Abstract
Fibronectin (FN) and collagen are vital components of the extracellular matrix (ECM). These proteins are essential for tissue formation and cell alignment during the wound healing stage. In particular, FN interacts with collagens to activate various intracellular signaling pathways to maintain ECM stability. A novel recombinant extra domain-B fibronectin (EDB-FN)-COL3A1 fusion protein (rhFEB) was designed to mimic the ECM to promote chronic and refractory skin ulcer wound healing. rhFEB significantly enhanced cell adhesion and migration, vascular ring formation, and the production of new collagen I (COL1A1) in vitro. rhFEB decreased M1 macrophages and further modulated the wound microenvironment, which was confirmed by the treatment of db/db mice with rhFEB. Accelerated wound healing was shown during the initial stages in rhFEB-treated db/db mice, as was enhanced follicle regeneration, re-epithelialization, collagen deposition, granulation, inflammation, and angiogenesis. The wound chronicity of diabetic foot ulcers (DFUs) remains the main challenge in current and future treatment. rhFEB may be a candidate molecule for regulating M1 macrophages during DFU healing.
Key points
• A recombinant protein EDB-FN-collagen III (rhFEB) was highly expressed in Escherichia coli
• rhFEB protein induces COL1A1 secretion in human skin fibroblasts
• rhFEB protein accelerates diabetic wound healing
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The management of chronic wounds, such as diabetic ulcers, is a major societal concern on a global scale. In diabetes, hyperglycemia disrupts the sequential progression of cellular and molecular events in the healing cascade, consequently resulting in delayed wound healing (Sun et al. 2022). Wound healing involves a synergistic effect between endothelial cells, inflammatory cells, macrophages, and keratinocytes (Schreml et al. 2010). The proliferation, migration, and differentiation of the aforementioned cells can trigger inflammatory cell infiltration, angiogenesis, granulation structure formation, collagen deposition, re-epithelialization, and hair follicle development, ultimately promoting wound healing (Hu et al. 2018; Singla et al. 2017). Macrophages, functioning as innate immune cells, play critical roles in wound healing by suppressing inflammatory responses, eliminating cellular debris, and orchestrating tissue repair (Kim and Nair 2019; Okabe and Medzhitov 2016). Typically, nonpathological wounds undergo M1 (inflammatory) to M2 (anti-inflammatory) differentiation approximately 3 days after trauma, with the peak occurring on the seventh day (Louiselle et al. 2021). Conversely, in diabetic wounds, the macrophage phenotype is predominantly M1 (Miao et al. 2012), leading to an observed imbalance and persistent cell polarization (Ganesh and Ramkumar 2020). Therefore, inhibiting the excessive development of M1 macrophages is particularly important for shortening the inflammatory cycle and accelerating wound healing.
The ECM is a platform for the expression of active molecules and for cellular functions, and its remodeling is directly related to the effectiveness of wound healing. FNs and collagen are important bioactive molecules within the ECM that play essential roles in maintaining the structural integrity of the ECM and governing cellular behavior (Chermnykh et al. 2018; Ge et al. 2020). FNs are critical for tissue formation and cellular organization during the wound healing process, including inflammation, proliferation, and remodeling. FNs is usually divided into two types, plasma FNs and cellular FNs, which exhibit different and independent functions in tissue repair processes. (To and Midwood 2011). Plasma FN primarily functions in the initial phase of wound healing by facilitating clot formation and orchestrating the assembly of the ECM. Cellular FN regulates tissue remodeling in the later stages (Mao and Schwarzbauer 2005; Patten and Wang 2021). FN isoforms exhibit greater efficacy in selectively promoting wound healing than dose full-length FN owing to the varying availability of binding domains (Vogel 2018). Increased vascular endothelial growth factor (VEGF), endothelial cell proliferation, and angiogenesis were observed in the presence of EDB-FN (Khan et al. 2005). EDB-FN is being investigated as a promising therapeutic target for promoting cell proliferation to address endothelial dysfunction caused by diabetes (Khan and Chakrabarti 2006; Lemańska-Perek and Adamik 2019). Furthermore, EDB-FN has been implicated in the immune regulatory pathway. The synthesis and secretion of EDB-FN play a role in the phagocytosis of immune cells that target inflammatory factors, with potential enhancement in the presence of integrin (Kraft et al. 2016).
