Abstract
Background
T-cell immunoglobulin mucin 3 (TIM3) is a negative immune checkpoint and plays a crucial part in tumor-induced immune suppression. However, the mechanism of TIM3 in regulating immunosuppression in head and neck squamous cell carcinoma (HNSCC) was still not quite clear.
Methods
We carried out the immunohistochemistry staining of HNSCC tissue microarrays. Through quantification of the histoscore, we performed the correlation analysis among the TIM3, Galectin-9, Foxp3, CD68 and CD163. The effects of TIM3 on regulatory T cells (Tregs) and macrophages were detected by utilizing the Tgfbr1/Pten 2cKO HNSCC mouse model. Flow cytometry were used to analysis the percent of Tregs, macrophages and IFN-γ.
Results
We demonstrated the close association among TIM3/Galectin-9 pathway, regulatory T cell marker (Foxp3) and macrophage marker (CD68, CD163) in human HNSCC. In the transgenic HNSCC mouse model, blockade of TIM3 by the anti-TIM3 monoclonal antibody induced a reduction of CD4+CD25+Foxp3+ Tregs. Meanwhile, the population of TIM3+ Tregs was also decreased. However, the population of CD206+ macrophages was not significantly declined. The increased IFN-γ production on CD8+ T cells in anti-TIM3 treatment mice showed that the antitumor immune response was enhanced through suppression of these negative immune factors.
Conclusions
The present study demonstrated that TIM3 was associated with the immunosuppression in HNSCC. And targeting TIM3 can enhance anti-tumor immune response by decreasing Tregs in HNSCC.
Similar content being viewed by others
Background
Head and neck squamous cell carcinoma (HNSCC) is one of the most common malignancies around the world [1]. It mainly takes place in the oral cavity, mouth pharynx, larynx and laryngopharynx, and is characterized by local invasion and metastasis [2]. Tobacco and alcohol consumption are considered as the major cause of HNSCC, and HPV infection has emerged as another important risk factor in recent years [3]. Although treatments have improved and the use of targeted medicines (such as cetuximab) combined with radiotherapy have improved the living quality and prognosis of patients, the 5 year overall survival rate is still approximately 50% [4].
Recent studies have indicated that the development of HNSCC is closely related to immunosuppression and immune escape. The aberrant activities of T lymphocytes, B lymphocytes, dendritic cells (DC), macrophages, NK cells and various cytokines are involved in the initiation, promotion and progress of HNSCC [5]. Regulatory T cell (Treg) is a subset of CD4+ T cells and serves as an inhibitor of the antitumor immune response [6]. Tregs can inhibit immune effector cells by releasing suppressive cytokines [7]. The transcription factor Foxp3 is a necessary marker for activity of Treg cells [8]. Recent evidence has demonstrated that Treg activity is increased in HNSCC patients, but the prognostic value of Treg in HNSCC is still controversial [9].
In addition to Tregs, macrophages also take a part in tumor initiation and promotion. Macrophages have been sorted into two main subgroups: classically activated macrophages (M1) and alternatively activated macrophages (M2) [10]. M1 macrophages have anti-tumor effects on tumorigenesis, while M2 macrophages promote tumor development by inhibiting tumor-specific immune response [11]. M2 macrophages in solid tumors are comparatively more than M1 macrophages [12]. CD206 is one of the surface molecules specific to M2 macrophages. In addition, Zhu et al. have suggested that CD206 expression was related to the prognosis of hepatocellular carcinomas [13].
T-cell immunoglobulin mucin 3 (TIM3), an important immune checkpoint protein, was initially shown to be expressed on CD4+ Th1 cells and CD8+ T cells [14]. Then, studies successively demonstrated TIM3 expression on macrophages, monocytes and CD11b+ DCs [14,15,16]. Although several molecules have been reported bind to TIM3 [16,17,18], Galectin-9 is considered as the major ligand [19]. By binding to Galectin-9, TIM3 induces T lymphocytes exhaustion or apoptosis [20]. While blockade of TIM3 could promote IFN-γ-mediated antitumor immunity of T cells [21]. A research showed that TIM3 was expressed on Tregs and correlated with rheumatoid arthritis activity [22]. An in vitro experiment suggested that TIM3 on Tregs was correlated with tumor size of ovarian carcinoma [23]. Moreover, TIM3 could be upregulated by stimuli and may be associated with macrophage activity [24]. Our previous study has demonstrated TIM3 is overexpressed in HNSCC and is associated with myeloid-derived suppressor cells MDSCs [25]. However, the role of TIM3 in modulating Tregs and macrophages in HNSCC is still unknown.
