Abstract
Purpose
Pancreatic cancer is a malignant tumor with a high degree of malignancy, strong heterogeneity, and high lethality. Trop2 is a transmembrane glycoprotein associated with the occurrence, development, and poor prognosis of pancreatic cancer. This study aims to develop 64Cu/177Lu-labeled anti-Trop2 monoclonal antibody (hIMB1636) for positron emission tomography (PET) imaging and radioimmunotherapy (RIT) application in pancreatic cancer tumor models.
Methods
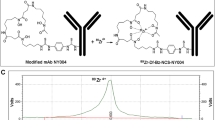
The binding kinetics of hIMB1636 to Trop2 antigen was measured by Biolayer interferometry (BLI). Western blotting was used to screen the Trop2 expression of pancreatic cancer cell lines. Flow cytometry and cell immunofluorescence were used to evaluate the binding ability of hIMB1636 and Trop2 on the cell surface. hIMB1636 were conjugated with p-SCN-Bn-NOTA (NOTA) and DOTA-NHS-ester (DOTA) for 64Cu and 177Lu radiolabeling respectively. ImmunoPET imaging and RIT studies were performed using 64Cu-NOTA-hIMB1636 and 177Lu-DOTA-hIMB1636 in subcutaneous pancreatic cancer tumor models.
Results
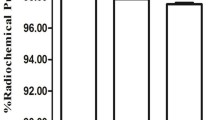
hIMB1636 had a strong binding affinity to Trop2 according to the results of BLI. The T3M-4 cell line showed the strongest expression of Trop2 and specific binding ability of hIMB1636 according to the results of Western blotting, flow cytometry, and cell immunofluorescence. The radiochemical purity of 64Cu-NOTA-hIMB1636 and 177Lu-DOTA-hIMB1636 exceeded 95%. PET imaging showed gradually an accumulation of 64Cu-NOTA-hIMB1636 in T3M-4 tumor models. The maximum tumor uptake was 8.95 ± 1.07%ID/g (n = 4) at 48 h post injection (p.i.), which had significant differences with T3M-4-blocked and PaTu8988-negative groups (P < 0.001). The high-177Lu-hIMB1636 group demonstrated the strongest tumor suppression with standardized tumor volume about 94.24 ± 14.62% (n = 5) at 14 days p.i., significantly smaller than other groups (P < 0.05). Ex vivo biodistribution and histological staining verified the in vivo PET imaging and RIT results.
Conclusions
This study demonstrated that 64Cu/177Lu-labeled hIMB1636 could noninvasively evaluate the expression level of Trop2 and inhibit the Trop2-overexpressed tumor growth in pancreatic cancer tumor models. Further clinical evaluation and translation of Trop2-targeted drug may be of great help in the stratification and management of pancreatic cancer patients.







Similar content being viewed by others
Change history
15 September 2022
The article was modified to change the order of the corresponding authors in the corresponding authors field.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71:7–33. https://doi.org/10.3322/caac.21654.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–705. https://doi.org/10.3748/wjg.v22.i44.9694.
Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6:321–37. https://doi.org/10.1177/1756283X13478680.
Wu M, Shu J. Multimodal molecular imaging: current status and future directions. Contrast Media Mol Imaging. 2018;2018:1382183. https://doi.org/10.1155/2018/1382183.
Avril S, Muzic RF Jr, Plecha D, Traughber BJ, Vinayak S, Avril N. (1)(8)F-FDG PET/CT for monitoring of treatment response in breast cancer. J Nucl Med. 2016;57(Suppl 1):34S-S39. https://doi.org/10.2967/jnumed.115.157875.
Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology. 2019;156:2024–40. https://doi.org/10.1053/j.gastro.2019.01.259.
Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY, Cai W. ImmunoPET: concept, design, and applications. Chem Rev. 2020;120:3787–851. https://doi.org/10.1021/acs.chemrev.9b00738.
