumor-infiltrating CD4(+) T cell subsets in colorectal cancer patients. Front Immunol 10:2936
Jain N, Nguyen H, Chambers C, Kang J (2010) Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A 107:1524–1528
Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A (2012) Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 119:5155–5163
Rochman Y, Yukawa M, Kartashov AV, Barski A (2015) Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig. PLoS ONE 10:e0122198
Keam SJ (2023) Tremelimumab: first approval. Drugs 83:93–102
Seidel JA, Otsuka A, Kabashima K (2018) Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 8:86–86
Fife B, Bluestone J (2008) Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 224:166–182
Huang RY, Francois A, McGray AR, Miliotto A, Odunsi K (2017) Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 6:e1249561
Wei SC, Anang NAS, Sharma R, Andrews MC, Reuben A, Levine JH, Cogdill AP, Mancuso JJ, Wargo JA, Pe’er D, Allison JP (2019) Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A 116:22699–22709
Tay RE, Richardson EK, Toh HC (2021) Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther 28:5–17
Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, Andre T (2018) Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 36:773–779
Lote H, Starling N, Pihlak R, Gerlinger M (2022) Advances in immunotherapy for MMR proficient colorectal cancer. Cancer Treat Rev 111:102480
Jonathan M, Loree JTT, Kennecke Hagen Fritz, Feilotter Harriet, Lee Young S, Virk Shakeel, Kopetz Scott, Duose Dzifa Yawa, Manyam Ganiraju C, Morris Jeffrey S, Maru Dipen M, Renouf Daniel, Jonker Derek J, Dongsheng Tu, O’Callaghan Christopher J, Chen Eric Xueyu (2022) Impact of consensus molecular subtyping (CMS) on survival in the CO.26 trial of durvalumab plus tremelimumab versus best supportive care (BSC) in metastatic colorectal cancer (mCRC). J Clin Oncol 40:3551
Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa EMF, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S (2015) The consensus molecular subtypes of colorectal cancer. Nat Med 21:1350–1356
Falcon BL, O’Clair B, McClure D, Evans GF, Stewart J, Swearingen ML, Chen Y, Allard K, Lee LN, Neote K, McEwen DP, Uhlik MT, Chintharlapalli S (2013) Development and characterization of a high-throughput in vitro cord formation model insensitive to VEGF inhibition. J Hematol Oncol 6:31
Rahma OE, Hodi FS (2019) The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res 25:5449–5457
Lee WS, Yang H, Chon HJ, Kim C (2020) Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 52:1475–1485
Lanitis E, Irving M, Coukos G (2015) Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 33:55–63
Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, Vardam TD, Weis EL, Passanese J, Wang WC, Gollnick SO, Dewhirst MW, Rose-John S, Repasky EA, Baumann H, Evans SS (2011) IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest 121:3846–3859
Barsoum IB, Smallwood CA, Siemens DR, Graham CH (2014) A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 74:665–674
Chen J, Jiang CC, Jin L, Zhang XD (2016) Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 27:409–416
Zhang W, Ding EX, Wang Q, Zhu DQ, He J, Li YL, Wang YH (2005) Fas ligand expression in colon cancer: a possible mechanism of tumor immune privilege. World J Gastroenterol 11:3632–3635
Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G (2011) Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 475:226–230
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70:5728–5739
Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI (1998) Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol 160:1224–1232
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2:1096–1103