We have previously demonstrated the function of TIM3 in regulating effector T cells in HNSCC [25]. In this study, we explored the role of TIM3 in regulating Tregs and macrophages in HNSCC. HNSCC tissue arrays were used to analyze the association among the TIM3/Galectin-9 signal, the Treg marker (Foxp3) and macrophage markers (CD68, CD163). By utilizing an HNSCC mouse model, we explored TIM3 function in regulating Tregs and M2 macrophages.
Methods
Patient samples and HNSCC tissue microarray
The Medical Ethics Committee of the School and Hospital of Stomatology Wuhan University approved this study. Human HNSCC tissue samples were acquired from the Hospital of Stomatology Wuhan University. All the patients accepted the informed consent before the surgery. The HNSCC samples, including 27 normal mucosa, 122 primary HNSCC, were used to construct tissue microarrays and applied to immunohistochemistry staining.
Immunohistochemistry
The immunohistochemistry staining of sections were performed according to the procedure as previously described [25]. The following antibodies were used: TIM3, Galectin9, Foxp3 (Cell Signaling Technology, USA), CD68 (Zymed, China), or CD163 (CW Biotech, China).
Animals
The animal experiments were performed under the guidelines of the Institutional Animal Care and Use Committee of Wuhan University. The spontaneous HNSCC mouse model is a transgenic mouse with a combined Tgfbr1/Pten knockout (K14-CreERtam+/−;Tgfbr1flox/flox; Ptenflox/flox) and with the background of CD1/129/FVBN/ C57/BL/6. Transforming Growth Factor-β (TGF-β) and components of the PTEN/PI3K/Akt signal pathways are the most common mutation molecules associated with HNSCC progress. Tgfbr1 and Pten knockout by tamoxifen induction in head and neck epithelium of mice could result in the occurrence of squamous cell carcinoma with full penetrance. This Tgfbr1/Pten 2cKO mouse model is immunocompetent, and it is suitable for cancer immunotherapy research. After five sequential days of tamoxifen treatments by oral gavage, Tgfbr1/Pten were knocked out in epithelium of oral cavity and head-neck region. The course of tamoxifen usage was illustrated as before [26]. During the induction process, squamous cell carcinoma occurred in head-neck region of the mice. This mouse model was maintained and genotyped as the previous description [26].
Mice treatment
After tamoxifen induction for 5 days, the mice were divided into control group (n = 6) and anti-TIM3 group (n = 6) at random. Rat isotype IgG2a was applied to the control group. The prophylactic administration of isotype IgG2a (clone 2A3) or anti-TIM3 (RMT3–23) (BioXCell, West Lebanon, NH) in mice was carried out by intraperitoneal injections (100 μg i.p.) for 3 days since day 12 and then once a week for the following weeks. The tumor size of mice was measured every five days. Finally, the mice were executed by euthanasia.
Flow cytometry
The single cell suspensions were obtained from the draining lymph node and the spleens and then stained with antibodies. The following antibodies were used: PE/Cy7-conjugated CD8, FITC-conjugated CD11b, CD4, PE-conjugated CD25, F4/80 and Foxp3 (eBioscience, San Diego, CA). APC-conjugated TIM3 and TIGIT, PE-conjugated CD206, BV-421 conjugated PD1 and LAG3, and PE/Cy7-conjugated CTLA4 (BioLegend, San Diego, CA). 7AAD (Invitrogen) was applied to exclude dead cells. The CytExpert software (Beckman Coulter, CA, USA) was used for flow cytometry analysis.
Scoring system
Aperio Scan Scope CS scanner (Vista, CA, USA) was used to scan the HNSCC tissue microarray. The interested area of the section was chosen for quantification. The score of the IHC staining was quantified with background subtraction using Aperio Quantification software (Version 9.1). The histoscore of the nucleus and membrane staining were worked out using the following formula for the percentage of cells with different positive degrees: (3+) × 3 + (2+) × 2 + (1+) × 1 [27]. Histoscores were translated to numerical scores from − 3 to 3 using Microsoft Excel. Cluster 3.0 was used to perform the hierarchical analysis. Finally, the cluster picture was completed with Java TreeView 1.0.5.