Collagen regeneration and deposition are important criteria for evaluating the efficacy of wound healing (Wu and Chen 2016). In the inflammatory phase, the degradation of collagen can activate immune cells to eliminate necrotic tissue and bacteria (Yeung and Kelly 2021), thereby regulating the extent of the inflammatory response and mitigating the likelihood of wound infection (Sun et al. 2022). The dynamic imbalance between the synthesis and decomposition of collagen is an important factor leading to the slow healing of diabetic wounds in the inflammatory stage (Dinh et al. 2012). In the later stages of wound healing, collagen facilitates the migration, re-epithelization, and deposition of keratinocytes (Chen et al. 2019; Dong et al. 2022) as well as the synthesis of FN in the wound tissue (Lai et al. 2020). Wound healing is accompanied by the conversion of COL3A1 to COL1A1, which is the result of FN recognizing and binding to COL3A1 during the proliferative phase (Beyeler et al. 2019). In addition, COL1A1 promotes angiogenesis and is important for elastic formation after skin repair (Fisher et al. 2009; Reilly and Lozano 2021).
The investigation of wound dressings and the exploration of efficacious strategies to enhance wound healing and mitigate inflammatory responses are highly important for addressing chronic and refractory skin ulcer wound healing. In this study, the active fragment of cellular FN and EDB-FN, and the triple-helix fragment of COL3A1, which is involved in cell adhesion, were carefully selected, and a novel recombinant human FN, referred to as rhFEB, was successfully obtained using recombination technology. The therapeutic efficacy of rhFEB on chronic and refractory skin ulcer healing was evaluated using a full-thickness skin defect model in db/db mice. The findings of this research show good potential for advancing the development of novel functional biomaterials that are based on FN and collagen.
Materials and methods
Materials
Vectors pET-20b (Invitrogen, Guangzhou, China) and E. coli BL21(DE3) (Invitrogen, Guangzhou, China, ATCC® BAA-1025TM) were used for cloning and heterologous expression. HaCaT (Human Immortalised Keratinocytes, Chinese Academy of Sciences, Shanghai, China, ATCC® no.BNCC101683) were cultured in H-DMEM medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS). HSF (human fibroblasts, Guangzhou Saiye Biotech Co., Ltd, ATCC® PCS-200–011) were cultured in DMEM-F12 (Gibco, Carlsbad, CA, USA) containing 10% FBS. ECV304-eGFP (human umbilical vein endothelial cells–enhanced green fluorescent protein, Chinese Academy of Sciences, Shanghai, China, ATCC® PCS-100–010) were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) containing 10% FBS. Male C57BL/6 mice (6–8 weeks, 20–25 g) purchased from the Guangdong Medical Laboratory Animal Centre (Guangdong, China, certificate no. 44007200069979).
Expression and purification of rhFEB protein in E. coli
Based on the nucleic acid sequences of human EDB-FN (ID: AB191261.1, 4596–4902 nt) and COL3A1 (ID: BC028178.1, 1562-1651nt) in the GeneBank database, the cDNA sequence of recombinant human EDB-FN-collagen III (rhFEB) was acquired. The cDNA sequence of rhFEB was optimized (Supplemental Table S1) and synthesized by Kingsley Biotechnology Co., Ltd., in accordance with the codon preference of E. coli. Subsequently, the sequence was integrated into the pET-20b vector utilizing the NdeI and XhoI sites, resulting in the generation of pET20b-rhFEB. The recombinant plasmid pET20b-rhFEB was introduced into E. coli BL21(DE3), followed by screening for positive transformants on LB medium with 100 µg/mL ampicillin.
The strains were cultured in 5 mL of LB media at 37 °C until an optical density of 600 nm (OD600) between 0.8 and 1.0 was reached. Subsequently, the strains were induced with 1 mM isopropylthio-β-D-galactoside (IPTG) for 3.5 h. The expression of rhFEB was monitored via SDS‒PAGE. The high-expression strain was then cultured overnight in 50 mL of LB medium at 37 °C, after which 5 mL of the culture was added to 500 mL of LB medium. The cultures were grown at 37 °C for approximately 3 h and induced with 1 mM IPTG for 3.5 h. Biomass was obtained through centrifugation at a temperature of 4 °C. Subsequently, the purification of rhFEB was carried out using Ni column affinity chromatography and Sephadex G25 gel filtration. The purified samples were then subjected to analysis via SDS‒PAGE and Western blotting (WB), and their purity was assessed via HPLC.
