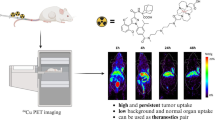
Abstract
Purpose
Triple-negative breast cancer (TNBC) has a poor prognosis due to the absence of effective therapeutic targets. Vascular endothelial growth factor (VEGF) family are expressed in 30–60% of TNBC, therefore providing potential therapeutic targets for TNBC. Aflibercept (Abe), a humanized recombinant fusion protein specifically bound to VEGF-A, B and placental growth factor (PIGF), has proven to be effective in the treatment in some cancers. Therefore, 89Zr/177Lu-labeled Abe was investigated for its theranostic role in TNBC.
Methods
Abe was radiolabeled with 89Zr and 177Lu via the conjugation of chelators. Flow cytometry and cell immunofluorescent staining were performed to evaluate the binding affinity of Abe. Sequential PET imaging and fluorescent imaging were conducted in TNBC tumor bearing mice following the injection of 89Zr-labeled Abe and Cy5.5-labeled Abe. Treatment study was performed after the administration of 177Lu-labeled Abe. Tumor volume and survival were monitored and SPECT imaging and biodistribution studies were conducted. Safety evaluation was performed including body weight, blood cell measurement, and hematoxylin–eosin (H&E) staining of major organs. Expression of VEGF and CD31 was tested by immunohistochemical staining. Dosimetry was estimated using the OLINDA software.
Results
FITC-labeled Abe showed a strong binding affinity to VEGF in TNBC 4T1 cells and HUVECs by flow cytometry and cell immunofluorescence. Tumor uptake of 89Zr-labeled Abe peaked at 120 h (SUVmax = 3.2 ± 0.64) and persisted before 168 h (SUVmax = 2.54 ± 0.42). The fluorescence intensity of the Cy5.5-labeled Abe group surpassed that of the Cy5.5-labeled IgG group, implying that Cy5.5-labeled Abe is a viable candidate monitoring in vivo tumor targeting and localization. 177Lu-labeled Abe (11.1 MBq) served well as the therapeutic component to suppress tumor growth with standardized tumor volume at 16 days, significantly smaller than PBS group (about 815.66 ± 3.58% vs 3646.52 ± 11.10%, n = 5, P < 0.01). Moreover, SPECT images confirmed high contrast between tumors and normal organs, indicating selective tumor uptake of 177Lu-labeled Abe. No discernible abnormalities in blood cells, and no evident histopathological abnormality observed in liver, spleen, and kidney. Immunohistochemical staining showed that 177Lu-labeled Abe effectively inhibited the expression of VEGF and CD31 of tumor, suggesting that angiogenesis may be suppressed by 177Lu-labeled Abe. The whole-body effective dose for an adult human was estimated to be 0.16 mSv/MBq.
Conclusion
89Zr/177Lu-labeled Abe could be a TNBC-specific marker with diagnostic value and provide insights into targeted therapy in the treatment of TNBC. Further clinical evaluation and translation may be of high significance for TNBC.







Similar content being viewed by others
Data availability
Original data are available on request.
Change history
12 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00259-023-06590-w
References
Pitt JJ, Riester M, Zheng Y, Yoshimatsu TF, Sanni A, Oluwasola O, et al. Characterization of Nigerian breast cancer reveals prevalent homologous recombination deficiency and aggressive molecular features. Nat Commun. 2018;9:4181. https://doi.org/10.1038/s41467-018-06616-0.
Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17:152–63. https://doi.org/10.2174/1871520616666160502122724.
Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293:247–69. https://doi.org/10.1007/s00404-015-3859-y.
Sukumar J, Gast K, Quiroga D, Lustberg M, Williams N. Triple-negative breast cancer: promising prognostic biomarkers currently in development. Expert Rev Anticancer Ther. 2021;21:135–48. https://doi.org/10.1080/14737140.2021.1840984.
Gianni-Barrera R, Butschkau A, Uccelli A, Certelli A, Valente P, Bartolomeo M, et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis. 2018;21:883–900. https://doi.org/10.1007/s10456-018-9634-5.
Kim W, Park A, Jang HH, Kim SE, Park KS. Breast tumor cell-stimulated bone marrow-derived mesenchymal stem cells promote the sprouting capacity of endothelial cells by promoting VEGF expression, mediated in part through HIF-1α increase. Cancers (Basel). 2022;14. https://doi.org/10.3390/cancers14194711.
Sun H, Zhang D, Yao Z, Lin X, Liu J, Gu Q, et al. Anti-angiogenic treatment promotes triple-negative breast cancer invasion via vasculogenic mimicry. Cancer Biol Ther. 2017;18:205–13. https://doi.org/10.1080/15384047.2017.1294288.