Statistical analysis
Statistical data analysis was performed by utilizing the GraphPad Prism 6 software (La Jolla, CA) and was shown as the mean values ± SEM. The Mann-Whitney test was used to analyze the differences between two different groups. And the value of Cohen’s d of each data have been calculated to confirm the significant difference between two different groups. The two-tailed Pearson correlation was used to evaluate the expression relevance of TIM3, Galectin-9, Foxp3, CD68 and CD163. Statistical significance was defined as P value < 0.05.
Results
TIM3/Galectin-9 pathway was correlated with the expression of Foxp3, CD68 and CD163 in HNSCC
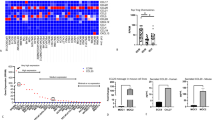
To evaluate the TIM3/Galectin-9 signaling pathway in HNSCC, we detected TIM3 and Galectin-9 expression in human HNSCC tissue arrays. The IHC photographs demonstrated that TIM3 was specifically expressed on immune cells in the tumor stroma. As the ligand of TIM3, Galectin-9 was not only expressed on tumor cells of invasive front but also on immune cells in the tumor stroma. We also examined the Treg marker (Foxp3) and macrophage markers (CD68 and CD163) in the HNSCC tissue array. These markers (Foxp3, CD68, and CD163) were expressed on immune cells in the tumor stroma (Fig. 1a). The cluster analysis showed the IHC scores of TIM3, Galectin-9, Foxp3, CD68 and CD163 for each patient and the correlation of their expression in HNSCC patients (Fig. 1b). Further correlation analysis shows that TIM3 expression was closely associated with Galectin-9 expression (Fig. 2a). Foxp3 expression was correlated with TIM3 and Galectin-9 expression (Fig. 2b). Additionally, the expression of TIM3 and Galectin-9 were significantly correlated with CD68 and CD163 expression (Fig. 2c, d).
Percent of Tregs and CD206+ macrophages increased in the HNSCC mouse model
The phenotypic and pathological features of head and neck squamous cell carcinoma of Tgfbr1/Pten 2cKO mouse model are shown in Fig. 3a and b. Tregs and M2 macrophages are considered as the immunosuppression-mediated cells which suppress the immune response to cancer cells. We detected the percent of CD4+CD25+Foxp3+ Tregs and CD11b+F4/80+CD206+ macrophages (M2) in the HNSCC mouse model. The flow cytometry analysis showed that in the HNSCC mice, the percent of Tregs was increased compared with that of wild-type (WT) mice (Fig. 3c and d). Meanwhile, the percent of CD11b+F4/80+CD206+ macrophages was also increased in the HNSCC mouse model (Fig. 3e and f). These results showed that the negative immune regulators, Tregs and M2 macrophages, accumulate in the development of HNSCC.
The number of TIM3+ Tregs increased in the Tgfbr1/Pten 2cKO HNSCC mouse model. a Photographs of a wild-type mouse and a Tgfbr1/Pten 2cKO HNSCC mouse. b HE staining of squamous cell specific tumor tissue in the 2cKO HNSCC mouse model. c Flow cytometry analysis photographs of CD25+Foxp3+ cells gated from CD4+ T cells. d The percent of Tregs from the draining lymph node (LN) and spleen (SP) in WT (n = 6) and HNSCC (n = 6) mice. e Flow cytometry analysis photographs of CD206+F4/80 cells in the CD11b+ cell populations. f The ratio of CD11b+CD206+F4/80 macrophages from LN and spleen SP in WT and HNSCC mice. (mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, Mann-Whitney test)
Blockade of TIM3 induced a decrease of Tregs in HNSCC mice
We next explored the role of TIM3 in immune suppression by using the HNSCC mouse model. The tumors with the anti-TIM3 therapy grew at a slower rate than those of the control group (Fig. 4a). Through flow cytometry analysis, we found that TIM3 expression was reduced in the anti-TIM3 group (Fig. 4b). Furthermore, we examined the population of Tregs in each group. The results showed that the percent of CD25+Foxp3+ cells in CD4+ T cells was significantly decreased in anti-TIM3 therapy mice compared with that of control mice (Fig. 4c and d), which means that Tregs were reduced in response to TIM3 blockade. Furthermore, these CD4+CD25+Foxp3+ Tregs were labeled with TIM3. Interestingly, the percent of TIM3+ Tregs was also down-regulated in the anti-TIM3 therapy group (Fig. 4e). It suggested that TIM3 may participate in the differentiation of Tregs, and that the blockade of TIM3 induced a decline in the number of Tregs.