Filippi L, Nervi C, Proietti I, Pirisino R, Potenza C, Martelli O, et al. Molecular imaging in immuno-oncology: current status and translational perspectives. Expert Rev Mol Diagn. 2020;20:1199–211. https://doi.org/10.1080/14737159.2020.1854090.
Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9:28989–9006. https://doi.org/10.18632/oncotarget.25615.
Zeng P, Chen MB, Zhou LN, Tang M, Liu CY, Lu PH. Impact of TROP2 expression on prognosis in solid tumors: a systematic review and meta-analysis. Sci Rep. 2016;6:33658. https://doi.org/10.1038/srep33658.
Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–5. https://doi.org/10.1038/sj.bjc.6604677.
Li C, Yang Q, Chen Z, Qiu Y, Du Y, Wang R, et al. Noninvasive evaluation of multidrug resistance via imaging of ABCG2/BCRP multidrug transporter in lung cancer xenograft models. Mol Pharm. 2022. https://doi.org/10.1021/acs.molpharmaceut.1c00939.
Yang Y, Hernandez R, Rao J, Yin L, Qu Y, Wu J, et al. Targeting CD146 with a 64Cu-labeled antibody enables in vivo immunoPET imaging of high-grade gliomas. Proc Natl Acad Sci U S A. 2015;112:E6525–34. https://doi.org/10.1073/pnas.1502648112.
Kang L, Li C, Rosenkrans ZT, Huo N, Chen Z, Ehlerding EB, et al. CD38-targeted theranostics of lymphoma with (89)Zr/(177)Lu-labeled daratumumab. Adv Sci (Weinh). 2021;8:2001879. https://doi.org/10.1002/advs.202001879.
Kang L, Li C, Rosenkrans ZT, Engle JW, Wang R, Jiang D, et al. Noninvasive evaluation of CD20 expression using (64)Cu-Labeled F(ab’)2 fragments of obinutuzumab in lymphoma. J Nucl Med. 2021;62:372–8. https://doi.org/10.2967/jnumed.120.246595.
Song IH, Lee TS, Park YS, Lee JS, Lee BC, Moon BS, et al. Immuno-PET imaging and radioimmunotherapy of 64Cu-/177lu-labeled anti-EGFR antibody in esophageal squamous cell carcinoma model. J Nucl Med. 2016;57:1105–11. https://doi.org/10.2967/jnumed.115.167155.
Li C, Kang L, Fan K, Ferreira CA, Becker KV, Huo N, et al. ImmunoPET of CD146 in orthotopic and metastatic breast cancer models. Bioconjug Chem. 2021;32:1306–14. https://doi.org/10.1021/acs.bioconjchem.0c00649.
Liu Q, Liao Q, Zhao Y. Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int. 2017;17:68. https://doi.org/10.1186/s12935-017-0437-3.
Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–48. https://doi.org/10.1038/s41575-018-0005-x.
Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84–105. https://doi.org/10.18632/genesandcancer.40.
Lenart S, Lenart P, Smarda J, Remsik J, Soucek K, Benes P. Trop2: jack of all trades, master of none. Cancers (Basel). 2020;12:3328. https://doi.org/10.3390/cancers12113328.
Pavone G, Motta L, Martorana F, Motta G, Vigneri P. A new kid on the block: sacituzumab govitecan for the treatment of breast cancer and other solid tumors. Molecules. 2021;26:7294. https://doi.org/10.3390/molecules26237294.
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–41. https://doi.org/10.1056/NEJMoa2028485.
Li S, England CG, Ehlerding EB, Kutyreff CJ, Engle JW, Jiang D, et al. ImmunoPET imaging of CD38 expression in hepatocellular carcinoma using 64Cu-labeled daratumumab. Am J Transl Res. 2019;11:6007–15.
Chen W, Li M, Younis MH, Barnhart TE, Jiang D, Sun T, et al. ImmunoPET of trophoblast cell-surface antigen 2 (Trop-2) expression in pancreatic cancer. Eur J Nucl Med Mol Imaging. 2022;49:861–70. https://doi.org/10.1007/s00259-021-05563-1.
