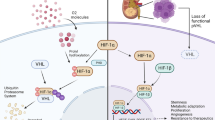
Abstract
Renal cell carcinoma (RCC) is one of the most lethal urologic malignancies. Its incidence continues to rise worldwide with a rate of 2% per year. Approximately, one-third of the RCC patients are diagnosed at advanced stages due to the asymptomatic nature of its early stages. This represents a great hurdle, since RCC is largely chemoresistant/radioresistant, and targeted therapy of mRCC still has limited efficacy. The 5-year survival rate of metastatic RCC (mRCC) is only around 10%. Adoptive cell transfer (ACT), a particular form of cell-based anticancer immunotherapy, is a promising approach in the treatment of mRCC. The vaccination principle, however, faces unique challenges that preclude the efficacy of ACT. In this article, we review the main challenges of ACT in the treatment of mRCC and describe multiple methods that can be used to overcome these challenges. In this respect, the ultimate purpose of this review is to provide a descriptive tool by which to improve the development of novel protocols for ACT of mRCC.



Similar content being viewed by others
Abbreviations
- ACT:
-
Adoptive cell transfer
- ccRCC:
-
Clear cell RCC
- IL:
-
Interleukin
- ITF:
-
Intratumoral fibrosis
- mRCC:
-
Metastatic RCC
- NK cell:
-
Natural killer cell
- non-ccRCC:
-
Non-clear cell RCC
- PBMC:
-
Peripheral blood mononuclear cell
- PECAM:
-
Platelet endothelial cell adhesion molecule
- RCC:
-
Renal cell carcinoma
- TIL:
-
Tumor-infiltrating lymphocyte
- TRAIL:
-
TNF-related apoptosis-inducing ligand
References
Kabaria R, Klaassen Z, Terris MK (2016) Renal cell carcinoma: links and risks. Int J Nephrol Renovasc Dis 9:45–52
Shea MW (2013) A proposal for a targeted screening program for renal cancer. Front Oncol 3:207
Santoni M, Massari F, Di Nunno V, Conti A, Cimadamore A, Scarpelli M et al (2018) Immunotherapy in renal cell carcinoma: latest evidence and clinical implications. Drugs Context 7:212528
Cohen JE, Merims S, Frank S, Engelstein R, Peretz T, Lotem M (2017) Adoptive cell therapy: past, present and future. Immunotherapy 9(2):183–196. https://doi.org/10.2217/imt-2016-0112
Rosenberg SA, Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348(6230):62–68
Phan GQ, Rosenberg SA (2013) Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy. Cancer Control 20(4):289–297
Tang X, Liu T, Zang X, Liu H, Wang D, Chen H et al (2013) Adoptive cellular immunotherapy in metastatic renal cell carcinoma: a systematic review and meta-analysis. PLoS ONE 8(5):e62847
Kalos M, June CH (2013) Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 39(1):49–60
Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L et al (2010) Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 16(9):2646–2655
Bellone M, Calcinotto A, Corti A (2012) Won’t you come on in? How to favor lymphocyte infiltration in tumors. Oncoimmunology 1(6):986–988
Torcellan T, Stolp J, Chtanova T (2017) In vivo imaging sheds light on immune cell migration and function in cancer. Front Immunol 8:309
Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A et al (2012) Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 122(3):899–910
Bougherara H, Mansuet-Lupo A, Alifano M, Ngo C, Damotte D, Le Frere-Belda MA et al (2015) Real-time imaging of resident T cells in human lung and ovarian carcinomas reveals how different tumor microenvironments control T lymphocyte migration. Front Immunol 6:500
Idorn M, Thor Straten P (2018) Chemokine receptors and exercise to tackle the inadequacy of T cell homing to the tumor site. Cells. https://doi.org/10.3390/cells7080108
Nayar S, Dasgupta P, Galustian C (2015) Extending the lifespan and efficacies of immune cells used in adoptive transfer for cancer immunotherapies—a review. Oncoimmunology 4(4):e1002720
Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G (2011) Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 71(17):5601–5605. https://doi.org/10.1158/0008-5472.CAN-11-1316
Lee S, Margolin K (2012) Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep 14(5):468–474
Giraldo NA, Becht E, Pages F, Skliris G, Verkarre V, Vano Y, Mejean A, Saint-Aubert N, Lacroix L, Natario I, Lupo A, Alifano M, Damotte D, Cazes A, Triebel F, Freeman GJ, Dieu-Nosjean MC, Oudard S, Fridman WH, Sautes-Fridman C (2015) Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin Cancer Res 21(13):3031–3040. https://doi.org/10.1158/1078-0432.CCR-14-2926
Geissler K, Fornara P, Lautenschlager C, Holzhausen HJ, Seliger B, Riemann D (2015) Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology 4(1):e985082
Strizova Z, Taborska P, Stakheev D, Partlova S, Havlova K, Vesely S, Bartunkova J, Smrz D (2019) NK and T cells with a cytotoxic/migratory phenotype accumulate in peritumoral tissue of patients with clear cell renal carcinoma. Urol Oncol 37(7):503–509. https://doi.org/10.1016/j.urolonc.2019.03.014
Crossey F, Marx S, Holters S, Schmitt K, Bohle RM, Schmidt T et al (2018) Robust method for isolation of tumor infiltrating lymphocytes with a high vital cell yield from small samples of renal cell carcinomas by a new collagenase-free mechanical procedure. Urol Oncol 36(9):402e1–402e10
Mayor P, Starbuck K, Zsiros E (2018) Adoptive cell transfer using autologous tumor infiltrating lymphocytes in gynecologic malignancies. Gynecol Oncol 150(2):361–369
