Abstract
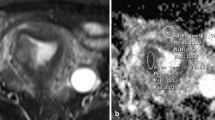
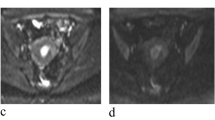
To determine the diagnostic performance of mean ADC values in the characterization of endometrial carcinoma (EC) from benign lesions by systematic review of the literature and performing meta-analysis. A systematic search of major electronic bibliographic databases was performed to find studies that used ADC values for differentiating EC from benign lesions. Two reviewers independently screened the titles and abstracts of the search results and then by reading the full texts selected the pertinent studies for final analyses. A bivariate random-effects model with pooled sensitivity and specificity values with 95% CI (confidence interval) was used. Summary receiver operating characteristic (SROC) curve and area under curve (AUC) were created. Between-study heterogeneity was measured using I squared (I2) index. Eleven studies including 269 ECs and 208 benign lesions were analyzed. Pooled average (95% CI) ADC in EC and benign lesions groups were, respectively, 0.82 (0.77–0.87) × 10–3 mm2/s and 1.41 (1.29–1.52) × 10–3 mm2/s. The combined (95% CI) sensitivity and specificity of mean ADC values for differentiating EC from benign lesions were 93% (87–96%; I2 = 41.19%) and 94% (88–97%; I2 = 46.91%), respectively. The AUC (95% CI) of the SROC curve was 98% (96–99%). ADC values had good diagnostic accuracy for differentiating EC from benign lesions. In order to recommend ADC measurement for detecting endometrial lesions in routine clinical practice, more primary studies, especially trials and comparative studies including hysteroscopically-guided biopsy method, with larger sample sizes are still required.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DW-MRI:
-
Diffusion-weighted MRI
- EC:
-
Endometrial carcinoma
- SROC:
-
Summary receiver operating characteristic
- AUC:
-
Area under curve
- CI:
-
Confidence interval
References
Cramer DW (2012) The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am 26:1-12. https://doi.org/10.1016/j.hoc.2011.10.009
Narice BF, Delaney B, Dickson JM (2018) Endometrial sampling in low-risk patients with abnormal uterine bleeding: a systematic review and meta-synthesis. BMC Fam Pract 19:135. https://doi.org/10.1186/s12875-018-0817-3
Svirsky R, Smorgick N, Rozowski U et al (2008) Can we rely on blind endometrial biopsy for detection of focal intrauterine pathology? Am J Obstet Gynecol 199:115.e1-3. https://doi.org/10.1016/j.ajog.2008.02.015
Vermoolen MA, Kwee TC, Nievelstein RA (2012) Apparent diffusion coefficient measurements in the differentiation between benign and malignant lesions: a systematic review. Insights Imaging 3:395-409. https://doi.org/10.1007/s13244-012-0175-y
Manfredi R, Gui B, Maresca G, Fanfani F, Bonomo L (2005) Endometrial cancer: magnetic resonance imaging. Abdom Imaging 30:626-636. https://doi.org/10.1007/s00261-004-0298-9
Beddy P, Moyle P, Kataoka M et al (2012) Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 262:530-537. https://doi.org/10.1148/radiol.11110984
Jacobs MA, Pan L, Macura KJ (2009) Whole-body diffusion-weighted and proton imaging: a review of this emerging technology for monitoring metastatic cancer. Semin Roentgenol 44:111-122. https://doi.org/10.1053/j.ro.2009.01.003
Kang SK, Zhang A, Pandharipande PV, Chandarana H, Braithwaite RS, Littenberg B (2015) DWI for Renal Mass Characterization: Systematic Review and Meta-Analysis of Diagnostic Test Performance. AJR Am J Roentgenol 205:317-324. https://doi.org/10.2214/ajr.14.13930
Caravan I, Ciortea CA, Contis A, Lebovici A (2018) Diagnostic value of apparent diffusion coefficient in differentiating between high-grade gliomas and brain metastases. Acta Radiol 59:599-605. https://doi.org/10.1177/0284185117727787
Kim JY, Suh HB, Kang HJ et al (2016) Apparent diffusion coefficient of breast cancer and normal fibroglandular tissue in diffusion-weighted imaging: the effects of menstrual cycle and menopausal status. Breast Cancer Res Treat 157:31-40. https://doi.org/10.1007/s10549-016-3793-0
