Abstract
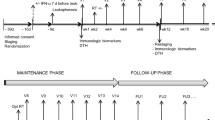
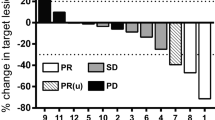
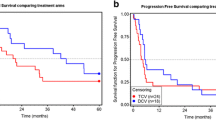
Adoptive transfer of in vitro-expanded tumor-infiltrating lymphocytes (TIL) has shown great clinical benefit in patients with malignant melanoma. TIL therapy itself has little side effects, but conditioning chemo- or radiotherapy and postinfusion interleukin 2 (IL-2) injections are associated with severe adverse advents. We reasoned that combining TIL infusion with dendritic cell (DC) vaccination could circumvent the need for conditioning and IL-2 support and thus represent a milder treatment approach. Eight patients with stage IV melanoma were enrolled in the MAT01 study, consisting of vaccination with autologous tumor-lysate-loaded DC, followed by TIL infusion. Six of eight patients were treated according to protocol, while one patient received only TIL and one only DC. Treatments were well tolerated with a single grade 3 adverse event. The small study size precludes analysis of clinical responses, though interestingly one patient showed a complete remission and two had stable disease. Analysis of the infusion products revealed that mature DC were generated in all cases. TIL after expansion were CD3+ T cells, dominated by effector memory CD8+ cytotoxic T cells. Analysis of the T cell receptor repertoire revealed presence of highly dominant clones in most infusion products, and many of these could be detected in the circulation for weeks after T cell transfer. Here, we report the first combination of DC vaccination and TIL infusion in malignant melanoma. This combined treatment was safe and feasible, though after evaluating both clinical and immunological parameters, we expect that administration of lymphodepleting chemotherapy and IL-2 will likely increase treatment efficacy.



Similar content being viewed by others
Abbreviations
- ACT:
-
Adoptive T cell transfer
- AJCC:
-
American Joint Committee on Cancer
- CDR3:
-
Complimentarity determining region 3
- Ct:
-
Threshold cycle value
- CR:
-
Complete response
- CT:
-
Computer tomography
- CYP:
-
Cyclophosphamide
- DC:
-
Dendritic cells
- DTH:
-
Delayed type hypersensitivity
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- IL:
-
Interleukin
- MDSC:
-
Myeloid-derived suppressor cells
- MRI:
-
Magnetic resonance imaging
- LN:
-
Lymph node
- OS:
-
Overall survival
- PBMC:
-
Peripheral blood mononuclear cells
- PD:
-
Progressive disease
- PET:
-
Positron emission tomography
- PR:
-
Partial response
- RESIST:
-
Response evaluation criteria in solid tumors
- SD:
-
Stable disease
- SOP:
-
Standard operating procedure
- TCR:
-
T cell receptor
- TIL:
-
Tumor-infiltrating lymphocytes
- TNF:
-
Tumor necrosis factor
- TNM:
-
Tumor node metastasis
- Tregs:
-
Regulatory T cells
- SC:
-
Subcutaneous
- WHO/ECOG:
-
World Health Organization/Eastern Cooperative Oncology Group
References
Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, Shalmon B, Hardan I, Catane R, Markel G, Apter S, Ben-Nun A, Kuchuk I, Shimoni A, Nagler A, Schachter J (2010) Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 16(9):2646–2655
Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA (2005) Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 23(10):2346–2357
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26(32):5233–5239
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH (2011) T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3(95):95ra73
Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, Albelda SM, June CH (2010) Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res 70(22):9053–9061
Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365(8):725–733
Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, Phan GQ, Kammula US, Hughes MS, Citrin DE, Restifo NP, Wunderlich JR, Prieto PA, Hong JJ, Langan RC, Zlott DA, Morton KE, White DE, Laurencot CM, Rosenberg SA (2010) CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res 16(24):6122–6131
Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA (2009) Gene therapy with human and mouse T cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114(3):535–546
Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA (2011) T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19(3):620–626
Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP (2006) Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nat Clin Pract Oncol 3(12):668–681
Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, Yu Z, Rosenberg SA, Restifo NP (2010) Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother 33(1):1–7
Petrella T, Quirt I, Verma S, Haynes AE, Charette M, Bak K (2007) Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev 33(5):484–496
Ellebaek E, Iversen TZ, Junker N, Donia M, Engell-Noerregaard L, Met O, Holmich LR, Andersen RS, Hadrup SR, Andersen MH, thor Straten P, Svane IM (2012) Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med 10:169
Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G (2012) Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology 1(7):1111–1134
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10(9):909–915
Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF (2005) Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother 28(5):496–504
Lopez MN, Pereda C, Segal G, Munoz L, Aguilera R, Gonzalez FE, Escobar A, Ginesta A, Reyes D, Gonzalez R, Mendoza-Naranjo A, Larrondo M, Compan A, Ferrada C, Salazar-Onfray F (2009) Prolonged survival of dendritic cell-vaccinated melanoma patients correlates with tumor-specific delayed type IV hypersensitivity response and reduction of tumor growth factor beta-expressing T cells. J Clin Oncol 27(6):945–952