Cell viability assay
HaCaT cells were cultured in 96-well plates (4.0 × 103 cells/well) for 15–18 h at 37 °C and 5% CO2. The suspended cells were removed by three rinses with PBS and the remaining cells were treated with 100 µL of tissue culture containing rhFEB (31.3 nmol/L, 62.5 nmol/L, 125.0 nmol/L, 250.0 nmol/L, 500.0 nmol/L) for 24 h and 48 h. After 10 µL of MTT was applied for another 4 h, the tissue culture was removed and the process was terminated with 100 µL of DMSO. Cell viability was quantified using a microplate reader at 570 nm, with 630 nm serving as the reference wavelength.
Cell adhesion assay
Ninety-six-well plates were coated with 50 µL of either 31.3 nmol/L or 125 nmol/L rhFEB, or 125 nmol/L EGF as a positive control and ddH2O as a normal control. Next, the plates were washed three times with PBS. One hundred microliters of tissue culture mixture containing 1.0 × 104 HaCaT cells was seeded onto the treated plates, and the cell were allowed to attach for 4 h at 37 °C and 5% CO2. After incubation, the nonadhesive cells were removed by three rinses with PBS. The remaining cells were incubated with fresh tissue culture containing 10 µL of MTT for another 4 h. The tissue culture was subsequently removed, and the process was terminated with 100 µL of DMSO. Adhesive cells were quantified using a microplate reader at 570 nm, with 630 nm as the reference wavelength.
Cell migration assay
Cell migration was assessed using an in vitro scratch wound healing model. HaCaT cells were cultured in 12-well plates at a density of 1.0 × 105 cells/well until they reached 95% confluence at 37 °C and 5% CO2. A scratch was created using a 200-µL pipette tip, and nonadhesive cells were removed using PBS. Subsequently, the cells were treated with 1 mL of sample containing either 31.3 nmol/L or 125 nmol/L rhFEB, or 125 nmol/L EGF in DMEM with 1% FBS. Images of the scratch area were captured using an inverted microscope at 0 h and 48 h. ImageJ software was used to determine percentage closure (%).
Tube formation assay
A tube formation assay was conducted using ECV304-eGFP cells. Forty microliter samples (31.3 nmol/L or 125 nmol/L rhFEB or 125 nmol/L EGF in RPMI-1640 medium without FBS) mixed with 10 µL of Matrigel were incubated in precooled 96-well plates for 2 h at 4 °C, followed by solidification at 37 °C for 16 h. Subsequently, 100 µL of culture containing 1.5 × 104 ECV304-eGFP cells was added to the wells and incubated at 37 °C and 5% CO2 for 12 h. The tubular structures were observed and captured with an inverted microscope (Olympus IX70, Tokyo, Japan). And the number of tubular structures was measured by Image J software.
COL1A1 secretion assay
A COL1A1 secretion assay was performed with fibroblasts in vitro. Two milliliters of culture containing 6.0 × 104 HSF cells was inoculated in 6-well plates at 37 °C and 5% CO2 until the cell confluence reached 45 ~ 60%. The culture was removed and the cells were treated with 2 mL of rhFEB (2 nmol/L, 20 nmol/L, 200 nmol/L), TGFβ1 (200 nmol/L) as the positive control, and DMEM without FBS as the normal control. After 24 h of incubation at 37 °C and 5% CO2, the supernatant of the cell culture was collected, and the COL1A1 concentration was subsequently measured via WB analysis.
Western blot analysis
Cells or tissues were lysed in ice-cold RIPA lysis buffer and centrifuged for 15 min at 15,000 × g. Insoluble debris was removed from the supernatant lysate, and a protease inhibitor cocktail was added. The protein concentration was determined using a BCA protein assay (Thermo Scientific, Waltham MA). Total protein in equal amounts was separated via SDS‒PAGE and transferred onto nitrocellulose membranes. After the nonspecific binding was blocked using 5% fat-free milk in 0.1 M TBST, the membranes were incubated with primary antibodies against COL1A1 (1:1000; CST, #72,026) and GAPDH (1:5000; Bioworld, AMM04690G) overnight at 4 °C. The membranes were then washed five times with 0.1 M TBST, followed by incubation with horseradish peroxidase (HRP)–conjugated secondary antibodies for 1 h at room temperature. The blots were developed and assessed using Pierce ECL chemiluminescent substrates (Thermo Scientific, Waltham, MA).
