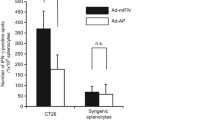
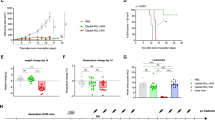
Abstract
The need for an intact immune system for cancer radiation therapy to be effective suggests that radiation not only acts directly on the tumor but also indirectly, through the activation of host immune components. Recent studies demonstrated that endogenous type I interferons (type I IFNs) play a role in radiation-mediated anti-tumor immunity by enhancing the ability of dendritic cells to cross-prime CD8+ T cells. However, it is still unclear to what extent endogenous type I IFNs contribute to the recruitment and function of CD8+ T cells. Little is also known about the effects of type I IFNs on myeloid cells. In the current study, we demonstrate that type I and type II IFNs (IFN-γ) are both required for the increased production of CXCL10 (IP-10) chemokine by myeloid cells within the tumor after radiation treatment. Radiation-induced intratumoral IP-10 levels in turn correlate with tumor-infiltrating CD8+ T cell numbers. Moreover, type I IFNs promote potent tumor-reactive CD8+ T cells by directly affecting the phenotype, effector molecule production, and enhancing cytolytic activity. Using a unique inducible expression system to increase local levels of IFN-α exogenously, we show here that the capacity of radiation therapy to result in tumor control can be enhanced. Our preclinical approach to study the effects of local increase in IFN-α levels can be used to further optimize the combination therapy strategy in terms of dosing and scheduling, which may lead to better clinical outcome.






Similar content being viewed by others
Abbreviations
- IFN:
-
Interferon
- MFI:
-
Mean fluorescence intensity
- RT:
-
Radiation therapy
- SBRT:
-
Stereotactic body radiation therapy
- tdLN:
-
Tumor-draining lymph nodes
- TILs:
-
Tumor-infiltrating lymphocytes
- Veh:
-
Vehicle control
References
Connell PP, Hellman S (2009) Advances in radiotherapy and implications for the next century: a historical perspective. Cancer Res 69:383–392. doi:10.1158/0008-5472.CAN-07-6871
Hoopes DJ, Tann M, Fletcher JW, Forquer JA, Lin PF, Lo SS, Timmerman RD, McGarry RC (2007) FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 56:229–234. doi:10.1016/j.lungcan.2006.12.009
Nedzi LA (2008) The implementation of ablative hypofractionated radiotherapy for stereotactic treatments in the brain and body: observations on efficacy and toxicity in clinical practice. Semin Radiat Oncol 18:265–272. doi:10.1016/j.semradonc.2008.04.009
Ritter M (2008) Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin Radiat Oncol 18:249–256. doi:10.1016/j.semradonc.2008.04.007
Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, Zatcky J, Zelefsky MJ, Fuks Z (2008) High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 71:484–490. doi:10.1016/j.ijrobp.2007.11.046
Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM (2005) Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 174:7516–7523
Lee Y, Auh SL, Wang Y et al (2009) Therapeutic effects of ablative radiation on local tumor require CD8 + T cells: changing strategies for cancer treatment. Blood 114:589–595. doi:10.1182/blood-2009-02-206870
Shiao SL, Coussens LM (2010) The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia 15:411–421. doi:10.1007/s10911-010-9194-9
Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL (2011) The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res 71:2488–2496. doi:10.1158/0008-5472.CAN-10-2820
Ferrantini M, Capone I, Belardelli F (2007) Interferon-alpha and cancer: mechanisms of action and new perspectives of clinical use. Biochimie 89:884–893. doi:10.1016/j.biochi.2007.04.006
Gresser I, Bourali C, Levy JP, Fontaine-Brouty-Boye D, Thomas MT (1969) Increased survival in mice inoculated with tumor cells and treated with interferon preparations. Proc Natl Acad Sci USA 63:51–57
Picaud S, Bardot B, De Maeyer E, Seif I (2002) Enhanced tumor development in mice lacking a functional type I interferon receptor. J Interferon Cytokine Res 22(4):457–462. doi:10.1089/10799900252952244
Belardelli F, Ferrantini M, Proietti E, Kirkwood JM (2002) Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev 13:119–134