Lau DK, Mencel J, Chau I. Safety and efficacy review of aflibercept for the treatment of metastatic colorectal cancer. Expert Opin Drug Saf. 2022;21:589–97. https://doi.org/10.1080/14740338.2022.2008905.
Azanza Perea JR, Sádaba B. Aflibercept. An approach to pharmacology. Arch Soc Esp Oftalmol. 2015;90(Suppl 1):6–10. https://doi.org/10.1016/s0365-6691(15)30003-4.
Eissing T, Stewart MW, Qian CX, Rittenhouse KD. Durability of VEGF suppression with intravitreal aflibercept and brolucizumab: using pharmacokinetic modeling to understand clinical outcomes. Transl Vis Sci Technol. 2021;10:9. https://doi.org/10.1167/tvst.10.4.9.
Jiang D, Xu T, Zhong L, Liang Q, Hu Y, Xiao W, et al. Research progress of VEGFR small molecule inhibitors in ocular neovascular diseases. Eur J Med Chem. 2023;257: 115535. https://doi.org/10.1016/j.ejmech.2023.115535.
Thakur A, Chorawala MR, Patel RS. A systemic review and meta-analysis of Aflibercept plus FOLFIRI regimen as a second-line treatment for metastatic colorectal cancer: A PRISMA compliant pooled analysis of randomized controlled trials and single arm studies to assess efficacy and safety. Crit Rev Oncol Hematol. 2023;188: 104034. https://doi.org/10.1016/j.critrevonc.2023.104034.
Erol C, Sendur Mehmet AN, Bilgetekin I, Garbioglu DB, Hamdard J, Akbas S, et al. Efficacy and safety of folfiri plus aflibercept in second-line treatment of metastatic colorectal cancer: Real-life data from Turkish oncology group. J Cancer Res Ther. 2022;18:S347–53. https://doi.org/10.4103/jcrt.jcrt_1104_21.
Nakatsumi H, Komatsu Y, Muranaka T, Yuki S, Kawamoto Y, Harada K, et al. Study protocol for HGCSG1801: a multicenter, prospective, phase II trial of second-line FOLFIRI plus aflibercept in patients with metastatic colorectal cancer refractory to anti-EGFR antibodies. Front Oncol. 2022;12: 939425. https://doi.org/10.3389/fonc.2022.939425.
Raga MG, Pérez IP, Veiga RC, Sosa MM, Aguilera MJS, Rodríguez PL, et al. Maintenance of angiogenesis inhibition with aflibercept after progression to bevacizumab in metastatic colorectal cancer: real life study in the Valencian community. Clin Transl Oncol. 2023;25:1455–62. https://doi.org/10.1007/s12094-022-03047-8.
García García Y, Marín Alcalá M, Martínez VC. Anti-angiogenic therapy for ovarian cancer. EJC Suppl. 2020;15:77–86. https://doi.org/10.1016/j.ejcsup.2020.02.003.
Andersson Y, Fleten KG, Abrahamsen TW, Reed W, Davidson B, Flatmark K. Anti-angiogenic treatment in Pseudomyxoma peritonei-still a strong preclinical rationale. Cancers (Basel). 2021;13. https://doi.org/10.3390/cancers13112819.
Perez K, Kulke MH, Horick NK, Regan E, Graham C, Scheutz S, et al. A phase II study of ziv-aflibercept in patients with advanced extrapancreatic neuroendocrine tumors. Pancreas. 2022;51:763–8. https://doi.org/10.1097/mpa.0000000000002099.
Zhao P, Anami Y, Gao P, Fan X, Li L, Tsuchikama K, et al. Enhanced anti-angiogenetic effect of transferrin receptor-mediated delivery of VEGF-trap in a glioblastoma mouse model. MAbs. 2022;14:2057269. https://doi.org/10.1080/19420862.2022.2057269.
Shi H, Guo N, Zhao Z, Liu L, Ni T, Zhang J, et al. Comparison of the second-line treatments for patients with small cell lung cancer sensitive to previous platinum-based chemotherapy: a systematic review and Bayesian network analysis. Front Oncol. 2023;13:1154685. https://doi.org/10.3389/fonc.2023.1154685.
Camera S, Deleporte A, Bregni G, Trevisi E, Pretta A, Telli TA, et al. Momentum: a phase I trial investigating 2 schedules of capecitabine with aflibercept in patients with gastrointestinal and breast cancer. Clin Colorectal Cancer. 2020;19:311-8.e1. https://doi.org/10.1016/j.clcc.2020.05.007.
