Abstract
Adoptive cell therapy using chimeric antigen receptor (CAR)-engineered T cells has emerged as a very promising approach to combating cancer. Despite its ability to eliminate tumors shown in some clinical trials, CAR-T cell therapy involves some significant safety challenges, such as cytokine release syndrome (CRS) and “on-target, off-tumor” toxicity, which is related to poor control of the dose, location, and timing of T cell activity. In the past few years, some strategies to avoid the side effects of CAR-T cell therapy have been reported, including suicide gene, inhibitory CAR, dual-antigen receptor, and the use of exogenous molecules as switches to control the CAR-T cell functions. Because of the advances of the CAR paradigm and other forms of cancer immunotherapy, the most effective means of defeating the cancer has become the integration therapy with the combinatorial control system of switchable dual-receptor CAR-T cell and immune checkpoint blockade.
Similar content being viewed by others
Background
A specific type of immune therapy, known as a green therapy, has emerged as an exciting new approach for cancer therapy, especially the adoptive cells transfer (ACT) therapy [1–3]. Unlike surgery, radiotherapy, and chemotherapy, the cell-based therapy can facilitate accurate decisions and execute highly complex behaviors [4–6]. The autologous lymphocytes isolated from a patient’s own peripheral blood are endowed with the ability of tumor antigen specificity and rendered capable of eliminating cancer cells expanded ex vivo, and then reinfused into the patient to attack any malignant tumor [7–10]. The majority of preclinical and clinical data regarding autologous T cells have highlighted the safety of using the cell therapy and the lack of potential for graft-versus-host disease (GvHD) mediated by the allogeneic T cells [11–13]. The T cell receptor (TCR), an α/β heterodimer, has the ability to redirect the tumor antigens in a major histocompatibility-complex-dependent (MHC) manner [14–16]. The conventional T cells are activated upon TCR combining with other cell surface molecules, termed TCR/CD3 complex, which contains 10 immunoreceptor tyrosine activation motifs (ITAMs) and 20 tyrosine-phosphorylation sites [17–19]. The tumor escape mechanism, however, is associated with the downregulation of the MHC molecule on the surface of the tumor cell, which restrains the homing of T cells because the interaction between T cell receptor and peptide-MHC is a prerequisite for T cell activation [20–22]. In contrast, the structure and signaling pathway of the chimeric antigen receptor (CAR) are delicate. CARs permanently endow the patient-derived T cells with the ability to recognize and kill any tumor cells expressing the antigens without MHC molecules, and render the tumor cell “visible” to T cell immune surveillance [23–25]. Recent advances in genetic engineering and improved recognition of T cells have resulted in the design of new receptor mechanisms, termed CARs. Human T cells modified with this synthetic receptor can specifically redirect tumor antigens and undertake the striking efficacy for many human malignancies [26–28].
Like that of the conventional T cell, the structure of CAR-modified T cell contains three moieties, i.e., an extracellular domain, single-chain antibody fragments (scFv), that recognize and bind a specific tumor antigen independent of MHC molecule, a transmembrane domain that usually comprises the homodimer of CD3 or CD8 molecule, and an intracellular signaling domain including a signal-transduction component of the T-cell receptor (e.g., CD3ζ or FcεRIγ) and a costimulatory receptor (e.g., 4-1BB, CD28, or OX40) (Fig. 1) [29–32]. The initial CAR-T cell comprises the scFv element and the CD3ζ signaling domain, which endows the T cell with the abilities of homing and activation (Fig. 1b). However, the cytotoxicity of first-generation CAR-T cells is transient in vivo. To enhance the durability of CAR-T cell cytotoxicity, the second- and third-generation CAR were developed by addition of single and dual costimulatory signaling domains respectively (Fig. 1c, d) [33, 34]. During the last decade, CAR-T cells have shown impressive results in patients with hematological tumor, but have limitations in treating solid tumors probably due to the blunt immune-surveillance that the immune suppressor cells, cytokines, and some proteins hinder T cell functions in tumor microenvironment [35–39]. To overcome this weakness, Koneru et al. recently developed a new module of CAR-T cells simultaneously transduced with both CAR and IL-12 genes, known as armored CAR-T cells, which can penetrate the ovarian tumor site with surmounting the tumor microenvironment [40–42]. Some researchers have also demonstrated that the release of specific enzymes by T cells, known as heparanase (HPSE), which can help immunocytes pass through physical barriers with degradation of extracellular matrix (ECM) that possesses an ability to prevent the T cells homing to tumor site. Some chemokine receptors have also been introduced into CAR-T cell, which can drive effective T cell infiltration into the tumor bed (Fig. 1e) [43–45].