Gresser I, Belardelli F (2002) Endogenous type I interferons as a defense against tumors. Cytokine Growth Factor Rev 13:111–118
Curtsinger JM, Mescher MF (2010) Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22:333–340. doi:10.1016/j.coi.2010.02.013
Sorensen EW, Gerber SA, Frelinger JG, Lord EM (2010) IL-12 suppresses vascular endothelial growth factor receptor 3 expression on tumor vessels by two distinct IFN-gamma-dependent mechanisms. J Immunol 184:1858–1866. doi:10.4049/jimmunol.0903210
Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM (2008) Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 180:3132–3139
Gerber SA, Pober JS (2008) IFN-alpha induces transcription of hypoxia-inducible factor-1alpha to inhibit proliferation of human endothelial cells. J Immunol 181:1052–1062
Vogel R, Mammeri H, Mallet J (2008) Lentiviral vectors mediate nonimmunosuppressive rapamycin analog-induced production of secreted therapeutic factors in the brain: regulation at the level of transcription and exocytosis. Hum Gene Ther 19:167–178. doi:10.1089/hum.2007.125
Gerber SA, Sorensen EW, Sedlacek AL, Lim JY, Skrombolas D, Frelinger JG, Lord EM (2013) Local expression of interleukin-2 by B16 melanoma cells results in decreased tumour growth and long-term tumour dormancy. Immunology 138:280–292. doi:10.1111/imm.12037
Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM (2013) IFN-gamma mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. doi:10.1016/j.ajpath.2013.02.041
Brassard DL, Grace MJ, Bordens RW (2002) Interferon-alpha as an immunotherapeutic protein. J Leukoc Biol 71:565–581
Taylor JL, Grossberg SE (1998) The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol 25:23–29
Hong M, Puaux AL, Huang C et al (2011) Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res 71:6997–7009. doi:10.1158/0008-5472.CAN-11-1466
Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF (2011) Host type I IFN signals are required for antitumor CD8 + T cell responses through CD8{alpha} + dendritic cells. J Exp Med 208:2005–2016. doi:10.1084/jem.20101159
Kline J, Zhang L, Battaglia L, Cohen KS, Gajewski TF (2012) Cellular and molecular requirements for rejection of B16 melanoma in the setting of regulatory T cell depletion and homeostatic proliferation. J Immunol 188:2630–2642. doi:10.4049/jimmunol.1100845
Klemm JD, Schreiber SL, Crabtree GR (1998) Dimerization as a regulatory mechanism in signal transduction. Annu Rev Immunol 16:569–592. doi:10.1146/annurev.immunol.16.1.569
Pollock R, Clackson T (2002) Dimerizer-regulated gene expression. Curr Opin Biotechnol 13:459–467
Voloshin T, Voest EE, Shaked Y (2013) The host immunological response to cancer therapy: an emerging concept in tumor biology. Exp Cell Res. doi:10.1016/j.yexcr.2013.03.007
Kondo T, Kobayashi J, Saitoh T, Maruyama K, Ishii KJ, Barber GN, Komatsu K, Akira S, Kawai T (2013) DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci USA 110:2969–2974. doi:10.1073/pnas.1222694110
Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M (1994) Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921
Wong LH, Hatzinisiriou I, Devenish RJ, Ralph SJ (1998) IFN-gamma priming up-regulates IFN-stimulated gene factor 3 (ISGF3) components, augmenting responsiveness of IFN-resistant melanoma cells to type I IFNs. J Immunol 160:5475–5484
Taniguchi T, Takaoka A (2001) A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol 2:378–386. doi:10.1038/35073080
Rayamajhi M, Humann J, Kearney S, Hill KK, Lenz LL (2010) Antagonistic crosstalk between type I and II interferons and increased host susceptibility to bacterial infections. Virulence 1:418–422. doi:10.4161/viru.1.5.12787
Acknowledgments
The authors thank Dr. John G. Frelinger for suggestions and thoughtful discussions pertinent to this study. This project was financially supported by National Institutes of Health, Grant CA 28332.
Conflict of interest
The authors disclose no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, J.Y.H., Gerber, S.A., Murphy, S.P. et al. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8+ T cells. Cancer Immunol Immunother 63, 259–271 (2014). https://doi.org/10.1007/s00262-013-1506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1506-7