Ridolfi L, Petrini M, Fiammenghi L, Granato AM, Ancarani V, Pancisi E, Scarpi E, Guidoboni M, Migliori G, Sanna S, Tauceri F, Verdecchia GM, Riccobon A, Valmorri L, Ridolfi R (2010) Unexpected high response rate to traditional therapy after dendritic cell-based vaccine in advanced melanoma: update of clinical outcome and subgroup analysis. Clin Dev Immunol 2010:504979
Salcedo M, Bercovici N, Taylor R, Vereecken P, Massicard S, Duriau D, Vernel-Pauillac F, Boyer A, Baron-Bodo V, Mallard E, Bartholeyns J, Goxe B, Latour N, Leroy S, Prigent D, Martiat P, Sales F, Laporte M, Bruyns C, Romet-Lemonne JL, Abastado JP, Lehmann F, Velu T (2006) Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother 55(7):819–829
Schwaab T, Schwarzer A, Wolf B, Crocenzi TS, Seigne JD, Crosby NA, Cole BF, Fisher JL, Uhlenhake JC, Mellinger D, Foster C, Szczepiorkowski ZM, Webber SM, Schned AR, Harris RD, Barth RJ Jr, Heaney JA, Noelle RJ, Ernstoff MS (2009) Clinical and immunologic effects of intranodal autologous tumor lysate-dendritic cell vaccine with Aldesleukin (Interleukin 2) and IFN-{alpha}2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res 15(15):4986–4992
Boniface JD, Poschke I, Mao Y, Kiessling R (2012) Tumor-dependent down-regulation of the zeta-chain in T cells is detectable in early breast cancer and correlates with immune cell function. Int J Cancer 131(1):129–139
Poschke I, De Boniface J, Mao Y, Kiessling R (2012) Tumor-induced changes in the phenotype of blood-derived and tumor-associated T cells of early stage breast cancer patients. Int J Cancer 131(7):1611–1620
Palma M, Hansson L, Choudhury A, Nasman-Glaser B, Eriksson I, Adamson L, Rossmann E, Widen K, Horvath R, Kokhaei P, Vertuani S, Mellstedt H, Osterborg A (2012) Vaccination with dendritic cells loaded with tumor apoptotic bodies (Apo-DC) in patients with chronic lymphocytic leukemia: effects of various adjuvants and definition of immune response criteria. Cancer Immunol Immunother 61(6):865–879
Adamson L, Palma M, Choudhury A, Eriksson I, Nasman-Glaser B, Hansson M, Hansson L, Kokhaei P, Osterborg A, Mellstedt H (2009) Generation of a dendritic cell-based vaccine in chronic lymphocytic leukaemia using CliniMACS platform for large-scale production. Scand J Immunol 69(6):529–536
Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R (2012) Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother 61(6):827–838
Koike N, Pilon-Thomas S, Mule JJ (2008) Nonmyeloablative chemotherapy followed by T cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother 31(4):402–412
Lutz-Nicoladoni C, Wallner S, Stoitzner P, Pircher M, Gruber T, Wolf AM, Gastl G, Penninger JM, Baier G, Wolf D (2012) Reinforcement of cancer immunotherapy by adoptive transfer of cblb-deficient CD8+ T cells combined with a DC vaccine. Immunol Cell Biol 90(1):130–134
Park MY, Kim CH, Sohn HJ, Oh ST, Kim SG, Kim TG (2007) The optimal interval for dendritic cell vaccination following adoptive T cell transfer is important for boosting potent anti-tumor immunity. Vaccine 25(42):7322–7330
Song S, Zhang K, You H, Wang J, Wang Z, Yan C, Liu F (2010) Significant anti-tumour activity of adoptively transferred T cells elicited by intratumoral dendritic cell vaccine injection through enhancing the ratio of CD8(+) T cell/regulatory T cells in tumour. Clin Exp Immunol 162(1):75–83
Tamai H, Watanabe S, Zheng R, Deguchi K, Cohen PA, Koski GK, Shu S (2008) Effective treatment of spontaneous metastases derived from a poorly immunogenic murine mammary carcinoma by combined dendritic-tumor hybrid vaccination and adoptive transfer of sensitized T cells. Clin Immunol 127(1):66–77
Kandalaft LE, Powell DJ Jr, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, June CH, Coukos G (2013) Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology 2(1):e22664
Goff SL, Smith FO, Klapper JA, Sherry R, Wunderlich JR, Steinberg SM, White D, Rosenberg SA, Dudley ME, Yang JC (2010) Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J Immunother 33(8):840–847
Rosenberg SA, Dudley ME (2009) Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 21(2):233–240
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA (2008) Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26(32):5233–5239
de Vries IJ, Castelli C, Huygens C, Jacobs JF, Stockis J, Schuler-Thurner B, Adema GJ, Punt CJ, Rivoltini L, Schuler G, Coulie PG, Lucas S (2011) Frequency of circulating Tregs with demethylated FOXP3 intron 1 in melanoma patients receiving tumor vaccines and potentially Treg-depleting agents. Clin Cancer Res 17(4):841–848
Acknowledgments
We would like to thank B. Näsman-Glaser and K. Heimersson for their expert technical assistance. R. Tell is supported by a 50 % research position from the Karolinska Institutet Thematic Network IMTAC. The study was supported by grants to R. Kiessling from the Swedish Cancer Society (120598 Cancerfonden), the Cancer Society of Stockholm (121103 Cancerföreningen, Radiumhemmets Forskningsfonder), Torsten Söderbergs stiftelse, the Swedish Medical Research Council (K2011-66X-15387-07-3 VR), an ALF-Project grant from Stockholm City Council (20110070 ALF Medicin 2012), the Knut and Alice Wallenberg Foundations and a grant from Magnus Bergvalls Foundation to T. Lövgren.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Giuseppe V. Masucci and Rolf Kiessling have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Poschke, I., Lövgren, T., Adamson, L. et al. A phase I clinical trial combining dendritic cell vaccination with adoptive T cell transfer in patients with stage IV melanoma. Cancer Immunol Immunother 63, 1061–1071 (2014). https://doi.org/10.1007/s00262-014-1575-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1575-2