Schematic diagram of TCR- and CAR-modified T cells in adoptive T cells therapy. a Activation, proliferation, and cytotoxicity of the T cell are dependent upon the dual signal pathway that includes the T cell receptors (TCRs) that recognize peptide antigens which were processed by the antigen-presenting cells and presented upon the major histocompatibility complex (MHC) of a target cells, and the costimulatory receptor of T cell simultaneously engages a ligand, such as CD28 and B7 molecules. b The first-generation CAR contains only the antigen recognition signal, CD3ζ domain, resulting in the transient activation and proliferation of the CAR-T cell based on scFv specificity. c–d The second- and third-generation CARs contain one and two additional costimulatory signaling domains, respectively, such as CD28, CD137 (4-1BB), and CD134 (OX40). The costimulatory signaling domains can facilitate greater proliferation of modified-T cell and greater cytotoxicity than first-generation CAR. e To significantly enhance the overall cytotoxicity of the modified-T cell, the fourth-generation CAR-T cell is generally modified to express CARs with an inducible cytokine genes, such as IL-12 or heparinase, which can stimulate T cell to reach the surface of tumor cells in degrading the extracellular matrix (ECM) within the tumor microenvironment and blocking the inhibitory signaling pathway. f The next-generation structure of the CARs with effective specificity for target cells lacking several side effects to the body will be generated in the near future, including reconstruction of endogenous structure and introduction of exogenous regulatory
Despite the promising clinical results, CAR-T cell therapy also involves several deleterious types of toxicity due to the inability to control T cell activity and some tumor-associated antigens that are presented by both diseased and healthy tissue. The prominent toxicity of CAR-T cell therapy involves cytokine release syndrome (CRS) and “on-target, off-tumor” toxicity [46–49]. The CRS effect, so-called cytokine-associated toxicity, is a result of intense tumor-killing responses mediated by large numbers of activated lymphocytes (B cells, T cells, and natural killer cells) [50]. According to a previous clinical trial, NCT0265014, the levels of several cytokines including interleukin 6 (IL-6) and interferon γ (IFN-γ) are markedly elevated in patient serum after receiving genetically engineered T cells. At the early stage of CAR-T cell therapy, large numbers of CAR-T cells are reinfused into patients with refractory and relapse malignancies. This results in rapid elimination of tumor cell and extends the patient survival considerably [51, 52]. Concomitantly, the obtained cytokine levels on patients with the administration of adoptive T-cell therapy are several hundred times higher than the baseline level, which typically causes a clinical syndrome including fever, hypotension, and neurological changes, potentially leading to rapid death [53, 54]. Lee et al. published a grading system for assessing the severity of CRS. In this system, the CRS has five grades based on the clinical signs and symptoms [55]. As conventional adverse effects derived from drugs, CRS toxicity can be controlled by reducing the dosage of the active T cells. However, the numbers of T cell are difficult to control and may eventually exceed a threshold, resulting in some severe side responses. In addition to CRS, the second safety concern associated with the CAR-T cell therapy is a normal tissue damage attributable to the presence of the tumor antigens on the peritumoral tissues [56, 57]. In current reports, tumor antigens that are expressed on cancer cells but not on normal cells are rare, especially for solid tumors [58]. In this way, tumor-associated antigens are often currently used as targeting molecules for CARs. The lethal toxicity described as “on-target, off-tumor” effect, to date, has been reported in some cancer immunotherapy clinical trials covering the infusion of engineered-T cells. For instance, the most successful therapy for targeting the B-cell malignancies has been reported after infusion of CAR-T cell with specificity to CD19 in an ongoing clinical trial NCT00924326. Owing to the targeting and eradication of normal B cells, long-term B-cell aplasia symptoms appear in patients with autologous T cell expressing the second-generation CAR with CD19 scFv [59]. This off-tumor expression of the interesting target molecule in normal cell leads to rapid cardiopulmonary toxicity [60]. For another case, a patient with the metastatic renal cell carcinoma expressing carboxyanhydrase-IX (CAIX) obtains the common toxicity criteria (CTC) grade 2–4 liver enzyme disturbances after receiving the CAR-modified T cell against CAIX antigen [61]. All of these types of on-target toxicity generally stem from the inability of CAR-T cell to distinguish between normal cell and cancer cell.
During the past few years, many modulations of the CAR-T cell have been developed and proved to be effective and safe [62–64]. In this review, the characteristics of these new technologies are summarized and analyzed. Based on previous research into technology to improve the safety of CAR-T cells (Fig. 2), the autonomous control (e.g., homing and specific recognition of the CAR-T cell) is the first-choice way of ameliorating T cell security. This involves autocrine cytokines against the tumor immunosuppressive microenvironment and the multireceptor presented on the T cells surface that specifically reject the cancer tissue with some tumor associated antigens. Later, some synthetic control devices that can alter the CAR-T cell activity can be implemented in some studies combined with exogenous molecules, which can produce “smart T cell” whose therapeutic functions are precisely controlled over the timing and dosage, thereby alleviating toxicity. These strategies offer specific advantages, and the combinatorial strategy involving the dual-receptor T cell and a dependent-molecule may become increasingly viable. In this system, the effective CAR-T cells can target the cancer cell attributed to the receptor with a specificity for the tumor antigen, and then be activated over the exogenous molecules targeting the other receptor (Fig. 3). Additionally, the receptor recognition domain of controlled molecules was coupled to another molecule carrying antitumor activity as a way to achieve higher cytotoxicity.
