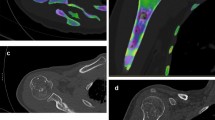
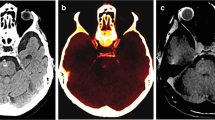
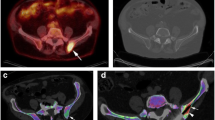
Abstract
Computed tomography (CT) is routinely used to diagnose and evaluate metastatic lesions in oncology. CT alone suffers from lack of sensitivity, especially for skeletal lesions in the bone marrow and lesions that have similar attenuation profiles to surrounding bone. Magnetic resonance imaging and nuclear medicine imaging remain the gold standard in evaluating skeletal lesions. However, compared to CT, these modalities are not as widely available or suitable for all patients. Dual energy computed tomography (DECT) exploits variations in linear attenuation coefficient of materials at different photon energy levels to reconstruct images based on material composition. DECT in musculoskeletal imaging is used in the imaging of crystal arthropathy and detecting subtle fractures, but it is not broadly utilized in evaluating infiltrative skeletal lesions. Malignant skeletal lesions have different tissue and molecular compositions compared to normal bone. DECT may exploit these physical differences to delineate infiltrative skeletal lesions from surrounding bone better than conventional monoenergetic CT. Studies so far have examined the utility of DECT in evaluating skeletal metastases, multiple myeloma lesions, pathologic fractures, and performing image-guided biopsies with promising results. These studies were mostly retrospective analyses and case reports containing small samples sizes. As DECT becomes more widely used clinically and more scientific studies evaluating the performance of DECT are published, DECT may eventually become an important modality in the work-up of infiltrative skeletal lesions. It may even challenge MRI and nuclear medicine because of relatively faster scanning times and ease of access.






Copyright Clearance Center: Springer. Skeletal Radiology. Initial experience with dual-energy computed tomography-guided bone biopsies of bone lesions that are occult on monoenergetic CT, Burke et al. [11]
Similar content being viewed by others
References
Yang P, Wu G, Chang X. Diagnostic accuracy of dual-energy computed tomography in bone marrow edema with vertebral compression fractures: a meta-analysis. Eur J Radiol. 2018;99:124–9.
Heindel W, Gübitz R, Vieth V, Weckesser M, Schober O, Schäfers M. The diagnostic imaging of bone metastases. Dtsch Arzteblatt Int Deutscher Arzte Verlag. 2014;111:741–7.
Yang H-L, Liu T, Wang X-M, Xu Y, Deng S-M. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–17.
Palmedo H, Marx C, Ebert A, et al. Whole-body SPECT/CT for bone scintigraphy: diagnostic value and effect on patient management in oncological patients. Eur J Nucl Med Mol Imaging. 2014;41:59–67.
Chen H, Zhang Y, Pang J, et al. The differentiation of soft tissue infiltration and surrounding edema in an animal model of malignant bone tumor: evaluation by dual-energy CT. Technol Cancer Res Treat. 2019;18:1533033819846842. https://doi.org/10.1177/1533033819846842.
Alvarez RE, Macovski A. Energy-selective reconstructions in X-ray computerized tomography. Phys Med Biol. 1976;21:733–44.
Flohr TG, McCollough CH, Bruder H, et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol. 2006;16:256–68.
McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 2015;276:637–53.
Mallinson PI, Coupal TM, McLaughlin PD, Nicolaou S, Munk PL, Ouellette HA. Dual-energy CT for the musculoskeletal system. Radiology. 2016;281:690–707.
Hubbell JH, Seltzer SM. X-ray mass attenuation coefficients. NIST Standard Reference Database 126 [Internet]. 2004 [cited 2021 Oct 31]. Available from: https://doi.org/10.18434/T4D01F
Burke MC, Garg A, Youngner JM, Deshmukh SD, Omar IM. Initial experience with dual-energy computed tomography-guided bone biopsies of bone lesions that are occult on monoenergetic CT. Skeletal Radiol. 2019;48:605–13.
Abdullayev N, Große Hokamp N, Lennartz S, Holz JA, Romman Z, Pahn G, et al. Improvements of diagnostic accuracy and visualization of vertebral metastasis using multi-level virtual non-calcium reconstructions from dual-layer spectral detector computed tomography. Eur Radiol. 2019;29:5941–9.
Palmer WE, Simeone FJ. Can dual-energy CT challenge MR imaging in the diagnosis of focal infiltrative bone marrow lesions? Radiology. 2018;286:214–6.