Shen G, Jia Z, Deng H (2016) Apparent diffusion coefficient values of diffusion-weighted imaging for distinguishing focal pulmonary lesions and characterizing the subtype of lung cancer: a meta-analysis. Eur Radiol 26:556-566. https://doi.org/10.1007/s00330-015-3840-y
Suh CH, Yun SJ, Jin W, Lee SH, Park SY, Ryu CW (2018) ADC as a useful diagnostic tool for differentiating benign and malignant vertebral bone marrow lesions and compression fractures: a systematic review and meta-analysis. Eur Radiol 28:2890-2902. https://doi.org/10.1007/s00330-018-5330-5
Pi S, Cao R, Qiang JW, Guo YH (2018) Utility of DWI with quantitative ADC values in ovarian tumors: a meta-analysis of diagnostic test performance. Acta Radiol 59:1386-1394. https://doi.org/10.1177/0284185118759708
Kim HJ, Lee SY, Shin YR, Park CS, Kim K (2016) The value of diffusion-weighted imaging in the differential diagnosis of ovarian lesions: a meta-analysis. PLoS One 11:e0149465. https://doi.org/10.1371/journal.pone.0149465
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529-536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58:982-990. https://doi.org/10.1016/j.jclinepi.2005.02.022
Deeks JJ (2001) Systematic reviews of evaluations of diagnostic and screening tests. In: Egger M, Davey-Smith G, Altman D (ed) Systematic Reviews in Healt Care: Meta analysis in Context, 2nd edn. BMJ Puublishing Group, Londonn, pp 248-282.
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557-560. https://doi.org/10.1136/bmj.327.7414.557
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58:882-893. https://doi.org/10.1016/j.jclinepi.2005.01.016
Chen Y, Cheng J, Bai J, Zhang Y, Xue K, Zhang C (2017) DWI and dynamic contrast-enhanced MRI in differential diagnosis of stage-I a endometrial carcinomas and endometrial polyps. Chin J Med Imaging Technol 33:70-74
Davarpanah AH, Kambadakone A, Holalkere NS, Guimaraes AR, Hahn PF, Lee SI (2016) Diffusion MRI of uterine and ovarian masses: identifying the benign lesions. Abdom Radiol (NY) 41:2466-2475. https://doi.org/10.1007/s00261-016-0909-2
Elsammak A, Shehata S, Abulezz M, Gouhar G (2017) Efficiency of diffusion weighted magnetic resonance in differentiation between benign and malignant endometrial lesions. Egypt J Radiol Nucl Med 48:751-759. https://doi.org/10.1016/j.ejrnm.2017.02.008
Fujii S, Matsusue E, Kigawa J et al (2008) Diagnostic accuracy of the apparent diffusion coefficient in differentiating benign from malignant uterine endometrial cavity lesions: initial results. Eur Radiol 18:384-389. https://doi.org/10.1007/s00330-007-0769-9
Gharibvand MM, Ahmadzadeh A, Asadi F, Fazelinejad Z (2019) The diagnostic precision of apparent diffusion coefficient (ADC) in grading of malignant endometrial lesions compared with histopathological findings. J Family Med Prim Care 8:3372-3378. https://doi.org/10.4103/jfmpc.jfmpc_142_19
Karakas O, Karakas E, Dogan F et al (2015) Diffusion-weighted MRI in the differential diagnosis of uterine endometrial cavity tumors. Wien Klin Wochenschr 127:266-273. https://doi.org/10.1007/s00508-015-0709-7
Kececi IS, Nural MS, Aslan K, Danaci M, Kefeli M, Tosun M (2016) Efficacy of diffusion-weighted magnetic resonance imaging in the diagnosis and staging of endometrial tumors. Diagn Interv Imaging 97:177-186. https://doi.org/10.1016/j.diii.2015.06.013
Keriakos NN, Darwish E (2018) Diffusion weighted imaging in suspicious uterine tumors; how efficient is it? Egypt J Radiol Nucl Med 49:838-845. https://doi.org/10.1016/j.ejrnm.2018.04.003
Mansour TMM, Ahmed YAA, Ahmed GAE (2019) The usefulness of diffusion-weighted MRI in the differentiation between focal uterine endometrial soft tissue lesions. Egypt J Radiol Nucl Med 50:102. https://doi.org/10.1186/s43055-019-0076-x
Takeuchi M, Matsuzaki K, Nishitani H (2009) Diffusion-weighted magnetic resonance imaging of endometrial cancer: differentiation from benign endometrial lesions and preoperative assessment of myometrial invasion. Acta Radiol 50:947-953. https://doi.org/10.1080/02841850903099981