Blockade of TIM3 reduced the number of TIM3+ Tregs. a The tumor growth curve of each group. b Percent of TIM3+ cells from the draining lymph node (LN) and spleen (SP) in the anti-TIM3 therapy group (n = 6) and control group (n = 6). c CD4+ T cells were gated from CD25+ Foxp3+; then, TIM3+ cells in CD4+CD25+Foxp3+ Treg population was assessed. d Percent of CD25+Foxp3+ Tregs in the CD4+ T cell population from LN and SP of anti-TIM3 therapy group (n = 6) and control group (n = 6). e Percent of TIM3+ cells in the CD4+CD25+Foxp3+ Treg population (mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, Mann-Whitney test)
Impact of TIM3 blockade on immune checkpoints and macrophages
Immune checkpoints play a vital role in T cell dysfunction and exhaustion. Here, the expression levels of PD1, CTLA4, LAG3 and TIGIT were detected by flow cytometry. The expression levels of PD1 and LAG3 were not changed significantly, while the expression levels of CTLA4 and TIGIT were significantly decreased (Fig. 5a). Moreover, since CD206 is considered an important marker of M2 macrophages, we examined CD11b+F4/80+CD206+ macrophages. However, flow cytometry analysis demonstrated that the number of CD11b+F4/80+CD206+ macrophages was not substantially decreased by blocking TIM3 (Fig. 5b and c).
Impact of TIM3 blockade on immune checkpoints, M2 macrophages and IFN-γ production. a Percent of PD1, CTLA4, LAG3 and TIGIT expressed in the LN and SP of each group. b F4/80+CD206+ macrophages gated from the CD11+ cells in each group. c Percent of CD11b+F4/80+CD206+ macrophages in each group. d The flow cytometry photos of CD8+ T cells and IFN-γ+ production on CD8+ T cells (gated from CD3+ cells) in LN and SP of each group. e Percent of CD8+ T cells in each group. f Percent of IFN-γ+ cells in CD8+ T cells in each group (mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, Mann-Whitney test)
Blockade of TIM3 promoted IFN-γ production on CD8 T cells
To verify whether the inhibition of Tregs and immune checkpoints by blocking TIM3 enhanced the anti-tumor immune response, we assessed the IFN-γ expression on CD8+ T cells. Flow cytometry analysis showed that the percent of CD8+ T cells was significantly increased in the anti-TIM3 therapy mice compared with that in the control mice (Fig. 5d, e). Furthermore, the IFN-γ production on CD8+ T cells was also remarkably elevated by anti-TIM3 therapy (Fig. 5f). TIM3 blockade enhanced the antitumor immune response in HNSCC mouse model.
Discussion
The immune system acts as a supervisor during the initiation and development of HNSCC. Suppression of the immune system leads to the tumor escape [5]. Tumor cell co-option of immune checkpoints act as the major pathways of immune suppression and immune evasion in cancer [28]. Recent evidence has demonstrated that TIM3/Gal9 is an important inhibitory pathway in the immune response of cancer [29]. TIM3 has been proved to be expressed on multiple immune cells, and blocking TIM3/Gal9 has an effect on various immune cells, such as effector T cells, Tregs, macrophages and monocytes [20, 22, 30, 31]. Our previous study has identified the overexpression of TIM3 in HNSCC patients and the association of TIM3 expression with MDSCs [25]. Evidences showed a general increase in the number of circulating and infiltrating Tregs in HNSCC patients [32, 33]. However, the actual association among TIM3, Tregs and macrophages is not very clear in HNSCC. In this study, we determined that the TIM3/Galectin-9 pathway is closely related to the expression of the Treg marker (Foxp3) and macrophage markers (CD68 and CD163) in a HNSCC tissue array.