Building strategies to improve the safety of CAR-modified T cells therapy. For upper left, when the release of cytokines by CAR-T cells after killing tumor cells becomes more pronounced than at basic levels, such as interleukin-2 (IL-2) and interferon-γ (IFN-γ), the inducible caspase 9 (iCasp9) can be dimerized, which usually leads to the rapid apoptosis of T cells expressing the iCasp9 suicide gene by addition of a synthetic dimerizing drug AP1903. For upper middle, to achieve the precise regulation of the CAR-T cells, the inhibitory strategy usually harnesses an inhibitory receptor structure comprising an antigen recognition domain with specificity to antigens only presented on normal tissue, and an inhibitory signaling domain to abort T cell behavior despite concurrent engagement of an activating receptor. In this way, the iCAR-T cell can distinguish the tumor and off-tumor cells, and reversibly restrict CAR-T cell functions in an antigen-selective manner, such as the PD-1- and CTLA-4-based iCARs. For upper right, the modified-T cell cotransduced with a CAR, which stimulates an activation signaling pathway upon binding to the first antigen and a chimeric costimulatory receptor (CCR) that recognizes a second antigen to provide the costimulatory signal, can eliminate the cancer cells expressing both antigens rather than either antigen alone. This dual receptor pattern provides a safe path to restricting the activity of engineered T cell in vitro and in vivo. For lower left and lower middle, the split CAR-T cell with two physically separate ports exert the therapeutic functions in the presence of tumor antigens and a heterodimerizing small molecule, AP21967. For lower right, bispecific antibodies are used as a switch to control the interaction between the cancer cell and CAR-T cell. The lower three strategies are similar, in which the cytotoxicity of CAR-T cell are dependent upon presence of exogenous molecule and tumor antigens
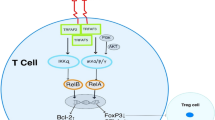
Combinatorial strategy for therapeutic T cell that integrates activation signaling pathway and inhibitory signaling pathway. In the promotion of the activation signaling pathway, this design conformation of the modified-T cell can yield safer therapeutic function upon the safe platform, including the dual receptor engineering T cell and bifunctional molecules. In this system, the T cell that carry two receptors can engage the molecules and tumor surface antigens, and the bifunctional molecule can target T cells and cancer cells. On the other hand, blocking the inhibitory signaling pathway by adding some monoclonal antibodies, such as tremelimumab and nivolumab with specificity for CTLA-4 and PD-1, respectively, was also found to improve the efficacy and persistence of the infused CAR-T cell in vitro and in vivo
Strategies to improve the safety and efficacy of CAR modified-T cells
Suicide gene
A safe and efficient means of resolving these adverse effects is the incorporation of suicide genes into the engineered T cells. In this approach, a molecule inciting the cells to apoptosis is administered in an adverse event for killing the transduced-T cell with a suicide-gene product. Based on the previous reports, there are, to date, two types of the suicide genes that have been integrated into CAR-T cells. Initial research into this field showed the herpes simplex virus thymidine kinase (HSV-TK) to be expressed in donor T cells, which has shown noticeable function as a safety switch in clinical trials for cellular therapies [65, 66]. The cell death caused by this suicide strategy, however, may take a long time and remove some of the engineered T cells resulting from the functional mechanism related to blockage of the DNA synthesis [67, 68]. Nevertheless, because it is derived from the virus, HSV-TK may be immunogenic in humans [69].
With the advance in suicide-gene technology, a suicide system based on an inducible caspase-9 (iCasp9) protein was activated through a specific chemical molecule, which has been shown to be safe in vivo [70]. This “safety switch” can be inveterately and efficiently expressed in human T lymphocytes and facilitate the maintenance of natural phenotype and antigen specificity [71–73]. In this suicide system, the suicide gene is composed of the sequence of the FK506-binding protein with an F36V mutation (FKBP12-F36V) that has a high affinity to a small-molecule, AP1903, and a gene encoding human caspase 9 switch (Fig. 2 upper left) [73]. Experiments show that iCasp9 suicide gene can induce approximately 99% of transduced cells apoptosis in vitro and in vivo with a 10 nM dose of AP1903 [73]. The iCasp9-based cell has some potential advantages over the HSV-TK suicide gene for cellular therapy [74]. For instance, the iCasp9 system is humanized through the involvement of a human gene, resulting in the low potential immunogenicity. Furthermore, administration of the exogenous AP1903 has an effective and specific ability to eliminate the cell with a transgene expression rather than untransduced cells. A novel 4th generation CD30 CAR-T cell engineered with a self-withdrawal mechanism (FKBP-iCasp9) has shown both efficacy and safety in lymphoma patients in clinical trial NCT02274584. Another clinical case on iCasp9 suicide switch, NCT02414269, the purpose of this phase I study is to test the safety of different doses of the mesothelin-targeted CAR-T cell in patients with malignant pleural disease. Although this method is an important part of the toolbox for engineering therapeutic T cells, it has several intrinsic defects [75, 76]. The suicide switches thoroughly abort the activity of infused cell with a complex and expensive manufacture in an irreversible fashion. In addition, the suicide gene in some modified cells may not act quickly enough to eliminate the off-tumor toxicity during initial cell transfer in vivo because the death of target cells may take several minutes after drug administration.