Fu C, Jiang A (2018) Dendritic cells and CD8 T cell immunity in tumor microenvironment. Front Immunol 9:3059
Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J (2013) VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 73:539–549
Yang J, Yan J, Liu B (2018) Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol 9:978
Kim CG, Jang M, Kim Y, Leem G, Kim KH, Lee H, Kim TS, Choi SJ, Kim HD, Han JW, Kwon M, Kim JH, Lee AJ, Nam SK, Bae SJ, Lee SB, Shin SJ, Park SH, Ahn JB, Jung I, Lee KY, Park SH, Kim H, Min BS, Shin EC (2019) VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci Immunol. https://doi.org/10.1126/sciimmunol.aay0555
Doleschel D, Hoff S, Koletnik S, Rix A, Zopf D, Kiessling F, Lederle W (2021) Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res 40:288
Fiegle E, Doleschel D, Koletnik S, Rix A, Weiskirchen R, Borkham-Kamphorst E, Kiessling F, Lederle W (2019) Dual CTLA-4 and PD-L1 blockade inhibits tumor growth and liver metastasis in a highly aggressive orthotopic mouse model of colon cancer. Neoplasia 21:932–944
Zhou K, Zhang JW, Wang QZ, Liu WY, Liu JL, Yao L, Cai MM, Ni SY, Cai QY, Wang GJ, Zhou F (2019) Apatinib, a selective VEGFR2 inhibitor, improves the delivery of chemotherapeutic agents to tumors by normalizing tumor vessels in LoVo colon cancer xenograft mice. Acta Pharmacol Sin 40:556–562
Wang Y, Wei B, Gao J, Cai X, Xu L, Zhong H, Wang B, Sun Y, Guo W, Xu Q, Gu Y (2020) Combination of fruquintinib and anti-PD-1 for the treatment of colorectal cancer. J Immunol 205:2905–2915
Cai X, Wei B, Li L, Chen X, Liu W, Cui J, Lin Y, Sun Y, Xu Q, Guo W, Gu Y (2020) Apatinib enhanced anti-PD-1 therapy for colon cancer in mice via promoting PD-L1 expression. Int Immunopharmacol 88:106858
Wang Q, Gao J, Di W, Wu X (2020) Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunol Immunother 69:1781–1799
Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, Matsuoka Y, Ghosh S, Kitano H, Nomoto K, Matsui J, Funahashi Y (2019) Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE 14:e0212513
Mettu NB, Ou FS, Zemla TJ, Halfdanarson TR, Lenz HJ, Breakstone RA, Boland PM, Crysler OV, Wu C, Nixon AB, Bolch E, Niedzwiecki D, Elsing A, Hurwitz HI, Fakih MG, Bekaii-Saab T (2022) Assessment of capecitabine and bevacizumab with or without atezolizumab for the treatment of refractory metastatic colorectal cancer: a randomized clinical trial. JAMA Netw Open 5:e2149040
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, Investigators IM (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894–1905
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M, Group IMS (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 378:2288–2301
Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL, Hawkins R, Ravaud A, Alekseev B, Staehler M, Uemura M, De Giorgi U, Mellado B, Porta C, Melichar B, Gurney H, Bedke J, Choueiri TK, Parnis F, Khaznadar T, Thobhani A, Li S, Piault-Louis E, Frantz G, Huseni M, Schiff C, Green MC, Motzer RJ, Group IMS (2019) Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 393:2404–2415
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 380:1116–1127
Hochster HS, Bendell JC, Cleary JM, Foster P, Zhang W, He X, Hernandez G, Iizuka K, Eckhardt SG (2017) Efficacy and safety of atezolizumab (atezo) and bevacizumab (bev) in a phase Ib study of microsatellite instability (MSI)-high metastatic colorectal cancer (mCRC). J Clin Oncol 35:673
Bocobo AGWR, Behr S et al (2022) Phase II study of pembrolizumab plus capecitabine and bevacizumab in microsatellite stable (MSS) metastatic colorectal cancer (mCRC). JCO 2022:40
Zhou Z, Xie X, Wang X, Zhang X, Li W, Sun T, Cai Y, Wu J, Dang C, Zhang H (2021) Correlations between tumor mutation burden and immunocyte infiltration and their prognostic value in colon cancer. Front Genet 12:623424