The canonical Tregs is a subset of T lymphocyte identified by CD4+CD25+Foxp3+. Tregs can restrain CD8+ T cell activation and inflammation through direct contact or production of TGF-β and interleukin (IL)-10 [34]. Early research on ovarian carcinoma demonstrated that blockade of TIM3 reverted Treg-mediated immune suppression [35]. We found that in the transgenic HNSCC mice, the number of Tregs was elevated compared with that of WT mice, while blockade of TIM3 induced a decrease in the Tregs population. Thus, the Treg-mediated inhibition of the immune response mediated was neutralized. Interestingly, the number of TIM3+ Tregs was also reduced by anti-TIM3 therapy, suggesting that TIM3 may participate in the regulation of Tregs. Coincidentally, recent studies have shown that TIM3 participated in the regulation of Tregs. Sun et al. observed that the number of TIM3+ Tregs was correlated with rheumatoid arthritis activity. Moreover, IL-10 expression on TIM3+ Tregs was higher than TIM− Tregs [22]. In hepatitis C viral infections, TIM3 was found to be expressed on Tregs and to regulate the balance between Tregs and effector T cells. And it has been reported that TIM3+ Tregs represent the highly suppressive Tregs due to their high production of IL-10, perforin, granzyme A and granzyme G [36]. These findings suggest that TIM3 acts as a vital regulator of Tregs and affects the function of Tregs.
TIM3 has been shown to be involved in regulating the macrophage activity [30]. Zhang et al. reported that TIM3 expression is increased on macrophages in autoimmune diseases. In addition, increased TIM3 expression on M2 macrophages participated in immune regulation by inhibiting macrophage activation [37]. Another research also indicated that the upregulation of TIM3 expression on M2 macrophages in mice mediated the anti-inflammatory response [38]. However, in the present study, blocking TIM3 did not reduce the number of CD11b+F4/80+CD206+ (M2) macrophages significantly in the HNSCC mouse model. This may be due to the limited expression of TIM3 on CD11b+F4/80+CD206+ macrophages in this mouse. The negative immune checkpoints (such as PD-1, LAG3, CTLA4) also play vital roles in immune suppression through multiple pathways in HNSCC development. In the vivo study, although PD-1 and LAG3 expression levels were not obviously decreased by TIM3 blockade, CTLA4 and TIGIT expression levels were significantly decreased. CTLA4 acts as a negative regulator of T cell activation and the maintenance of T cell homeostasis [39]. TIGIT is expressed on memory T cells, Tregs and NK cells, and can suppress the activation of T cells [40]. Thus, the downregulation of CTLA4 and TIGIT may alleviate the inhibition of T cell activation and strengthen the immune response. In addition to these mechanisms, the reduced expression of TIM3 on T cells can directly increase IFN-γ production and enhance the anti-tumor immune effect.
Conclusion
Taken together, HNSCC is a malignant tumor characterized by a substantially suppressed immune system. There are various mechanisms that contribute to the failed anti-tumor immune response. We showed that TIM3 participates in the regulation of Tregs, and that blockade of TIM3 relieves the immune suppression by reducing Treg activation and decreasing CTLA4 and TIGIT in HNSCC, supporting the therapeutic value of anti-TIM3 treatment in HNSCC.
Abbreviations
- DC:
-
Dendritic cells
- HNSCC:
-
Head and neck squamous cell carcinoma
- IL-10:
-
Interleukin-10
- MDSCs:
-
Myeloid-derived suppressor cells
- TGF-β:
-
Transforming Growth Factor-β
- TIM3:
-
T-cell immunoglobulin mucin 3;
- Tregs:
-
Regulatory T cells
- WT:
-
Wild type
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–709.
Msaouel P, Massarelli E. Immune checkpoint therapy in head and neck cancers. Cancer J. 2016;22(2):108–16.
Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22.
Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33(29):3293–304.
Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64.
Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–45.
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6.
Maggioni D, Pignataro L, Garavello W. T-helper and T-regulatory cells modulation in head and neck squamous cell carcinoma. Oncoimmunology. 2017;6(7):e1325066.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20.
Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37.
Rhee I. Diverse macrophages polarization in tumor microenvironment. Arch Pharm Res. 2016;39(11):1588–96.