Expression of M1-type macrophage markers
Primary precursor cells were obtained and cultured from the bone marrow of male C57BL/6 mice (6–8 weeks, 20–25 g). These cells were then stimulated to differentiate into monocyte-derived macrophages with 25 ng/mL macrophage colony–stimulating factor (M-CSF1) and cultured in 10% FBS-DMEM for 7 days. Then, differentiated macrophages were exposed to inducers of M1 macrophages (100 ng/mL LPS and 20 ng/mL IFN-γ) for 24 h, followed by stimulation with rhFEB at concentrations of 31.3 nmol/L and 125.0 nmol/L for an additional 24 h. Total RNA was extracted from cells using a HiPure Fibrous RNA Plus Kit (Magen, Guangzhou, China) and was subsequently transcribed into cDNA via a reverse transcription kit (Tiangen Biotech, Beijing, China). qRT‒PCR was subsequently performed with a SYBR-Green Quantitative PCR Kit (Bio-Rad, Hercules, CA, USA) and the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Relative expression of IL-1β, TNF-α, CD206, and Arg-1 was quantified by the 2−ΔΔCt method, with GAPDH serving as the reference gene.
Full-thickness skin defect model in diabetes mice
Female mice with diabetes and a weight of 40 ± 2 g were administered anesthesia with 3% pentobarbital sodium. Depilatories were applied to remove hair from an approximately 3 cm × 3 cm area on the back of the mice. A skin wound, measuring approximately 0.8 cm2 and reaching the fascia, was then created using a 10-mm diameter corneal trephine. This wound was located approximately 2 mm below the scapula and on both sides of the midline of the spine. Following the modeling procedure, 0.1 g of rhFEB gel complex (50 nmol/L, 200 nmol/L) was evenly applied to the wounds of the mice, and the EGF gel complex (125 nmol/L) was used as a positive control. The basic gel complex consisting of 14% Poloxamer 407 and 6% Poloxamer 188 and ddH2O was used as model. The day of the wound incision was designated day 0, and subsequently, the dimensions and healing progress of the wounds were assessed and documented at 7, 10, 14, 21, and 28 days. Subsequently, the mice were humanely euthanized, and a section of normal skin approximately 3–4 mm from the wound edge was excised for further analysis.
Histological and immunohistochemical staining
The skin tissue of the mice was collected and preserved overnight at a temperature of 4 °C in a 4% paraformaldehyde solution. Subsequently, the tissue was rinsed with water and subjected to dehydration using ethanol and xylene. The dehydrated tissue was then embedded in paraffin and sliced into thin sections measuring 5 µm using a microtome. These sections were subsequently placed onto glass slides and dried at 65 °C. Hematoxylin–eosin staining (H&E) and Masson trichrome staining were performed to analyze the tissue morphology of the repaired wounds. Immunofluorescence analysis was performed to stain the nuclei using DAPI (Beyotime, C1005), rabbit anti-mouse iNOS primary antibody (Proteintech, 80,517–1-RR) and FITC-labeled anti-rabbit secondary antibody (Servicebio, China) to label M1 macrophages. For immunohistochemical (IHC) staining, the slices were immersed in xylene twice for 5 min followed soaked in 100–70% ethanol for 3 min, and subsequently washed in PBS 3 times for 3 min. Citric acid buffer was used for antigen repair by boiling for 8 min and holding for 8 min, followed by natural cooling to room temperature, and rinsing with PBS 3 times for 3 min. Endogenous peroxidase activity was blocked by adding 3% H2O2 for 10 min in the dark, followed by blocking with 3% BSA-PBS for 30 min at room temperature. The slices were covered with CD31 Polyclonal antibody (1:500, Proteintech, 28,083–1-AP) at 4 °C overnight, followed by incubation with a goat anti-rabbit IgG secondary antibody (Gene Tech, #GK500705) for 30 min. After incubation, the slices were rinsed with PBS 3 times for 3 min and a fresh DAB chromogenic agent (Gene Tech, #GK500705) was added dropwise. The color development was controlled using a microscope and terminated by rinsing with water. After hematoxylin staining, the slices were dehydrated in 70–100% ethanol for 3 min and xylene twice for 10 min. The slices were sealed with neutral resin and observed with an inverted microscope (Olympus IX70, Tokyo, Japan).
Statistical analysis
All data are represented by Mean ± SEM, and statistical analysis was conducted using GraphPad Prism 9.0 software. T-tests or one-way ANOVA was used to analyze the differences. * P < 0.05 indicates a statistically significant difference (n ≥ 3).