Wang X, Zhao Y, Hu Y et al (2017) Evaluation and validation of the diagnostic value of the apparent diffusion coefficient for differentiating early-stage endometrial carcinomas from benign mimickers at 3T MRI. Oncotarget 8:46390-46397. https://doi.org/10.18632/oncotarget.18553
Bakir B, Sanli S, Bakir VL et al (2017) Role of diffusion weighted MRI in the differential diagnosis of endometrial cancer, polyp, hyperplasia, and physiological thickening. Clin Imaging 41:86-94. https://doi.org/10.1016/j.clinimag.2016.10.016
Cavusoglu M, Sozmen Ciliz D, Ozsoy A et al (2016) Diffusion-Weighted Mri of Postmenopausal Women with Vaginal Bleeding and Endometrial Thickening: Differentiation of Benign and Malignant Lesions. J Belg Soc Radiol 100:70. https://doi.org/10.5334/jbr-btr.1118
Kilickesmez O, Bayramoglu S, Inci E, Cimilli T, Kayhan A (2009) Quantitative diffusion-weighted magnetic resonance imaging of normal and diseased uterine zones. Acta Radiol 50:340-347. https://doi.org/10.1080/02841850902735858
Shen SH, Chiou YY, Wang JH et al (2008) Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. AJR Am J Roentgenol 190:481-488. https://doi.org/10.2214/ajr.07.2155
Wang J, Yu T, Bai R, Sun H, Zhao X, Li Y (2010) The value of the apparent diffusion coefficient in differentiating stage IA endometrial carcinoma from normal endometrium and benign diseases of the endometrium: initial study at 3-T magnetic resonance scanner. J Comput Assist Tomogr 34:332-337. https://doi.org/10.1097/RCT.0b013e3181d0f666
Kierans AS, Doshi AM, Dunst D, Popiolek D, Blank SV, Rosenkrantz AB (2016) Retrospective Assessment of Histogram-Based Diffusion Metrics for Differentiating Benign and Malignant Endometrial Lesions. J Comput Assist Tomogr 40:723-729. https://doi.org/10.1097/rct.0000000000000430
Koc Z, Erbay G, Ulusan S, Seydaoglu G, Aka-Bolat F (2012) Optimization of b value in diffusion-weighted MRI for characterization of benign and malignant gynecological lesions. J Magn Reson Imaging 35:650-659. https://doi.org/10.1002/jmri.22871
Cartayrade N, Lacombe S, Daures J-P, Chiavassa H, Viala-Trentini M (2018) Discriminant value of MRI for the diagnosis of benign and malignant endometrium in postmenopausal women with uterine bleeding or asymptomatic endometrium thickening: prospective prestudy. Imagerie de la Femme 28:196-207. https://doi.org/10.1016/j.femme.2018.07.004
Bharwani N, Miquel ME, Sahdev A et al (2011) Diffusion-weighted imaging in the assessment of tumour grade in endometrial cancer. Br J Radiol 84:997-1004. https://doi.org/10.1259/bjr/14980811
Cao K, Zhang XP, Tang J, Li J (2008) Clinical application of diffusion-weighted MRI in uterine tumors-evaluation of signal intensity and ADC values. Chin J Med Imaging Technol 8: 1231-1235.
van Hanegem N, Prins MM, Bongers MY et al (2016) The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 197:147-155. https://doi.org/10.1016/j.ejogrb.2015.12.008
Breijer MC, Visser NC, van Hanegem N et al (2016) A structured assessment to decrease the amount of inconclusive endometrial biopsies in women with postmenopausal bleeding. Int J Surg Oncol 2016:3039261. https://doi.org/10.1155/2016/3039261
Gkrozou F, Dimakopoulos G, Vrekoussis T et al (2015) Hysteroscopy in women with abnormal uterine bleeding: a meta-analysis on four major endometrial pathologies. Arch Gynecol Obstet 291:1347-1354. https://doi.org/10.1007/s00404-014-3585-x
Martinelli F, Ditto A, Bogani G et al (2017) Accuracy of pre-operative hysteroscopic guided biopsy for predicting final pathology in uterine malignancies. J Cancer Res Clin Oncol 143: 1275-1279. https://doi.org/10.1007/s00432-017-2371-0
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622-1635. https://doi.org/10.2214/ajr.06.1403
Page MJ, Higgins JPT, Sterne JAC (2019) Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (ed) Cochrane Handbook for Systematic Reviews of Interventions. 2nd edn. John Wiley & Sons, Chichester (UK), pp 349-374.