Chong CCW, Rai S, Nicolaou S. Dual energy CT in musculoskeletal tumors. In: De Cecco CN, Laghi A, Schoepf UJ, Meinel FG, editors. Dual energy in CT Oncology. Switzerland: Springer International Publishing; 2015. p. 147–52.
Ishiwata Y, Hieda Y, Kaki S, et al. Improved diagnostic accuracy of bone metastasis detection by water-HAP associated to non-contrast CT. Diagn Basel Switz. 2020;10:E853.
Borggrefe J, Neuhaus V-F, Le Blanc M, et al. Accuracy of iodine density thresholds for the separation of vertebral bone metastases from healthy-appearing trabecular bone in spectral detector computed tomography. Eur Radiol. 2019;29:3253–61.
Guillevin R, Vallee J-N, Lafitte F, Menuel C, Duverneuil N-M, Chiras J. Spine metastasis imaging: review of the literature. J Neuroradiol. 2007;34:311–21.
White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14:587–98.
Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64.
Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93.
Gdowski AS, Ranjan A, Vishwanatha JK. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J Exp Clin Cancer Res. 2017;36:108–108.
Ahmed F, Muzaffar R, Fernandes H, Tu Y, Albalooshi B, Osman MM. Skeletal metastasis as detected by 18F-FDG PET with negative CT of the PET/CT: frequency and impact on cancer staging and/or management. Front Oncol. 2016;6:208.
Yamaguchi T, Tamai K, Yamato M, Honma K, Ueda Y, Saotome K. Intertrabecular pattern of tumors metastatic to bone. Cancer. 1996;78:1388–94.
Suzuki A, Kashiwagi N, Doi H, Ishii K, Doi K, Kitano M, et al. Patterns of bone metastases from head and neck squamous cell carcinoma. Auris Nasus Larynx. 2020;47:262–7.
Issa G, Davis D, Mulligan ME. The ability of dual-energy computed tomography to distinguish normal bone marrow from metastases using bone marrow color Maps. J Comput Assist Tomogr. 2018;42:552–8.
Huang H-C, Srinivasan R, Sun Y, Kazakia GJ, Lin P-C, Yeh BM. Detection of lumbar spine osseous metastases using dual-energy CT: phantom results and preliminary clinical validation. Am J Roentgenol. 2019;212:402–10.
Neuhaus V, Lennartz S, Abdullayev N, et al. Bone marrow edema in traumatic vertebral compression fractures: diagnostic accuracy of dual-layer detector CT using calcium suppressed images. Eur J Radiol. 2018;105:216–20.
Lee YH, Kim S, Lim D, Suh J-S, Song H-T. Spectral parametric segmentation of contrast-enhanced dual-energy CT to detect bone metastasis: feasibility sensitivity study using whole-body bone scintigraphy. Acta Radiol. 2015;56:458–64.
Dimopoulos MA, Hillengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol. 2015;33:657–64.
Kosmala A, Weng AM, Heidemeier A, et al. Multiple myeloma and dual-energy CT: diagnostic accuracy of virtual noncalcium technique for detection of bone marrow infiltration of the spine and pelvis. Radiology. 2018;286:205–13.
Kosmala A, Weng AM, Krauss B, Knop S, Bley TA, Petritsch B. Dual-energy CT of the bone marrow in multiple myeloma: diagnostic accuracy for quantitative differentiation of infiltration patterns. Eur Radiol. 2018;28:5083–90.
Wang Q, Sun Z, Li S, et al. Bone marrow imaging by third-generation dual-source dual-energy CT using virtual noncalcium technique for assessment of diffuse infiltrative lesions of multiple myeloma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39:114–9.
Thomas C, Schabel C, Krauss B, et al. Dual-energy CT: virtual calcium subtraction for assessment of bone marrow involvement of the spine in multiple myeloma. Am J Roentgenol. 2015;204:W324–31.
Werner S, Krauss B, Horger M. Dual-energy CT-based bone marrow imaging in multiple myeloma: assessment of focal lesions in relation to disease status and MRI findings. Acad Radiol [Internet]. 2021 [cited 2021 Nov 29]; S1076-6332(21)00057-X. Epub 2021 Mar 8. Available from: https://doi.org/10.1016/j.acra.2021.01.029
Reinert CP, Krieg E, Esser M, Nikolaou K, Bösmüller H, Horger M. Role of computed tomography texture analysis using dual-energy-based bone marrow imaging for multiple myeloma characterization: comparison with histology and established serologic parameters. Eur Radiol. 2021;31:2357–67.