Zhu F, Li X, Jiang Y, Zhu H, Zhang H, Zhang C, Zhao Y, Luo F. GdCl3 suppresses the malignant potential of hepatocellular carcinoma by inhibiting the expression of CD206 in tumorassociated macrophages. Oncol Rep. 2015;34(5):2643–55.
Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–41.
Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–3.
DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, et al. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J Immunol. 2010;184(4):1918–30.
Wilker PR, Sedy JR, Grigura V, Murphy TL, Murphy KM. Evidence for carbohydrate recognition and homotypic and heterotypic binding by the TIM family. Int Immunol. 2007;19(6):763–73.
Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517(7534):386–90.
Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501–10.
Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–52.
Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71(10):3540–51.
Sun H, Gao W, Pan W, Zhang Q, Wang G, Feng D, Geng X, Yan X, Li S. Tim3(+) Foxp3 (+) Treg cells are potent inhibitors of effector T cells and are suppressed in rheumatoid arthritis. Inflammation. 2017;40(4):1342–50.
Bu M, Shen Y, Seeger WL, An S, Qi R, Sanderson JA, Cai Y. Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8(+) T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumour Biol. 2016;37(3):3949–56.
Han G, Chen G, Shen B, Li Y. Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol. 2013;4:449.
Liu JF, Ma SR, Mao L, Bu LL, Yu GT, Li YC, Huang CF, Deng WW, Kulkarni AB, Zhang WF, et al. T-cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Mol Oncol. 2017;11(2):235–47.
Bian Y, Hall B, Sun ZJ, Molinolo A, Chen W, Gutkind JS, Waes CV, Kulkarni AB. Loss of TGF-beta signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene. 2012;31(28):3322–32.
Sun ZJ, Zhang L, Hall B, Bian Y, Gutkind JS, Kulkarni AB. Chemopreventive and chemotherapeutic actions of mTOR inhibitor in genetically defined head and neck squamous cell carcinoma mouse model. Clin Cancer Res. 2012;18(19):5304–13.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014;2(5):393–8.
Frisancho-Kiss S, Nyland JF, Davis SE, Barrett MA, Gatewood SJ, Njoku DB, Cihakova D, Silbergeld EK, Rose NR, Fairweather D. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J Immunol. 2006;176(11):6411–5.
Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. J Leukoc Biol. 2012;91(2):189–96.
Lim KP, Chun NA, Ismail SM, Abraham MT, Yusoff MN, Zain RB, Ngeow WC, Ponniah S, Cheong SC. CD4+CD25hiCD127low regulatory T cells are increased in oral squamous cell carcinoma patients. PLoS One. 2014;9(8):e103975.
Sun W, Li WJ, Fu QL, Wu CY, Lin JZ, Zhu XL, Hou WJ, Wei Y, Wen YH, Wang YJ, et al. Functionally distinct subsets of CD4(+) regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol Rep. 2015;33(1):354–62.
Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32.
Bu M, Shen Y, Seeger WL, An S, Qi R, Sanderson JA, Cai Y. Ovarian carcinoma-infiltrating regulatory T cells were more potent suppressors of CD8(+) T cell inflammation than their peripheral counterparts, a function dependent on TIM3 expression. Tumour Biol. 2015;37(3):1–8.
Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC. TIM3+FOXP3+ regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology. 2013;2(4):e23849.
Zhang ZY, Schluesener HJ, Zhang Z. Distinct expression of Tim-3 during different stages of rat experimental autoimmune neuritis. Brain Res Bull. 2011;86(3–4):229–34.
Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather D. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23(5):649–57.
Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97.
Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (81672667, 81672668, 81472528, 81472529). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
JFL, LW and LLY performed experiments; WFZ, ZJS participated in the design of the study; JFL, WWD, LM and HW participated in data analysis and statistical analysis; JFL, LW, WFZ and ZJS drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All protocols dealing with the patients conformed to the ethical guidelines of the Helsinki Declaration and were approved by the Medical Ethics Committee of Hospital of Stomatology Wuhan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, JF., Wu, L., Yang, LL. et al. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J Exp Clin Cancer Res 37, 44 (2018). https://doi.org/10.1186/s13046-018-0713-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-018-0713-7