Results
Expression and purification of rhFEB
The coding sequence of the rhFEB fusion (1869 bp) was inserted into the pET-20b plasmid distal to the T7 promoter. The resulting recombinant plasmid, pET20b-rhFEB, contains a 6-histidine tag for facilitating protein purification (Fig. 1a and b). The rhFEB protein was expressed in E. coli BL21 (DE3) through recombinant techniques, and induction was achieved by IPTG. Upon induction, the rhFEB protein exhibited a significant difference in expression compared to that in the control group, in which the protein had an approximate molecular weight of 60 kDa (Fig. 1c). The rhFEB protein was acquired using Ni affinity chromatography in a 100 mM imidazole eluant, followed by purification through a molecular sieve to obtain the desalted product. The verification of rhFEB expression and purification were conducted via Western blot analysis utilizing an anti-rhFEB monoclonal antibody (Fig. 1d and e).
Expression and purification of rhFEB protein. a Construction schematic of the recombinant pET20b-rhFEB plasmid. b Nucleic acid electrophoresis of recombinant plasmid rhFEB. M: DNA Ladder 10,000; lane 1: negative control; lane 2: the bands of rhFEB were amplified by PCR. c Electrophoretic expression of rhFEB plasmid in BL21 (DE3) by SDS-PAGE; M: 14.4–116.0 kDa protein marker; lane 1: sediment of rhFEB before induction; lanes 2–6: sediment of different BL21 (DE3)/rhFEB monoclonal after induction. d Electrophoretic diagram of purified rhEGF protein; M: 14.4–116.0 kDa protein marker. e Western blotting analysis of recombinant rhFEB. M: 10–180 kDa protein marker. f HPLC analysis of the purity of rhFEB protein
Cell biological viability of rhFEB on adhesion, migration, and tube formation
HaCaT cells were used as a cellular model to examine the biological efficacy of rhFEB in terms of cell adhesion and migration, and the growth factor EGF was utilized as a positive control. Initially, a viability assessment of rhFEB demonstrated that concentrations ranging of 31.3–500 nmol/L for a duration of 48 h did not induce any toxicity in HaCaT cells (Fig. 2a). After 48 h of rhFEB or EGF administration, the wounds in the wound healing assay exhibited nearly complete closure (Fig. 2b). The wound healing rates of the rhFEB group at concentrations of 31.3 nmol/L and 125.0 nmol/L were 78.44% and 90.53% respectively, which were significantly greater than those of the control group, in which the healing rate was 58.02% (P < 0.001) (Fig. 2b). Additionally, the adhesion assay results revealed that the cells treated with rhFEB exhibited a spread-out morphology, whereas the control group exhibited a circular morphology (Fig. 2c). Moreover, compared with the control group, which had 133 adherent cells, the rhFEB group demonstrated a substantial increase in cell adhesion: 267 cells adhered at a concentration of 31.3 nmol/L, and 303 cells adhered at a concentration of 125.0 nmol/L (P < 0.001) (Fig. 2c). Additionally, the rhFEB protein displayed a remarkable ability to promote vascularization in the tube formation assay (Fig. 2d).
Cell biological visibility of rhFEB on proliferation, adhesion, and migration. a Proliferation of rhFEB to HaCaT cells. b Migration and healing rates of HaCaT cells by rhFEB (scale bar = 100 μm). c Adhesion of rhFEB to HaCaT cells (scale bar = 100 μm). d rhFEB-induced tube formation of ECV304-eGFP cells. n = 3, mean ± SD, ** P < 0.01, *** P < 0.001 vs. control group
rhFEB promoted COL1A1 secretion by HSF cells
The protein COL1A1 is the predominant elastic collagen protein found in the human body, and its intricate network architecture serves to safeguard and maintain the elasticity of the skin. With inadequate production or excessive degradation of COL1A1 within the dermis, the skin’s elasticity diminishes, resulting in signs of aging such as wrinkling and sagging. To investigate the impact of the recombinant rhFEB protein on the synthesis or release of COL1A1, we conducted in vitro experiments using fibroblasts. COL1A1 expression increased significantly in response to varying concentrations of rhFEB (2.0 nmol/L, 20.0 nmol/L, and 200.0 nmol/L), with corresponding fold changes of 1.2, 1.3, and 1.5, respectively, compared to that in the control group (P < 0.05) (Fig. 3a).