Noepel-Duennebacke S, Juette H, Schulmann K, Graeven U, Porschen R, Stoehlmacher J, Hegewisch-Becker S, Raulf A, Arnold D, Reinacher-Schick A, Tannapfel A (2021) J Cancer Res Clin Oncol. 147:3063–3072
Ou DL, Chen CW, Hsu CL, Chung CH, Feng ZR, Lee BS, Cheng AL, Yang MH, Hsu C (2021) Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J Immunother Cancer. 9:e001657
Wang F, He M, Yao Y, Wang Z, Jin YK, Wang F, Qiu M, Lv Z, Wang D, Luo H, Li Y, Zhang D, Xu R (2020) 433P A phase Ib/II clinical trial of tolerability, safety and efficacy of regorafenib in combination with toripalimab (a PD-1 antibody) in patients with relapsed or metastatic colorectal cancer. Ann Oncol 31:S425
Kim RD, Kovari BP, Martinez M, Xie H, Sahin IH, Mehta R, Strosberg J, Imanirad I, Ghayouri M, Kim YC, Kim DW (2022) A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur J Cancer 169:93–102
Carlos Gomez-Roca EY, Im Seock-Ah, Alvarez Eduardo Castanon, Senellart Helene, Doherty Mark, García-Corbacho Javier, Lopez Juanita Suzanne, Basu Bristi, Maurice-Dror Corinne, Gill Sanjeev Singh, Ghori Razi, Kubiak Peter, Jin Fan, Norwood Kevin Glen, Chung Hyun Cheol (2021) LEAP-005: a phase II multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—results from the colorectal cancer cohort. J Clin Oncol 39:94
Xiao LZY, Lin Q (2020) 442P Camrelizumab combined with apatinib in the treatment of patients with advanced gastric cancer and colorectal cancer: one-arm exploratory clinical trial. Ann Oncol 2020:31
Wortzel I, Seger R (2011) The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer 2:195–209
Dhillon AS, Hagan S, Rath O, Kolch W (2007) MAP kinase signalling pathways in cancer. Oncogene 26:3279–3290
Fremin C, Meloche S (2010) From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol 3:8
Allegrezza MJ, Rutkowski MR, Stephen TL, Svoronos N, Perales-Puchalt A, Nguyen JM, Payne KK, Singhal S, Eruslanov EB, Tchou J, Conejo-Garcia JR (2016) Trametinib drives T-cell-dependent control of KRAS-mutated tumors by inhibiting pathological myelopoiesis. Cancer Res 76:6253–6265
Baumann D, Hagele T, Mochayedi J, Drebant J, Vent C, Blobner S, Noll JH, Nickel I, Schumacher C, Boos SL, Daniel AS, Wendler S, Volkmar M, Strobel O, Offringa R (2020) Proimmunogenic impact of MEK inhibition synergizes with agonist anti-CD40 immunostimulatory antibodies in tumor therapy. Nat Commun 11:2176
Yarchoan M, Mohan AA, Dennison L, Vithayathil T, Ruggieri A, Lesinski GB, Armstrong TD, Azad NS, Jaffee EM (2019) MEK inhibition suppresses B regulatory cells and augments anti-tumor immunity. PLoS ONE 14:e0224600
Bendell JLC, Raghav KPS et al (2019) A phase Ib study of the safety and efficacy of atezolizumab (atezo) + bevacizumab (bev) + cobimetinib (cobi) in patients (pts) with metastatic colorectal cancer (mCRC). Ann Oncol 30:v227–v228
Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H (2008) Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest 118:3367–3377
Wang Y, Shen Y, Wang S, Shen Q, Zhou X (2018) The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett 415:117–128
Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, Ott M, Ochs K, Lutz C, Liu X, Anastasov N, Lehmann I, Hofer T, von Deimling A, Wick W, Platten M (2014) Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 5:1038–1051
Melillo JA, Song L, Bhagat G, Blazquez AB, Plumlee CR, Lee C, Berin C, Reizis B, Schindler C (2010) Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J Immunol 184:2638–2645
Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, Tveit KM, Gibson F (2015) A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer 14:1–10