Wang H, Li D, Liu S, Liu R, Yuan H, Krasnoperov V, et al. Small-animal PET imaging of pancreatic cancer xenografts using a 64Cu-labeled monoclonal antibody, MAb159. J Nucl Med. 2015;56:908–13. https://doi.org/10.2967/jnumed.115.155812.
Sugyo A, Tsuji AB, Sudo H, Nagatsu K, Koizumi M, Ukai Y, et al. Preclinical evaluation of (8)(9)Zr-labeled human antitransferrin receptor monoclonal antibody as a PET probe using a pancreatic cancer mouse model. Nucl Med Commun. 2015;36:286–94. https://doi.org/10.1097/MNM.0000000000000245.
Lamberts LE, Menke-van der Houven van Oordt CW, ter Weele EJ, Bensch F, Smeenk MM, Voortman J, et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody-drug conjugate treatment. Clin Cancer Res. 2016;22:1642–52. https://doi.org/10.1158/1078-0432.CCR-15-1272.
Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Arrojo R, Liu D, et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26:919–31. https://doi.org/10.1021/acs.bioconjchem.5b00223.
Mao Y, Wang X, Zheng F, Wang C, Tang Q, Tang X, et al. The tumor-inhibitory effectiveness of a novel anti-Trop2 Fab conjugate in pancreatic cancer. Oncotarget. 2016;7:24810–23. https://doi.org/10.18632/oncotarget.8529.
Nishimura T, Mitsunaga M, Sawada R, Saruta M, Kobayashi H, Matsumoto N, et al. Photoimmunotherapy targeting biliary-pancreatic cancer with humanized anti-TROP2 antibody. Cancer Med. 2019;8:7781–92. https://doi.org/10.1002/cam4.2658.
Chang CH, Gupta P, Michel R, Loo M, Wang Y, Cardillo TM, et al. Ranpirnase (frog RNase) targeted with a humanized, internalizing, anti-Trop-2 antibody has potent cytotoxicity against diverse epithelial cancer cells. Mol Cancer Ther. 2010;9:2276–86. https://doi.org/10.1158/1535-7163.MCT-10-0338.
Govindan SV, Stein R, Qu Z, Chen S, Andrews P, Ma H, et al. Preclinical therapy of breast cancer with a radioiodinated humanized anti-EGP-1 monoclonal antibody: advantage of a residualizing iodine radiolabel. Breast Cancer Res Treat. 2004;84:173–82. https://doi.org/10.1023/B:BREA.0000018417.02580.ef.
Goldsmith SJ. Targeted radionuclide therapy: a historical and personal review. Semin Nucl Med. 2020;50:87–97. https://doi.org/10.1053/j.semnuclmed.2019.07.006.
Sheridan C. Ablynx’s nanobody fragments go places antibodies cannot. Nat Biotechnol. 2017;35:1115–7. https://doi.org/10.1038/nbt1217-1115.
Funding
This work was supported by the Natural Science Foundation of Beijing Municipality (Grant numbers 7224365 and 7202133), the National Natural Science Foundation of China (Grant numbers 82171970, 81871385, 81971642, 82104052, and 82001860), the Beijing Science Foundation for Distinguished Young Scholars (Grant number JQ21025), the Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (Grant number BMU2022PY006), the Clinical Medicine Plus X-Youth Scholars Project of Peking University (Grant number PKU2020LCXQ023), the CAMS Innovation Fund for Medical Sciences (CIFMS, Grant number 2021-I2M-1-026), and the Natural Science Foundation of Shandong Province (Grant number ZR2020QH201).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Theragnostic.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Liu, J., Yang, X. et al. Theranostic application of 64Cu/177Lu-labeled anti-Trop2 monoclonal antibody in pancreatic cancer tumor models. Eur J Nucl Med Mol Imaging 50, 168–183 (2022). https://doi.org/10.1007/s00259-022-05954-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05954-y