Cell biological activity of rhFEB on COL1A1 secretion and inflammatory reaction. a Expression of COL1A1 protein in HSF cells treated with TGFβ1 and rhFEB. b Expression of inflammatory markers, TNF-α, IL-1β, Arg-1, and CD206 in M1 macrophage treated with rhFEB protein. n = 3, * P < 0.05, ** P < 0.01 vs. control group
rhFEB suppressed M1-type macrophage phenotypic differentiation
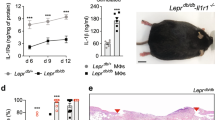
The expression of proinflammatory and anti-inflammatory factors is a typical characteristic of the inflammatory response, while alternative polarization typically leads to the expression of anti-inflammatory factors. Consequently, the gene expression of TNF-α, IL-1β, Arg-1, and CD206 was used as a representative measure of macrophage phenotype. Compared to the normal group (M0), the expression levels of TNF-α and IL-1β in M1 group were significantly increased (P < 0.01) (Supplemental Figure S1). After treatment with rhFEB for 24 h, the expression levels of the TNF-α and IL-1β in M1 macrophage were rapidly decreased (Fig. 3b). However, the expression levels of Arg-1 and CD206 were inhibited with the treatment of low concentration rhFEB and restored to the original levels when treated with higher concentration rhFEB (Fig. 3b). These results suggested that the rhFEB has capacity of inhibiting the expression of inflammatory factors but not able to promote the polarization from M1 to M2.
rhFEB improved wound healing of diabetes mice with full-thickness skin defects
The process of wound healing is a complex and well-coordinated phenomenon that occurs during tissue regeneration. To assess the impact of rhFEB on diabetic wounds, we generated a full-thickness skin defect model in vivo. The evaluation was based on the wound healing time and wound area measurements within a predetermined time interval. Over time, wound contraction from the margins was observed in all the experimental groups, with db/db mice treated with varying concentrations of rhFEB exhibiting faster wound area contraction than did the model group mice at 7, 10, and 14 days. Notably, the db/db mice treated with rhFEB exhibited significantly accelerated wound closure on the 14th day following surgery. However, the wounds observed in the model group still exhibited greater severity than those in the rhFEB group on the 14th day. Subsequently, the wound exhibited ongoing healing progress, and during the period from day 14 to day 28 in the advanced phase of wound healing, the speed and area of wound healing tended to remain consistent across all groups of mice. Notably, all wounds in mice achieved a healing area exceeding 95% by the 28th day (Fig. 4a and b).
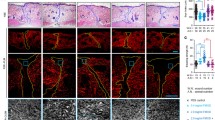
Effect of rhFEB on the skin wound healing. a Traces of wound closure were photographed at 0, 7, 10, 14, 21, and 28 days post-surgery. b Quantitative analysis of epithelial thickness at days 3, 7, 10, 14, 21, and 28 using ImageJ software. c H&E staining of wound sections at 28 days post-surgery (40 × , 200 ×). d and e Masson trichrome staining of wound sites at 28 days post-surgery(40 × , 200 ×). Arrows indicate hair follicle structure. n = 3, ** P < 0.01 vs. normal group
Histopathological analysis
Wound healing involves various cellular interactions, encompassing inflammatory cells, keratinocytes, and fibroblasts. This intricate process entails cell migration, proliferation, and differentiation, ultimately resulting in angiogenesis, collagen deposition, ECM formation, re-epithelialization, and the formation of sebaceous glands and hair follicles (Hu et al. 2018; Zhao et al. 2017). To further investigate the effect of rhFEB on diabetic wounds in vivo, we performed histopathological analysis through H&E staining and Masson trichrome staining. H&E staining analysis, conducted 28 days after the defect was initiated, revealed the presence of an intact skin stratum corneum with a basal layer in all experimental groups. In comparison to those in the model group, the repaired tissue in the rhFEB treatment group exhibited normal hair follicle structures, as indicated by the arrow. Additionally, the H&E staining results revealed greater granulation and vascular ring structures in the skin tissue of the rhFEB groups following wound healing, than in that of the model group (Fig. 4c). Masson’s trichrome staining serves as a valuable tool for evaluating the comprehensive integrity of the scar region through the quantification of collagen deposition and morphology. Masson’s trichrome staining revealed that the application of rhFEB to diabetic wounds resulted in the deposition of ECM on day 28, particularly in relation to the blue-stained collagen. Moreover, the collagen volume fractions in the rhFEB group reached 44.57% and 59.23% at doses of 50 nmol/L and 200 nmol/L, respectively, which were significantly greater than those observed in the normal and model groups. Furthermore, the application of rhFEB notably enhanced the organization and density of collagen fibers on the treated wound surfaces (Fig. 4d and e). Neovascularization, a crucial aspect of wound healing, was assessed using CD31, a widely accepted marker for angiogenesis. The rhFEB-50.0 and rhFEB-200.0 groups exhibited significantly treater CD31 expression than the normal group (Fig. 5).
