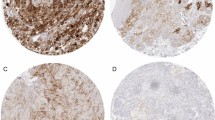
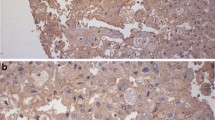
Abstract
Recently, the effectiveness of anti-programmed death 1 (PD-1) antibody therapy in the treatment of renal cell carcinoma (RCC) has been established. Nevertheless, efficacy has been reported to be limited to only 10–30% of patients. To develop more effective immunotherapy for RCC, we analyzed the immunological characteristics in RCC tissues by immunohistochemistry (IHC). We prepared a tissue microarray that consisted of tumor tissue sections (1 mm in diameter) from 83 RCC patients in Kanagawa Cancer Center between 2006 and 2015. IHC analysis was performed with antibodies specific to immune-related (CD8 and Foxp3) and immune checkpoint (programmed death ligand 1 (PD-L1) and 2 (PD-L2), B7-H4 and galectin-9) molecules. The numbers and proportions of positively stained tumor cells or immune cells were determined in each section. From multivariate analysis of all 83 patients, higher galectin-9 expression was detected as a factor associated with worse overall survival (OS) (P = 0.029) and that higher stage and higher B7-H4 expression were associated with worse progression-free survival (PFS) (P < 0.001 and P = 0.021, respectively). Similarly, in multivariate analysis of 69 patients with clear cell RCC, though not statistically significant, there was a trend for association between higher galectin-9 expression and worse OS (P = 0.067), while higher stage was associated with worse PFS (P < 0.001). This study suggests that higher galectin-9 expression is an independent adverse prognostic factor of OS in RCC patients. Therefore, to develop more effective personalized immunotherapy to treat RCC, it may be important to target not only PD-1/PD-L1, but also other immune checkpoint molecules such as galectin-9.



Similar content being viewed by others
Abbreviations
- ccRCC:
-
Clear cell renal cell carcinoma
- CSS:
-
Cancer-specific survival
- ICI:
-
Immune checkpoint inhibitor
- IHC:
-
Immunohistochemistry
- OS:
-
Overall survival
- PD-1:
-
Programmed death 1
- PD-L1:
-
Programmed death ligand 1
- PD-L2:
-
Programmed death ligand 2
- PFS:
-
Progression-free survival
- RCC:
-
Renal cell carcinoma
- RFS:
-
Recurrence-free survival
- TIM-3:
-
T cell immunoglobulin and mucin domain-containing protein 3
- TMA:
-
Tissue microarray
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. https://doi.org/10.1056/NEJMoa1200690
Motzer RJ, Rini BI, McDermott DF et al (2015) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 33:1430–1437. https://doi.org/10.1200/JCO.2014.59.0703
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813. https://doi.org/10.1056/NEJMoa1510665
Weber JS, D'Angelo SP, Minor D et al (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375–384. https://doi.org/10.1016/S1470-2045(15)70076-8
Daud AI, Wolchok JD, Robert C et al (2016) Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 34:4102–4109. https://doi.org/10.1200/JCO.2016.67.2477
Gandhi L, Rodriguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G (2017) The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14:717–734. https://doi.org/10.1038/nrclinonc.2017.101
Giraldo NA, Becht E, Vano Y et al (2017) Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin Cancer Res 23:4416–4428. https://doi.org/10.1158/1078-0432.CCR-16-2848
Tanaka A, Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell Res 27:109–118. https://doi.org/10.1038/cr.2016.151
Polimeno M, Napolitano M, Costantini S et al (2013) Regulatory T cells, interleukin (IL)-6, IL-8, vascular endothelial growth factor (VEGF), CXCL10, CXCL11, epidermal growth factor (EGF) and hepatocyte growth factor (HGF) as surrogate markers of host immunity in patients with renal cell carcinoma. BJU Int 112:686–696. https://doi.org/10.1111/bju.12068
Leite KR, Reis ST, Junior JP, Zerati M, Gomes Dde O, Camara-Lopes LH, Srougi M (2015) PD-L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn Pathol 10:189. https://doi.org/10.1186/s13000-015-0414-x
Shin SJ, Jeon YK, Kim PJ, Cho YM, Koh J, Chung DH, Go H (2016) Clinicopathologic analysis of PD-L1 and PD-L2 expression in renal cell carcinoma: association with oncogenic proteins status. Ann Surg Oncol 23:694–702. https://doi.org/10.1245/s10434-015-4903-7