Inhibitory chimeric antigen receptor
An effective strategy for the moderation of immunotoxicity derived from the therapeutic cells depends upon the use of exogenous inhibitors that possess some cytostatic or cytotoxic effects for CAR-T cells, such as corticosteroids [77]. However, this user control approach fails to discriminate between beneficial and deleterious T cell functions in some clinical trials. Additionally, exogenous drugs may trigger several other adverse effects, especially severe organ damage [78]. Next, the negative regulatory receptors engineering strategies, such as PD-1 and CTLA-4 immune inhibitor receptors, which can mitigate the cytotoxicity responses of CAR-T cell when a specific “protect me” ligand presented only on the healthy tissue is recognized and captured (Fig. 2 upper middle) [79]. In general, the inhibitory receptors can be upregulated to regulate the number of activated T cell when the immune responses were activated. Based on the mechanism of immune inhibitory receptors, Fedorov et al. described an inhibitory chimeric antigen receptor (iCAR) that can override the T cell responses, and results demonstrated that the iCARs can specifically hinder T cell behaviors from activation, proliferation, and cytokine secretion via the surface TCR or CAR domain with targeting tumor antigen [3, 79, 80].
To prevent the unwanted effects of T cell therapy, the iCAR-engineered T cell can selectively produce cytotoxicity only when the activating receptor comes into contact with tumor antigen, and then transition to a resting state when the inhibitory receptor is targeted with the antigens that are only presented on normal tissues. Notably, this method is a powerful brake for T cells because the inhibitory receptors specifically expressed on activated T cell are mobilized to effectively limit T cell responses. Generally, this genetically engineered receptor regulates T cell responses in an antigen-selective manner. However, unlike antibody-mediated checkpoint blockade, the iCAR-T cells cannot control their spatio-temporal activity.
Dual-antigen receptor
Another means of increasing the safety of engineered T cell can be demonstrated upon coexpression of two antigen receptors to target two different tumor-associated antigens (Fig. 2 upper right) [81]. Because of the rarity of real tumor specific antigens, to date, some engineering strategies that can specifically identify a tumor-specific antigen through an engineered CAR or TCR are restricted to apply for killing tumor, especially in solid-tumor cancers. Therefore, the dual-antigen receptor of engineered T cell module has been reported to have less intense side effects even in the absence of a truly tumor-restricted antigen. In previous studies, two types of this module have been characterized in combinatorial recognition manner [82, 83]. Here, we make a conclusion about their advantages and describe the different mechanisms of the killing pathway. First, based on the mechanism of antigen encounter and the stage of T cell activation, the dual pathway from the interaction between T cell and antigen-presenting cell is required for full activation of the T cells [84]. The first signal pathway involves tumor antigen recognition through the extracellular TCR complex and the activation signal transduction with the CD3ζ domain. If the engineered T cells have only the first signal upon the TCR-CD3ζ, however, the activation and durability of T cell are usually transient, such as the first generation of CAR-T cell. The complementary signal pathway provided by costimulatory molecules on antigen presenting cells promotes the survival and expansion of the modified-T cell. The split module of signal pathway helps the T cell control tumor accurately as a result of the dual targeting approach [82, 85]. In 2012, Kloss and coworkers presented a tumor-sensing approach to successfully redirect the T cell specificity for a tumor tissue in the absence of a truly tumor-specific antigen. In this way, the dual-receptor T cell can secrete cytokines and exhibit cytotoxicity once the CAR encounters the first antigen and a chimeric costimulatory receptor (CCR) targeting another antigen [82]. To investigate the antitumor activity of this engineered-T cell for prostate tumors expressing prostate stem cell antigen (PSCA), prostate-specific membrane antigen (PSMA) or both antigens in vivo, they intravenously injected the dual-receptor T cell showing CAR and CCR with a specificity for PSCA and PSMA, respectively, and then tested only for the robust proliferation and tumor eradication in mice bearing the double-positive tumors. The treatment resulted in complete long-term survival rather than in single-positive tissues. However, this tumor-sensing method may have a potential “on-target, off-tumor” effect due to the modified-T cell activated by dual-target tumor can eliminate the normal tissue expressing single tumor-associated antigen specificity for the CAR [86].