Discussion
Diabetic wounds are typical chronic wounds that can cause severe problems in diabetic patients. Chronic wounds are characterized by pathological inflammation, and persistent inflammation is an inhibitory factor of diabetic wound healing (Louiselle et al. 2021). Wound healing involves four distinct stages: hemostasis, inflammation, proliferation, and tissue remodeling. Inflammation, which follows hemostasis, involves the phagocytosis and elimination of pathogens by immune cells. During the proliferative phase, the formation of blood vessels, granulation, and ECM scaffolds are synergistically stimulated by growth factors. Subsequently, re-epithelialization and collagen deposition progress during tissue remodeling, and vascular density return to low levels (Campos et al. 2008; Malone-Povolny et al. 2019).
The ECM serves as a structural framework that facilitates cellular functional expression and possesses regenerative and reparative capabilities (Yang et al. 2021). FNs and their subtypes, which are functional glycoproteins in the ECM, are crucial for cell–matrix interactions (Lenselink 2015; Parisi et al. 2020). Following injury, FN undergoes alternative splicing to produce an isoform known as EDB-FN (Natal et al. 2006). EDB-FN is involved in endothelial cell proliferation and angiogenesis (Khan et al. 2005). Collagen is implicated in the complete wound healing process. During the early stages, breakdown products of collagen can activate immune cells and facilitate inflammatory reactions. As wound healing progresses, collagen contributes to vascular regeneration, keratinocyte migration, collagen deposition, hair follicle development, and re-epithelialization (Elbialy et al. 2020; Lai et al. 2020).
Wound healing is closely related to the synergistic effect of FN and collagen. FN contacts collagen to activate variable internal signaling pathways to maintain ECM stability (Dalton and Lemmon 2021; Singh et al. 2010). FNs actively participate in multiple stages of wound healing, including hemostasis, proliferation, and remodeling. Therefore, developing therapies that augment the healing potential of FN through the synthesis of FN peptides and the targeted utilization of growth factors is interesting (Llopis-Hernández et al. 2016; Martino et al. 2011). Therefore, in this study, the active fragment of cellular FN, EDB-FN, and the triple-helix fragment of COL3A1, which is involved in cell adhesion, were recombined and expressed in an E. coli system. Finally, recombinant FN with a molecular weight of 60 kDa was obtained.
Angiogenesis is essential for tissue regeneration and an important indicator of wound healing processes. The formation of fresh blood vessels is particularly important for chronic wound healing (Bauer et al. 2005). Research has indicated that the presence of the EDB-FN domain can enhance the expression of VEGF, thereby facilitating the proliferation of endothelial cells and angiogenesis (Khan et al. 2005). In this study, we simulated vascular differentiation of ECV304-eGFP cells in vitro, and the findings revealed that the induction of ECV304-eGFP cells by rhFEB resulted in the formation of well-defined and abundant lumen structures compared to those in the control group. Additionally, the efficacy of rhFEB was further validated through IHC analysis of tissues from diabetic rats with full-thickness skin defects.
Early initiation of effective treatment is crucial for ensuring stable and orderly wound repair and achieving early attainment of primary healing. FN is involved in the complete process of wound repair, especially in the early stages (Barrenas et al. 2019). To further demonstrate the effect of rhFEB on diabetic wounds, we established a full-thickness skin defect model in diabetic mice. During the initial 2-week period, compared with those undergoing natural healing, the mice treated with rhFEB exhibited significantly faster wound healing. Although chronic and refractory conditions are typical features of diabetic wounds (Louiselle et al. 2021), and the wound healing process in experimental mice lasts for 28 days, rhFEB could promote the initial stages of healing. Furthermore, H&E staining and Masson staining revealed that rhFEB notably enhanced the development of hair follicles and granulation structures, as well as collagen deposition and organized arrangement during the wound remodeling phase.