Iacovelli R, Nole F, Verri E et al (2016) Prognostic role of PD-L1 expression in renal cell carcinoma. a systematic review and meta-analysis. Target Oncol 11:143–148. https://doi.org/10.1007/s11523-015-0392-7
Kim SH, Park WS, Park EY, Park B, Joo J, Joung JY, Seo HK, Lee KH, Chung J (2017) The prognostic value of BAP1, PBRM1, pS6, PTEN, TGase2, PD-L1, CA9, PSMA, and Ki-67 tissue markers in localized renal cell carcinoma: a retrospective study of tissue microarrays using immunohistochemistry. PLoS ONE 12:e0179610. https://doi.org/10.1371/journal.pone.0179610
Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, Kim NW (2006) B7-H4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res 66:1570–1575. https://doi.org/10.1158/0008-5472.CAN-04-3550
Krambeck AE, Thompson RH, Dong H et al (2006) B7–H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA 103:10391–10396. https://doi.org/10.1073/pnas.0600937103
Shen L, Qian Y, Wu W et al (2017) B7-H4 is a prognostic biomarker for poor survival in patients with pancreatic cancer. Hum Pathol 66:79–85. https://doi.org/10.1016/j.humpath.2017.05.023
Thijssen VL, Heusschen R, Caers J, Griffioen AW (2015) Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta 1855:235–247. https://doi.org/10.1016/j.bbcan.2015.03.003
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393:1819–1830. https://doi.org/10.1016/S0140-6736(18)32409-7
Yeong J, Zhao Z, Lim JCT et al (2020) PD-L1 expression is an unfavourable prognostic indicator in Asian renal cell carcinomas. J Clin Pathol. https://doi.org/10.1136/jclinpath-2019-206092
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transpl 48:452–458. https://doi.org/10.1038/bmt.2012.244
Kageshita T, Kashio Y, Yamauchi A et al (2002) Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int J Cancer 99:809–816. https://doi.org/10.1002/ijc.10436
Irie A, Yamauchi A, Kontani K et al (2005) Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res 11:2962–2968. https://doi.org/10.1158/1078-0432.CCR-04-0861
Liang M, Ueno M, Oomizu S, Arikawa T, Shinonaga R, Zhang S, Yamauchi A, Hirashima M (2008) Galectin-9 expression links to malignant potential of cervical squamous cell carcinoma. J Cancer Res Clin Oncol 134:899–907. https://doi.org/10.1007/s00432-008-0352-z
Zhang ZY, Dong JH, Chen YW, Wang XQ, Li CH, Wang J, Wang GQ, Li HL, Wang XD (2012) Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev 13:2503–2509. https://doi.org/10.7314/apjcp.2012.13.6.2503
Fu H, Liu Y, Xu L, Liu W, Fu Q, Liu H, Zhang W, Xu J (2015) Galectin-9 predicts postoperative recurrence and survival of patients with clear-cell renal cell carcinoma. Tumour Biol 36:5791–5799. https://doi.org/10.1007/s13277-015-3248-y
Qi Y, Chang Y, Wang Z et al (2019) Tumor-associated macrophages expressing galectin-9 identify immunoevasive subtype muscle-invasive bladder cancer with poor prognosis but favorable adjuvant chemotherapeutic response. Cancer Immunol Immunother 68:2067–2080. https://doi.org/10.1007/s00262-019-02429-2
Li H, Wu K, Tao K et al (2012) Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 56:1342–1351. https://doi.org/10.1002/hep.25777
Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6:1245–1252. https://doi.org/10.1038/ni1271
Granier C, Dariane C, Combe P et al (2017) Tim-3 expression on tumor-infiltrating PD-1(+)CD8(+) T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res 77:1075–1082. https://doi.org/10.1158/0008-5472.CAN-16-0274
Podojil JR, Miller SD (2017) Potential targeting of B7-H4 for the treatment of cancer. Immunol Rev 276:40–51. https://doi.org/10.1111/imr.12530
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887–3895
Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. https://doi.org/10.1038/nature04444
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8:467–477. https://doi.org/10.1038/nri2326
Ohigashi Y, Sho M, Yamada Y et al (2005) Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 11:2947–2953. https://doi.org/10.1158/1078-0432.CCR-04-1469