Voigt H, Kleeberg UR (1986) Malignes melanom. Springer, Berlin, p 185
Cox TR, Erler JT (2016) Fibrosis and cancer: Partners in crime or opposing forces? Trends Cancer 2(6):279–282
Joung JW, Oh HK, Lee SJ, Kim YA, Jung HJ (2018) Significance of intratumoral fibrosis in clear cell renal cell carcinoma. J Pathol Transl Med 52(5):323–330
Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA (2010) Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 70:6171–6180. https://doi.org/10.1158/0008-5472.CAN-10-0153
Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R (2012) Targeted drug delivery and penetration into solid tumors. Med Res Rev 32(5):1078–1091
Gregorc V, Gaafar RM, Favaretto A, Grossi F, Jassem J, Polychronis A et al (2018) NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 19(6):799–811
Zhang E, Xu H (2017) A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J Hematol Oncol 10(1):1
Muller WA (2011) Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 6:323–344
Berman ME, Xie Y, Muller WA (1996) Roles of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and beta 2 integrin activation. J Immunol 156(4):1515–1524
Dasgupta B, Dufour E, Mamdouh Z, Muller WA (2009) A novel and critical role for tyrosine 663 in platelet endothelial cell adhesion molecule-1 trafficking and transendothelial migration. J Immunol 182(8):5041–5051
Andersen R, Westergaard MCW, Kjeldsen JW, Muller A, Pedersen NW, Hadrup SR et al (2018) T-cell responses in the microenvironment of primary renal cell carcinoma-implications for adoptive cell therapy. Cancer Immunol Res 6(2):222–235
Tian JQ, Wang ZP, Rodriguez R, Fu JS, Lu JZ, Ma BL (2006) In vitro enhanced cytotoxicity of tumor-infiltrating lymphocytes transfected with tumor necrosis factor-related apoptosis-inducing ligand and/or interleukin-2 gene in human renal cell carcinoma. Urology 67(5):1093–1098. https://doi.org/10.1016/j.urology.2005.11.030
de Bruyn M, Wei Y, Wiersma VR, Samplonius DF, Klip HG, van der Zee AG, Yang B, Helfrich W, Bremer E (2011) Cell surface delivery of TRAIL strongly augments the tumoricidal activity of T cells. Clin Cancer Res 17(17):5626–5637. https://doi.org/10.1158/1078-0432.CCR-11-0303
Strater J, Hinz U, Hasel C, Bhanot U, Mechtersheimer G, Lehnert T, Moller P (2005) Impaired CD95 expression predisposes for recurrence in curatively resected colon carcinoma: clinical evidence for immunoselection and CD95L mediated control of minimal residual disease. Gut 54(5):661–665. https://doi.org/10.1136/gut.2004.052696
Cacan E (2017) Enhancing sensitivity of chemoresistant ovarian cancer cells to TRAIL and FAS mediated apoptosis by radiation. Turk Hij Den Biyol Derg 74(3):185–192
de Miguel D, Lemke J, Anel A, Walczak H, Martinez-Lostao L (2016) Onto better TRAILs for cancer treatment. Cell Death Differ 23(5):733–747
Hinrichs C, Borman Z, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern S, Logun C et al (2009) Adoptively transferred effector cells derived from naïve rather than central memory CD8 T cells mediate superior antitumor immunity. PNAS 106:17469–17474
Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, Forman SJ (2010) Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 16:1245–1256
Poschke I, Lovgren T, Adamson L, Nystrom M, Andersson E, Hansson J et al (2014) A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma. Cancer Immunol Immunother 63(10):1061–1071
Cesana GC, DeRaffele G, Cohen S et al (2006) Characterization of CD4*CD25* regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol 24:1169–1177
Siddiqui SA, Frigola X, Bonne-Annee S et al (2007) Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res 13:2075–2081
Finke JH, Rini B, Ireland J et al (2008) Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res 14(20):6674–6682 Epub 2008/10/18
Nagaraj S, Youn JI, Weber H et al (2010) Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res 16(6):1812–1823
Nicholson IC, Mavrangelos C, Bird DR et al (2012) PI16 is expressed by a subset of human memory Treg with enhanced migration to CCL17 and CCL20. Cell Immunol 275(1–2):12–18
Knutson KL, Wagner W, Disis ML (2006) Adoptive T cell therapy of solid cancers. Cancer Immunol Immunother 55(1):96–103
Acknowledgement
We would like to thank Prof. Ilja Striz and Dr. Alasdair M. Gilfillan for a critical review of the manuscript.
Funding
Charles University—project GA UK No. 364218 and PRIMUS/MED/12 and funding by the Ministry of Health, Czech Republic—project AZV 16-28135A.
Author information
Authors and Affiliations
Contributions
Zuzana Strizova drafted and wrote the manuscript. Jirina Bartunkova contributed to the intellectual content and writing of the manuscript. Daniel Smrz contributed to the intellectual content and with Zuzana Strizova wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors Zuzana Strizova and Daniel Smrz declare that there is no conflict of interest regarding this article. Jirina Bartunkova is a part-time employee and a minority shareholder of SOTIO, a.s., a biotech company developing cell-based immunotherapy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Strizova, Z., Bartunkova, J. & Smrz, D. The challenges of adoptive cell transfer in the treatment of human renal cell carcinoma. Cancer Immunol Immunother 68, 1831–1838 (2019). https://doi.org/10.1007/s00262-019-02359-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02359-z