Synthetic Notch (synNotch) receptors were developed by Roybal et al. to serve as a general platform for generating novel cell-cell communications toward the purpose of producing a safer and more effective dual-receptor T cell. Results were made public in two recent reports in Cell [83, 87]. In this system, activating T cells requires two mechanical processes. First, the synNotch receptor can release a transcription factor to control a CAR expression when the T cell recognizes the first antigen, the tissue-specific antigen. Secondly, the CAR targeting the second antigen causes the T cell to enter a state in which the effect cell has a high activation, proliferation, and cytotoxicity for target cells. On account of the SynNotch receptor to gate and confine the expression of the CARs, it increases the landscape of targetable antigens for CARs and simultaneously reduces the toxicity derived from the use of conventional CARs [88]. This makes this is a unique way in which the synNotch receptors and CARs could improve the therapeutic ability of T cell to identify tumor sites with a high specificity and accuracy.
ON-switch CAR
With the advance of strategies for controlling the timing and intensity of engineered T cells, Wu and colleagues recently proposed a complementary positive-regulation manner that the therapeutic cell, termed an ON-switch CAR-T cell, can eliminate target cells bearing cognate antigens in the presence of an exogenous molecule, rapamycin analog AP21967 (Fig. 2 lower left) [89]. Unlike other positive regulations, this ON-switch structure can target the tumor tissue attributed to the CAR with a specificity to tumor antigens, facilitating the gradual titration of therapeutic activity to appropriate levels by varying the concentration of the molecules, and regulate the timing of T cell activation through addition or removal of the small molecule in order to mitigate some severe toxicities. They have constructed the ON-switch CAR with split synthetic receptor system in which the first part of the receptor mainly contains an antigen binding domain, scFv, and another part features two downstream signaling elements, CD3ζ and 4-1BB. In this way, the immunoreactivity of therapeutic cell depends upon the tumor antigen and a small molecule.
Similar to this design, Juillerat et al. describe a method to construct “transient” CAR-T cells with a new CAR architecture that is directly dimerized at the hinge domain with addition of the specific molecule (Fig. 2 lower middle) [90]. Finally, they confirmed that the convenient-to-operate strategy mentioned in their report can offer a basic platform to use alternative split-CARs and show a safer way toward the development of the engineered CAR-T cell. In summary, the type of exogenous control behavior based on small molecules here relates to the general principle of integrating autonomous signals with input control. The ON-switch CAR and transient CAR can be implemented for the modified-T cell resulting in ultimately altering conventional T cells into smart T cells whose therapeutic behaviors are precise and effective and subject to user control.
Bifunctional molecules as switches
With the rapid development of the bispecific antibodies in cancer therapy, using the bifunctional molecule as a switch to control the activity of T lymphocytes is another exogenous approach to enhance the safety and efficacy of infused immune cells. In the field of immune cell therapy, the bispecific antibodies can be developed as an efficacious bridge to recruit the cytotoxic T cells to kill cancer cells while simultaneously targeting CD3 molecules of T cell and tumor-associated antigen presented on cancer cell surface, resulting in T cell activation and then the destruction of the target cell (Fig. 2 lower right) [91]. This novel design may ultimately overcome some hurdles for the safety of current CAR-T cell immunotherapy and provide a promising approach to improve treatment effects. The results of research performed by Sun et al. show that the anti-CD19/CD3 bispecific T cell engager (BiTE) has had some encouraging clinical effects [92]. Then the anti-CD20/CD3 BiTE was also constructed using “knobs-into-holes” technique. Some efficacy studies in murine models demonstrate that the anti-CD20/CD3 molecule can kill B cells and show a high specificity for both effector and target cells even at low doses. The principal mechanistic features of the anti-CD20/CD3 described in their report involve broad activity against malignant B cells with very low CD20 expression levels, and the cytotoxicity for target cell through the granzyme and perforin pathway of T cell. Kim and collaborators have provided another form of bifunctional molecule, consisting of folate coupled with fluorescein isothiocyanate (folate-FITC), which can redirect and regulate the activity of the FITC-specific CAR-T cells toward tumor cells with folate receptors (FR) [93]. In their experiment, this switchable platform has shown a high specificity for FR-positive cells with no activity against FR-negative cells, which demonstrates the specific redirection of the CAR-T cell by folate-FITC molecule. Finally, they confirmed that the cytotoxicity of the modified-T cell is strictly dependent on the presence of both folate-FITC molecule and FR-positive cells, and the CAR-T cells eventually eliminate the target cells with this bifunctional molecule in a concentration-dependent manner.