Wu FT, Paez-Ribes M, Xu P, Man S, Bogdanovic E, Thurston G, et al. Aflibercept and Ang1 supplementation improve neoadjuvant or adjuvant chemotherapy in a preclinical model of resectable breast cancer. Sci Rep. 2016;6:36694. https://doi.org/10.1038/srep36694.
Sideras K, Dueck AC, Hobday TJ, Rowland KM Jr, Allred JB, Northfelt DW, et al. North central cancer treatment group (NCCTG) N0537: phase II trial of VEGF-trap in patients with metastatic breast cancer previously treated with an anthracycline and/or a taxane. Clin Breast Cancer. 2012;12:387–91. https://doi.org/10.1016/j.clbc.2012.09.007.
Kang L, Li C, Yang Q, Sutherlin L, Wang L, Chen Z, et al. (64)Cu-labeled daratumumab F(ab’)(2) fragment enables early visualization of CD38-positive lymphoma. Eur J Nucl Med Mol Imaging. 2022;49:1470–81. https://doi.org/10.1007/s00259-021-05593-9.
Kang L, Jiang D, England CG, Barnhart TE, Yu B, Rosenkrans ZT, et al. ImmunoPET imaging of CD38 in murine lymphoma models using (89)Zr-labeled daratumumab. Eur J Nucl Med Mol Imaging. 2018;45:1372–81. https://doi.org/10.1007/s00259-018-3941-3.
Kang L, Li C, Rosenkrans ZT, Engle JW, Wang R, Jiang D, et al. Noninvasive evaluation of CD20 expression using (64)Cu-labeled F(ab’)(2) fragments of obinutuzumab in lymphoma. J Nucl Med. 2021;62:372–8. https://doi.org/10.2967/jnumed.120.246595.
Li C, Liu J, Yang X, Yang Q, Huang W, Zhang M, et al. Theranostic application of (64)Cu/(177)Lu-labeled anti-Trop2 monoclonal antibody in pancreatic cancer tumor models. Eur J Nucl Med Mol Imaging. 2022;50:168–83. https://doi.org/10.1007/s00259-022-05954-y.
Kang L, Li C, Rosenkrans ZT, Huo N, Chen Z, Ehlerding EB, et al. CD38-targeted theranostics of lymphoma with (89)Zr/(177)Lu-labeled daratumumab. Adv Sci (Weinh). 2021;8:2001879. https://doi.org/10.1002/advs.202001879.
Li C, Kang L, Fan K, Ferreira CA, Becker KV, Huo N, et al. ImmunoPET of CD146 in orthotopic and metastatic breast cancer models. Bioconjug Chem. 2021;32:1306–14. https://doi.org/10.1021/acs.bioconjchem.0c00649.
Li C, Yang Q, Chen Z, Qiu Y, Du Y, Wang R, et al. Noninvasive evaluation of multidrug resistance via imaging of ABCG2/BCRP multidrug transporter in lung cancer xenograft models. Mol Pharm. 2022;19:3521–9. https://doi.org/10.1021/acs.molpharmaceut.1c00939.
Du Y, Huo Y, Yang Q, Han Z, Hou L, Cui B, et al. Ultrasmall iron-gallic acid coordination polymer nanodots with antioxidative neuroprotection for PET/MR imaging-guided ischemia stroke therapy. Exploration (Beijing). 2023;3:20220041. https://doi.org/10.1002/exp.20220041.
Saatci O, Kaymak A, Raza U, Ersan PG, Akbulut O, Banister CE, et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11:2416. https://doi.org/10.1038/s41467-020-16199-4.
Yudistiro R, Hanaoka H, Katsumata N, Yamaguchi A, Tsushima Y. Bevacizumab radioimmunotherapy (RIT) with accelerated blood clearance using the avidin chase. Mol Pharm. 2018;15:2165–73. https://doi.org/10.1021/acs.molpharmaceut.8b00027.
Mery B, Rowinski E, Vallard A, Jacquin JP, Simoens X, Magné N, et al. Advocacy for a new oncology research paradigm: the model of bevacizumab in triple-negative breast cancer in a French cohort study. Oncology. 2019;97:1–6. https://doi.org/10.1159/000499583.
Korobelnik JF, Larsen M, Eter N, Bailey C, Wolf S, Schmelter T, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend for macular edema in central retinal vein occlusion: the CENTERA study. Am J Ophthalmol. 2021;227:106–15. https://doi.org/10.1016/j.ajo.2021.01.027.
Mitchell P, Holz FG, Hykin P, Midena E, Souied E, Allmeier H, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the ARIES study: a randomized clinical trial. Retina. 2021;41:1911–20. https://doi.org/10.1097/iae.0000000000003128.