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N (2006) Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 108:19–24. https://doi.org/10.1016/j.acthis.2006.01.003
Hamanishi J, Mandai M, Iwasaki M et al (2007) Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 104:3360–3365. https://doi.org/10.1073/pnas.0611533104
Droeser RA, Hirt C, Viehl CT et al (2013) Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 49:2233–2242. https://doi.org/10.1016/j.ejca.2013.02.015
Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL (2014) In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 20:2773–2782. https://doi.org/10.1158/1078-0432.CCR-13-2702
Lipson EJ, Vincent JG, Loyo M et al (2013) PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res 1:54–63. https://doi.org/10.1158/2326-6066.CIR-13-0034
Topalian SL, Taube JM, Anders RA, Pardoll DM (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16:275–287. https://doi.org/10.1038/nrc.2016.36
Bu M, Shen Y, Seeger WL, An S, Qi R, Sanderson JA, Cai Y (2016) Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8(+) T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumour Biol 37:3949–3956. https://doi.org/10.1007/s13277-015-4237-x
Liu Z, McMichael EL, Shayan G, Li J, Chen K, Srivastava R, Kane LP, Lu B, Ferris RL (2018) Novel effector phenotype of Tim-3(+) regulatory T cells leads to enhanced suppressive function in head and neck cancer patients. Clin Cancer Res 24:4529–4538. https://doi.org/10.1158/1078-0432.CCR-17-1350
Liu JF, Wu L, Yang LL, Deng WW, Mao L, Wu H, Zhang WF, Sun ZJ (2018) Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J Exp Clin Cancer Res 37:44. https://doi.org/10.1186/s13046-018-0713-7
Acknowledgements
This study was supported by Kanagawa Cancer Center Hospital-Research Institute Joint Study.
Author information
Authors and Affiliations
Contributions
RJ, TK, and TS were involved in conceptualization; RJ, TK, TY, MY, AH, TT, NM, KM, SU, MK, MY, YN, YM, and TS contributed to data acquisition and methodology; RJ, TK, MS, TY, and TS contributed to formal analysis and investigation; RJ contributed to writing—original draft preparation; TK, MS, and TS contributed to writing—review and editing; RJ and TS contributed to funding acquisition; and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
262_2020_2608_MOESM1_ESM.pdf
Figure 1. Kaplan–Meier analysis of OS in the subgroups of RCC patients separated by stage or grade. Kaplan–Meier analysis of OS was performed according to galectin-9 expression in the subgroups of RCC patients (n=83) separated by stage or grade. The patients were separated by the stage to (a) lower stage subgroup consisting of stage I and II and (b) higher stage subgroup consisting of stage III and IV. They were also separated by the grade to (c) lower grade subgroup consisting of grade 1 and 2 and (d) higher grade subgroup consisting of grade 3. The patients were divided into “high” and “low” groups in each subgroup by setting the median value of galectin-9 expression as the cutoff. Log-rank p value was shown. (PDF 239 kb)
262_2020_2608_MOESM2_ESM.pdf
Figure 2. Kaplan–Meier analysis of OS in the subgroups of clear cell RCC patients separated by stage or grade. Kaplan–Meier analysis of OS was performed according to galectin-9 expression in the subgroups of clear cell RCC patients (n=69) separated by stage or grade. The patients were separated by the stage to (a) lower stage subgroup consisting of stage I and II and (b) higher stage subgroup consisting of stage III and IV. They were also separated by the grade to (c) lower grade subgroup consisting of grade 1 and 2 and (d) higher grade subgroup consisting of grade 3. The patients were divided into “high” and “low” groups in each subgroup by setting the median value of galectin-9 expression as the cutoff. Log-rank p value was shown. (PDF 237 kb)
Rights and permissions
About this article
Cite this article
Jikuya, R., Kishida, T., Sakaguchi, M. et al. Galectin-9 expression as a poor prognostic factor in patients with renal cell carcinoma. Cancer Immunol Immunother 69, 2041–2051 (2020). https://doi.org/10.1007/s00262-020-02608-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02608-6