The latest research papers on a peptide-specific switchable CAR-T (sCAR-T) published in PNAS have depicted a new engineered-T system which the sCAR-T cell, switch and target cell can assemble in a spatio-temporal control manner [94, 95]. In vivo in case of B-cell leukemia, the activation, tissue-homing, and cytokine release of sCAR-T cells can be controlled upon administration of small switches consisting of a peptide neo-epitope (PNE), and a scFv specificity for target antigen, such as PNE-CD19 and PNE-CD22. The ability of another universal CAR-T, called anti-FITC CAR-T cell, was confirmed using the FITC-CD19 molecule or FITC-CD20 molecule in xenograft models, resulting in potent, dose-dependent antitumor activity, and slight toxicity. In conclusion, the versatile strategy related to many different tumor antigens with a single-scFv CAR-T cell should be an effective means of surmounting the tumor escape variants and heterogeneity, and can also simplify manufacturing of engineered-T cells for different tumor antigens, which greatly reduces expense [96].
Conclusions and perspectives
Through some clinical trials on the impressive activity of the modified-T cells, such as ClinicalTrials.gov nos. NCT01029366, NCT02030847, and NCT02388828, this cell-based therapy has been given high expectations for tumor eradication. Even though successful tumor eradication, this form of immunotherapy can cause some systemic life-threatening adverse effects, such as CRS and “on-target, off-tumor” toxicity. In some previous reports, strategies toward amelioration of the adverse effects derived from the CAR-T cell therapy in patients with some serious diseases can be divided into two categories. First, some forms of the CAR structure and autocrime cytokines have been developed to enhance the CAR-T cell abilities for its specific recognition and homing. On the other hand, the timing, dose, and location of the CAR-T cell therapy can be precisely regulated in vitro and in vivo upon addition of some molecules that are used as a switch and rheostat to achieve a remote control for therapeutic T cells.
In this review, the forms of cellular control represent autonomous control (e.g., tumor antigens), user control (e.g., small molecules), or both. This strategy toward improving the safety of CAR-T cell therapy involves the use of active molecules, including dimerization molecules and bifunctional molecules. This exogenous approach to cellular regulation is likely to become increasingly safe and effective, and also allows for more precise control over the timing and location of the immune response. With advances in the dual-receptor paradigm in the field of cell therapy, the new mode of synthetic combinatorial system that includes the dual-receptor CAR with the bifunctional molecules comprising targeted molecule and antitumor molecule may provide an important platform for producing more controllable cellular therapeutic T cells (Fig. 3). The switchable dual-receptor CAR-T characterized above, termed sdCAR-T, may have some advantages with bifunctional molecule for antitumor effect. Firstly, the immune response of sdCAR-T cell against the tumor tissue is dependent on the bifunctional molecule as a switch to simultaneously redirect the tumor cell and molecule-specific CAR-T cell. Second, the therapeutic activity of the dual-receptor CAR-T cell can be titratable by varying concentration of the bifunctional molecule, resulting in precision-control of the therapeutic T cell. Third, the durability of the CAR-T therapeutic effects is greatly increased due to the bifunctional molecules with a long half-life in vivo and in vitro relative to the constitutive single-molecule. Finally, the overall antitumor activity of this combinatorial system will be improved associated with the exogenous molecule carrying the ability to suppress tumor growth. In this way, the combinatorial therapy, a promising pattern of the cell therapy in the war against cancer, now raises the possibility that the tumor cells could be killed based on autonomous tumor antigens and active molecules. This method may have better safety and efficacy than current CAR-T cell.
Conclusively, the cancer biotherapy, including the adoptive immunotherapy with genetically modified T cells and immune checkpoint blockade therapy, has produced better antitumor response than other therapies. Integration therapy involving modified-T cells and immune checkpoint blockade may be an effective means of ultimately eliminating tumor cells (Fig. 3). The switchable CAR-T cells have a controllable cytotoxicity for mitigating tumor burden with the bifunctional molecules. Additionally, using monoclonal antibodies to target the immune checkpoints may produce more efficient T cell behavior. In this way, this combinatorial control system may provide a valuable insight for further refining spatio-temporal control of CAR-T cell therapy.
Abbreviations
- ACT:
-
Adoptive cells transfer
- BiTE:
-
Bispecific T cell engager
- CAIX:
-
Carboxyanhydrase-IX
- CAR:
-
Chimeric antigen receptor
- CCR:
-
Chimeric costimulatory receptor
- CRS:
-
Cytokine release syndrome
- CTC:
-
Common toxicity criteria
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen 4
- ECM:
-
Extracellular matrix
- FITC:
-
Fluorescein isothiocyanate
- FKBP12-F36V:
-
FK506-binding protein with an F36V mutation
- FR:
-
Folate receptor
- GvHD:
-
Graft-versus-host disease
- HPSE:
-
Heparanase
- HSV-TK:
-
Herpes simplex virus thymidine kinase
- iCasp9:
-
Inducible caspase-9
- IFN-γ:
-
Interferon γ
- IL-12:
-
Interleukin 12
- IL-6:
-
Interleukin 6
- ITAMs:
-
Immunoreceptor tyrosine activation motifs
- MHC:
-
Major histocompatibility complex
- PD-1:
-
Programmed death 1
- PNE:
-
Peptide neo-epitope
- PSCA:
-
Prostate stem cell antigen
- PSMA:
-
Prostate-specific membrane antigen
- sCAR:
-
Switchable CAR
- scFv:
-
Single-chain antibody fragments
- sdCAR:
-
Switchable dual-receptor CAR
- synNotch:
-
Synthetic notch
- TCR:
-
T cell receptor
References
Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5.