Munker S, Gerken M, Fest P, Ott C, Schnoy E, Fichtner-Feigl S, Wiggermann P, Vogelhuber M, Herr W, Stroszczynski C, Schlitt HJ, Evert M, Reng M, Klinkhammer-Schalke M, Teufel A (2018) Chemotherapy for metastatic colon cancer: no effect on survival when the dose is reduced due to side effects. BMC Cancer 18:455
Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y, Sobrero A, Boucher E, Peeters M, Tran B, Lenz HJ, Zaniboni A, Hochster H, Cleary JM, Prenen H, Benedetti F, Mizuguchi H, Makris L, Ito M, Ohtsu A (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 372:1909–19
Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, Bordonaro R, Latiano TP, Tamburini E, Santini D, Passardi A, Marmorino F, Grande R, Aprile G, Zaniboni A, Murgioni S, Granetto C, Buonadonna A, Moretto R, Corallo S, Cordio S, Antonuzzo L, Tomasello G, Masi G, Ronzoni M, Di Donato S, Carlomagno C, Clavarezza M, Ritorto G, Mambrini A, Roselli M, Cupini S, Mammoliti S, Fenocchio E, Corgna E, Zagonel V, Fontanini G, Ugolini C, Boni L, Falcone A, Investigators GF (2020) Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 21:497–507
Dosset M, Vargas TR, Lagrange A, Boidot R, Vegran F, Roussey A, Chalmin F, Dondaine L, Paul C, Lauret Marie-Joseph E, Martin F, Ryffel B, Borg C, Adotevi O, Ghiringhelli F, Apetoh L (2018) PD-1/PD-L1 pathway: an adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. Oncoimmunology 7:e1433981
Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F (2010) 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70:3052–3061
Wu Y, Deng Z, Wang H, Ma W, Zhou C, Zhang S (2016) Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol 17:29
Duffy AG, Greten TF (2014) Immunological off-target effects of standard treatments in gastrointestinal cancers. Ann Oncol 25:24–32
Liu WM, Fowler DW, Smith P, Dalgleish AG (2010) Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer 102:115–123
Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G (2010) Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29:482–491
Melichar B, Touskova M, Vesely P (2002) Effect of irinotecan on the phenotype of peripheral blood leukocyte populations in patients with metastatic colorectal cancer. Hepatogastroenterology 49:967–970
Kim HS, Park HM, Park JS, Sohn HJ, Kim SG, Kim HJ, Oh ST, Kim TG (2010) Dendritic cell vaccine in addition to FOLFIRI regimen improve antitumor effects through the inhibition of immunosuppressive cells in murine colorectal cancer model. Vaccine 28:7787–7796
Suzuki N, Tsukihara H, Nakagawa F, Kobunai T, Takechi T (2017) Synergistic anticancer activity of a novel oral chemotherapeutic agent containing trifluridine and tipiracil in combination with anti-PD-1 blockade in microsatellite stable-type murine colorectal cancer cells. Am J Cancer Res 7:2032–2040
Lim JY, Gerber SA, Murphy SP, Lord EM (2014) Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol Immunother 63:259–271
Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC (2004) Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 58:862–870
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX (2014) Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–695
Funding
The study was supported by grants from the Canadian Institute of Health Research and the Natural Science and Engineering Research of Canada.
Author information
Authors and Affiliations
Contributions
KXL, AI, and XZ involved in manuscript writing and revision; DQ, AS, and ET involved in scientific discussion and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, K., Istl, A.C., Quan, D. et al. PD-1 and PD-L1 inhibitors in cold colorectal cancer: challenges and strategies. Cancer Immunol Immunother 72, 3875–3893 (2023). https://doi.org/10.1007/s00262-023-03520-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03520-5