Rajiah P, Sundaram M, Subhas N. Dual-energy CT in musculoskeletal imaging: what is the role beyond gout? Am J Roentgenol. 2019;213:493–505.
Saad F, Lipton A, Cook R, Chen Y-M, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–7.
Behnke NK, Baker DK, Xu S, Niemeier TE, Watson SL, Ponce BA. Risk factors for same-admission mortality after pathologic fracture secondary to metastatic cancer. Support Care Cancer. 2017;25:513–21.
Bae JH, Lee IS, Song YS, et al. Bone tumors with an associated pathologic fracture: differentiation between benign and malignant status using radiologic findings. J Korean Radiol Soc. 2004;2015(73):240–8.
Fayad LM, Kawamoto S, Kamel IR, et al. Distinction of long bone stress fractures from pathologic fractures on cross-sectional imaging: how successful are we? Am J Roentgenol. 2005;185:915–24.
Mauch JT, Carr CM, Cloft H, Diehn FE. Review of the imaging features of benign osteoporotic and malignant vertebral compression fractures. Am J Neuroradiol. 2018;39:1584.
Fayad LM, Kamel IR, Kawamoto S, Bluemke DA, Frassica FJ, Fishman EK. Distinguishing stress fractures from pathologic fractures: a multimodality approach. Skeletal Radiol. 2005;34:245–59.
Issa G, Mulligan M. Dual energy CT can aid in the emergent differentiation of acute traumatic and pathologic fractures of the pelvis and long bones. Emerg Radiol. 2020;27:285–92.
Zheng S, Dong Y, Miao Y, et al. Differentiation of osteolytic metastases and Schmorl’s nodes in cancer patients using dual-energy CT: advantage of spectral CT imaging. Eur J Radiol. 2014;83:1216–21.
Dong Y, Zheng S, Machida H, et al. Differential diagnosis of osteoblastic metastases from bone islands in patients with lung cancer by single-source dual-energy CT: advantages of spectral CT imaging. Eur J Radiol. 2015;84:901–7.
Jain RK. Determinants of tumor blood flow: a review. Cancer Res. 1988;48:2641–58.
Hauger O, Cotten A, Chateil J-F, Borg O, Moinard M, Diard F. Giant cystic Schmorl’s nodes. Am J Roentgenol. 2001;176:969–72.
Ulano A, Bredella MA, Burke P, Chebib I, Simeone FJ, Huang AJ, et al. Distinguishing untreated osteoblastic metastases from enostoses using CT attenuation measurements. Am J Roentgenol. 2016;207:362–8.
Yamamoto S, Kamei S, Tomita K, et al. CT-guided bone biopsy using electron density maps from dual-energy CT. Radiol Case Rep. 2021;16:2343–6.
Huddleston AL, Sackler JP. The determination of electron density by the dual-energy Compton scatter method. Med Phys. 1985;12:13–9.
Dwijendra S, Burke M. Application of dual-energy computed tomography in bone lesion biopsy. Adv Clin Radiol. 2020;2:273–84.
U.S. Food and Drug Administration. FDA clears first major imaging device advancement for computed tomography in nearly a decade [Internet]. FDA; 2021 [updated 2021 Sep 30; cited 2021 Nov 28]. Available from: https://www.fda.gov/news-events/press-announcements/fda-clears-first-major-imaging-device-advancement-computed-tomography-nearly-decade
Siemens Healthineers. NAEOTOM Alpha with quantum technology [Internet]. Siemens; 2021 [cited 2021 Nov 28]. Available from: https://www.siemens-healthineers.com/computed-tomography/photon-counting-ct-scanner/naeotom-alpha
Wehrse E, Sawall S, Klein L, et al. Potential of ultra-high-resolution photon-counting CT of bone metastases: initial experiences in breast cancer patients. NPJ Breast Cancer. 2021;7:1–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The Institutional Research Board had determined the review article to be compliant with the national ethical considerations in quality assurance and evaluation activities and waived the need for a human research ethics committee review.
Consent to participate
Informed consent was obtained from the patients at our institute whose radiologic images are used in this review article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, M.T., Lloyd, T.B. Utility of dual energy computed tomography in the evaluation of infiltrative skeletal lesions and metastasis: a literature review. Skeletal Radiol 51, 1731–1741 (2022). https://doi.org/10.1007/s00256-022-04032-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04032-6