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81.
Morrison C. CAR-T field booms as next-generation platforms attract big players. Nat Biotechnol. 2015;33:571–2.
Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8.
Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, et al. Trial watch: adoptive cell transfer immunotherapy. Oncoimmunology. 2012;1:306–15.
Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60.
Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81.
Mochizuki K, Meng L, Mochizuki I, Tong Q, He S, Liu Y, et al. Programming of donor T cells using allogeneic δ-like ligand 4-positive dendritic cells to reduce GVHD in mice. Blood. 2016;127:3270–80.
Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–58.
Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–23.
Kersh EN, Shaw AS, Allen PM. Fidelity of T cell activation through multistep T cell receptor ζ phosphorylation. Science. 1998;281:572–5.
Davis MM, Krogsgaard M, Huppa JB, Sumen C, Purbhoo MA, Irvine DJ, et al. Dynamics of cell surface molecules during T cell recognition. Annu Rev Biochem. 2003;72:717–42.
Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66.
Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, et al. Human TCR-binding affinity is governed by MHC class restriction. J Immunol. 2007;178:5727–34.
Birnbaum ME, Berry R, Hsiao YS, Chen Z, Shingu-Vazquez MA, Yu X, et al. Molecular architecture of the αβ T cell receptor-CD3 complex. Proc Natl Acad Sci U S A. 2014;111:17576–81.
Harris DT, Kranz DM. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol Sci. 2015;37:220–30.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8.
Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–3.
Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21:687–92.
Posey AD, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, et al. Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 2016;44:1444–54.
Barrett DM, Grupp SA, June CH. Chimeric antigen receptor- and TCR-modified T cells enter main street and wall street. J Immunol. 2015;195:755–61.
Figueroa JA, Reidy A, Mirandola L, Trotter K, Suvorava N, Figueroa A, et al. Chimeric antigen receptor engineering: a right step in the evolution of adoptive cellular immunotherapy. Int Rev Immunol. 2015;34:154–87.
Srivastava S, Riddell SR. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494–502.
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38.
Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35.
Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–98.
Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65:333–47.
Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–50.
Pegram HJ, Park JH, Brentjens RJ. CD28z CARs and armored CARs. Cancer J. 2014;20:127–33.
Zhao Z, Condomines M, van der Stegen SJ, Perna F, Kloss CC, Gunset G, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T Cells. Cancer Cell. 2015;28:415–28.
Han EQ, Li XL, Wang CR, Li TF, Han SY. Chimeric antigen receptor-engineered T cells for cancer immunotherapy: progress and challenges. J Hematol Oncol. 2013;6:47.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33.
Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR–T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med. 2012;18:377–84.
Davila ML, Bouhassira DC, Park JH, Curran KJ, Smith EL, Pegram HJ, et al. Chimeric antigen receptors for the adoptive T cell therapy of hematologic malignancies. Int J Hematol. 2014;99:361–71.
Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–706.
Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4:e994446.
Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–41.
Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21:524–9.
Ramani VC, Zhan F, He J, Barbieri P, Noseda A, Tricot G, et al. Targeting heparanase overcomes chemoresistance and diminishes relapse in myeloma. Oncotarget. 2016;7:1598–607.
June CH, Maus MV, Plesa G, Johnson LA, Zhao Y, Levine BL, et al. Engineered T cells for cancer therapy. Cancer Immunol Immun. 2014;63:969–75.
Xu XJ, Tang YM. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Cancer Lett. 2014;343:172–8.
Lee DW, Stetler-Stevenson M, Yuan CM, Fry TJ, Shah NN, Delbrook C, et al. Safety and response of incorporating CD19 chimeric antigen receptor T cell therapy in typical salvage regimens for children and young adults with acute lymphoblastic leukemia. Blood. 2015;126:684.
Hu Y, Sun J, Wu Z, Yu J, Cui Q, Pu C, et al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J Hematol Oncol 2016;9:70.
Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28.
Magee MS, Snook A. Challenges to chimeric antigen receptor (CAR)-T cell therapy for cancer. Discov Med. 2014;18:265–71.
Kershaw MH, Westwood JA, Slaney CY, Darcy PK. Clinical application of genetically modified T cells in cancer therapy. Clin Transl Immunol. 2014;3:e16.