Jhaveri CD, Glassman AR, Ferris FL 3rd, Liu D, Maguire MG, Allen JB, et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N Engl J Med. 2022;387:692–703. https://doi.org/10.1056/NEJMoa2204225.
Stahl A, Sukgen EA, Wu WC, Lepore D, Nakanishi H, Mazela J, et al. Effect of intravitreal aflibercept vs laser photocoagulation on treatment success of retinopathy of prematurity: the FIREFLEYE randomized clinical trial. JAMA. 2022;328:348–59. https://doi.org/10.1001/jama.2022.10564.
Malekian S, Rahmati M, Sari S, Kazemimanesh M, Kheirbakhsh R, Muhammadnejad A, et al. Expression of diverse angiogenesis factor in different stages of the 4T1 tumor as a mouse model of triple-negative breast cancer. Adv Pharm Bull. 2020;10:323–8. https://doi.org/10.34172/apb.2020.039.
Breeman WA, van der Wansem K, Bernard BF, van Gameren A, Erion JL, Visser TJ, et al. The addition of DTPA to [177Lu-DOTA0, Tyr3]octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur J Nucl Med Mol Imaging. 2003;30:312–5. https://doi.org/10.1007/s00259-002-1054-4.
Li WP, Ma DS, Higginbotham C, Hoffman T, Ketring AR, Cutler CS, et al. Development of an in vitro model for assessing the in vivo stability of lanthanide chelates. Nucl Med Biol. 2001;28:145–54. https://doi.org/10.1016/s0969-8051(00)00196-7.
Hicks RJ, Hofman MS. Is there still a role for SPECT-CT in oncology in the PET-CT era? Nat Rev Clin Oncol. 2012;9:712–20. https://doi.org/10.1038/nrclinonc.2012.188.
Sabet A, Haug AR, Eiden C, Auernhammer CJ, Simon B, Bartenstein P, et al. Efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in metastatic pulmonary neuroendocrine tumors: a dual-centre analysis. Am J Nucl Med Mol Imaging. 2017;7:74–83.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. https://doi.org/10.1084/jem.20052494.
Hecht M, Eckstein M, Rutzner S, von der Grün J, Illmer T, Klautke G, et al. Induction chemoimmunotherapy followed by CD8+ immune cell-based patient selection for chemotherapy-free radioimmunotherapy in locally advanced head and neck cancer. J Immunother Cancer. 2022;10. https://doi.org/10.1136/jitc-2021-003747.
Fu DX, Tanhehco Y, Chen J, Foss CA, Fox JJ, Chong JM, et al. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat Med. 2008;14:1118–22. https://doi.org/10.1038/nm.1864.
Sheng Y, You Y, Chen Y. Dual-targeting hybrid peptide-conjugated doxorubicin for drug resistance reversal in breast cancer. Int J Pharm. 2016;512:1–13. https://doi.org/10.1016/j.ijpharm.2016.08.016.
Chakraborty A, Hatzis C, DiGiovanna MP. Co-targeting the HER and IGF/insulin receptor axis in breast cancer, with triple targeting with endocrine therapy for hormone-sensitive disease. Breast Cancer Res Treat. 2017;163:37–50. https://doi.org/10.1007/s10549-017-4169-9.
Chen M, Lin Z, Yao G, Hong X, Xue X, Chen L. A novel NIR fluorescent nanoprobe targeting HER2-positive breast cancer: Tra-TTR-A. Bioinorg Chem Appl. 2021;2021:2495958. https://doi.org/10.1155/2021/2495958.
Sun P, Qu F, Zhang C, Cheng P, Li X, Shen Q, et al. NIR-II excitation phototheranostic platform for synergistic photothermal therapy/chemotherapy/chemodynamic therapy of breast cancer bone metastases. Adv Sci (Weinh). 2022;9: e2204718. https://doi.org/10.1002/advs.202204718.
Funding
This work was supported by the National Natural Science Foundation of China (82171970, 82202206), the Beijing Science Foundation for Distinguished Young Scholars (JQ21025), the Beijing Municipal Science & Technology Commission (Z221100007422027), Beijing Natural Science Foundation (7224365), National High Level Hospital Clinical Research Funding (Interdisciplinary Research Project of Peking University First Hospital, 2023IR17).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Animal Committee of Peking University First Hospital (No. 2022018).
Consent to participate and publication
The requirement for informed consent was waived.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors regret that the order of the names in the corresponding authors’ details section were interchanged. Dr. Kang should be listed before Dr. Li.
The original article has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Chen, Z., Qiu, Y. et al. Theranostic role of 89Zr- and 177Lu-labeled aflibercept in breast cancer. Eur J Nucl Med Mol Imaging 51, 1246–1260 (2024). https://doi.org/10.1007/s00259-023-06575-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06575-9