Schünemann HJ, Mustafa RA, Brozek J et al (2020) GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol 122:142-152. https://doi.org/10.1016/j.jclinepi.2019.12.021
Zhang M, Hu Z-D (2019) Suggestions for designing studies investigating diagnostic accuracy of biomarkers. Ann Transl Med 7:788-788. https://doi.org/10.21037/atm.2019.11.133
Cohen JF, Korevaar DA, Altman DG et al (2016) STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 6:e012799. https://doi.org/10.1136/bmjopen-2016-012799
Takwoingi Y, Riley RD, Deeks JJ (2015) Meta-analysis of diagnostic accuracy studies in mental health. Evid Based Ment Health 18:103-109. https://doi.org/10.1136/eb-2015-102228
Cronin P, Kelly AM, Altaee D, Foerster B, Petrou M, Dwamena BA (2018) How to perform a systematic review and meta-analysis of diagnostic imaging studies. Acad Radiol 25:573-593. https://doi.org/10.1016/j.acra.2017.12.007
Kim KW, Lee J, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. general guidance and tips. Korean J Radiol 16:1175-1187. https://doi.org/10.3348/kjr.2015.16.6.1175
Rutjes AW, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PM (2006) Evidence of bias and variation in diagnostic accuracy studies. CMAJ 174:469-476. https://doi.org/10.1503/cmaj.050090
Nalaboff KM, Pellerito JS, Ben-Levi E (2001) Imaging the endometrium: disease and normal variants. Radiographics 21:1409-1424. https://doi.org/10.1148/radiographics.21.6.g01nv211409
Sadro CT (2016) Imaging the endometrium: a pictorial essay. Can Assoc Radiol J 67:254-262. https://doi.org/10.1016/j.carj.2015.09.012
Takeuchi M, Matsuzaki K, Uehara H, Yoshida S, Nishitani H, Shimazu H (2005) Pathologies of the uterine endometrial cavity: usual and unusual manifestations and pitfalls on magnetic resonance imaging. Eur Radiol 15:2244-2255. https://doi.org/10.1007/s00330-005-2814-x
Kido A, Fujimoto K, Okada T, Togashi K (2013) Advanced MRI in malignant neoplasms of the uterus. J Magn Reson Imaging 37:249-264. https://doi.org/10.1002/jmri.23716
Takeuchi M, Matsuzaki K, Harada M (2016) Carcinosarcoma of the uterus: MRI findings including diffusion-weighted imaging and MR spectroscopy. Acta Radiol 57:1277-1284. https://doi.org/10.1177/0284185115626475
Sato K, Yuasa N, Fujita M, Fukushima Y (2014) Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol 210:368.e1-368.e8. https://doi.org/10.1016/j.ajog.2013.12.028
Lin G, Yang LY, Huang YT et al (2016) Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma/smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J Magn Reson Imaging 43:333-342. https://doi.org/10.1002/jmri.24998
Acknowledgements
The authors acknowledge Dr. Abbasali Keshtkar, Professor of Epidemiology at Tehran University of Medical Sciences, Tehran, Iran for his kind assistance in designing the study and interpretation of the results. We acknowledge Dr. Mohammad M. Gharibvand (Ahvaz Jundishapur University of Medical Sciences, Iran) and Dr. Tarek Mohamed M. Mansour (Al-Azhar University, Egypt) for their sincere help in sharing the individual patient data of their studies. The protocol of the systematic review was registered in PROSPERO (CRD42019123910).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors inform that there is no conflict of interest regarding the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
261_2020_2734_MOESM3_ESM.pdf
Supplementary Figure 1. Forest plot for the diagnostic significance of weighted mean ADC (apparent diffusion coefficient) value difference between endometrial carcinoma and benign lesions (PDF 258 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moharamzad, Y., Davarpanah, A.H., Yaghobi Joybari, A. et al. Diagnostic performance of apparent diffusion coefficient (ADC) for differentiating endometrial carcinoma from benign lesions: a systematic review and meta-analysis. Abdom Radiol 46, 1115–1128 (2021). https://doi.org/10.1007/s00261-020-02734-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02734-w