Xu XJ, Zhao HZ, Tang YM. Efficacy and safety of adoptive immunotherapy using anti-CD19 chimeric antigen receptor transduced T-cells: a systematic review of phase I clinical trials. Leuk Lymphoma. 2013;54:255–60.
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28.
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95.
Gross G, Eshhar Z. Therapeutic potential of T cell chimeric antigen receptors (CARs) in cancer treatment: counteracting off-tumor toxicities for safe CAR T cell therapy. Annu Rev Pharmacol Toxicol. 2016;56:59–83.
Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13:525–41.
Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46.
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor–transduced T cells. Blood. 2012;119:2709–20.
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51.
Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–12.
Turtle CJ, Hudecek M, Jensen MC, Riddell SR. Engineered T cells for anti-cancer therapy. Curr Opin Immunol. 2012;24:633–9.
Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther. 2013;21:2122–9.
Fedorov VD, Sadelain M, Kloss CC. Novel approaches to enhance the specificity and safety of engineered T cells. Cancer J. 2014;20:160–5.
Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–24.
Tiberghien P, Reynolds CW, Keller J, Spence S, Deschaseaux M, Certoux J, et al. Ganciclovir treatment of herpes simplex thymidine kinase-transduced primary T lymphocytes: an approach for specific in vivo donor T-cell depletion after bone marrow transplantation? Blood. 1994;84:1333–41.
Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009;10:489–500.
Tiberghien P, Ferrand C, Lioure B, Milpied N, Angonin R, Deconinck E, et al. Administration of herpes simplex-thymidine kinase-expressing donor T cells with a T-cell-depleted allogeneic marrow graft. Blood. 2001;97:63–72.
Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–23.
Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–54.
Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–802.
Ramos CA, Asgari Z, Liu E, Yvon E, Heslop HE, Rooney CM, et al. An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells. 2010;28:1107–15.
Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83.
Gargett T, Brown MP. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol. 2014;5:235.
Zhou X, Dotti G, Krance RA, Martinez CA, Naik S, Kamble RT, et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125:4103–13.
Yagyu S, Hoyos V, Del Bufalo F, Brenner MK. An inducible caspase-9 suicide gene to improve the safety of therapy using human induced pluripotent stem cells. Mol Ther. 2015;23:1475–85.
Akpek G, Lee SM, Anders V, Vogelsang GB. A high-dose pulse steroid regimen for controlling active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7:495–502.
Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61.
Fedorov VD, Themeli M, Sadelain M. PD-1– and CTLA-4–based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172.
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53.
Seton-Rogers S. Two antigens are better than one. Nat Rev Cancer. 2016;16:128–9.
Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–5.
Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–9.
Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat Biotechnol. 2002;20:70–5.
Liu JC, Voisin V, Bader GD, Deng T, Pusztai L, Symmans WF, et al. Seventeen-gene signature from enriched Her2/Neu mammary tumor-initiating cells predicts clinical outcome for human HER2+: ERα− breast cancer. Proc Natl Acad Sci U S A. 2012;109:5832–7.
Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32:1059–70.
Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164:780–91.
Irvine DJ. A Receptor for All Occasions. Cell. 2016;164:599–600.
Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule–gated chimeric receptor. Science. 2015;350:aab4077.
Juillerat A, Marechal A, Filhol JM, Valton J, Duclert A, Poirot L, et al. Design of chimeric antigen receptors with integrated controllable transient functions. Sci Rep. 2016;6:18950.
Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol. 2013;17:385–92.
Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, et al. Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 2015;7:287ra70.
Kim CH, Axup JY, Lawson BR, Yun H, Tardif V, Choi SH, et al. Bispecific small molecule-antibody conjugate targeting prostate cancer. Proc Natl Acad Sci U S A. 2013;110:17796–801.
Kim MS, Ma JS, Yun H, Cao Y, Kim JY, Chi V, et al. Redirection of genetically engineered CAR-T cells using bifunctional small molecules. J Am Chem Soc. 2015;137:2832–5.
Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A. 2016;113:E459–68.
Ma JS, Kim JY, Kazane SA, Choi SH, Yun HY, Kim MS, et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A. 2016;113:E450–8.
Acknowledgements
Not applicable.
Funding
This work was supported by the Project Program of State Key Laboratory of Natural Medicines (no. SKLNMBZ201403) and the National Science and Technology Major Projects of New Drugs (nos. 2012ZX09103301-004 and. 2014ZX09508007) in China. This project was also funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data and materials
The material supporting the conclusion of this review has been included within the article.
Authors’ contributions
EZ designed and drafted the manuscript. HX discussed and revised the manuscript. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
This is not applicable for this review.
Ethics approval and consent to participate
This is not applicable for this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, E., Xu, H. A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J Hematol Oncol 10, 1 (2017). https://doi.org/10.1186/s13045-016-0379-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-016-0379-6