Inflammation is an important process in wound healing. Inhibiting the expression of proinflammatory factors secreted by M1 macrophages or promoting the differentiation of inflammatory M1 macrophages into anti-inflammatory M2 macrophages is a conventional method for monitoring the development of inflammation (Van den Bossche et al. 2016). M1 and M2 macrophages and their corresponding marker genes are commonly used to evaluate the phenotypic transformation of proinflammatory and anti-inflammatory effects. In vitro findings demonstrated that rhFEB effectively suppressed the transcription of the proinflammatory factor TNF-α and IL-1β in M1 macrophages. However, we did not detect an increase in the expression of the anti-inflammatory factors Arg-1 or CD206, indicating the absence of M1 to M2 conversion. It may be necessary to increase the administration time. Additionally, these findings imply that rhFEB may play an important role in the early stage of inflammation, particularly in the healing of diabetic wounds where M1 macrophages persist in the wound bed (Van den Bossche et al. 2016).
COL1A1, the predominant elastic collagen protein in the human body, possesses essential mechanical characteristics that contribute to flexibility. In the proliferative phase of wound healing, FN identifies and attaches to COL3A1, thereby transforming it into COL1A1 (Beyeler et al. 2019). In vitro, we successfully evaluated the secretion of COL1A1 by stimulating fibroblasts with rhFEB. In the diabetic wound model, Masson trichrome staining revealed a greater collagen deposition score in the tissue repaired with rhFEB.
In conclusion, through recombination technology, we constructed a new recombinant FN construct containing the EDB-FN active fragment of cellular FN and the triple-helix fragment of COL3A1 that has cell adhesive activities. The impact of rhFEB on chronic and respiratory skin ulcer wound healing was evaluated through a diabetic wound model. We confirmed that rhFEB has the potential to increase the initial healing rate of diabetic wounds, potentially through its inhibitory effect on proinflammatory factors. In addition, rhFEB has been shown to stimulate the production of COL1A1 and to promote better wound tissue remodeling and re-epithelialization. The design of the rhFEB protein still has shortcomings, such as the risk of metal-binding activities with the His-rich sequences, which should be improved and optimized in subsequent experiments. However, the molecular mechanisms involved, specifically those pertaining to the regulatory effect of rhFEB on macrophages during the diabetic wound healing, require further research.
Data availability
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Code availability
Not applicable.
References
Barrenas F, Raehtz K, Xu C, Law L, Green RR, Silvestri G, Bosinger SE, Nishida A, Li Q, Lu W, Zhang J, Thomas MJ, Chang J, Smith E, Weiss JM, Dawoud RA, Richter GH, Trichel A, Ma D, Peng X, Komorowski J, Apetrei C, Pandrea I, Gale M Jr (2019) Macrophage-associated wound healing contributes to African green monkey SIV pathogenesis control. Nat Commun 10(1):5101. https://doi.org/10.1038/s41467-019-12987-9
Bauer SM, Bauer RJ, Velazquez OC (2005) Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovasc Surg 39(4):293–306. https://doi.org/10.1177/153857440503900401
Beyeler J, Katsaros C, Chiquet M (2019) Impaired contracture of 3D collagen constructs by fibronectin-deficient murine fibroblasts. Front Physiol 10:166. https://doi.org/10.3389/fphys.2019.00166
Campos AC, Groth AK, Branco AB (2008) Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr 11(3):281–288. https://doi.org/10.1097/MCO.0b013e3282fbd35a
Chen J, Gao K, Liu S, Wang S, Elango J, Bao B, Dong J, Liu N, Wu W (2019) Fish collagen surgical compress repairing characteristics on wound healing process in vivo. Mar Drugs 17(1). https://doi.org/10.3390/md17010033
Chermnykh E, Kalabusheva E, Vorotelyak E (2018) Extracellular matrix as a regulator of epidermal stem cell fate. Int J Mol Sci 19(4). https://doi.org/10.3390/ijms19041003
Dalton CJ, Lemmon CA (2021) Fibronectin: molecular structure, fibrillar structure and mechanochemical signaling. Cells 10(9). https://doi.org/10.3390/cells10092443
Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, Tellechea A, Pradhan L, Lyons TE, Giurini JM, Veves A (2012) Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 61(11):2937–2947. https://doi.org/10.2337/db12-0227
Dong Y, Zhu W, Lei X, Luo X, Xiang Q, Zhu X, Pan Q, Jin P, Cheng B (2022) Treatment of acute wounds with recombinant human-like collagen and recombinant human-like fibronectin in C57BL/6 mice individually or in combination. Fron Bioeng Biotechnol 10:908585. https://doi.org/10.3389/fbioe.2022.908585
Elbialy ZI, Atiba A, Abdelnaby A, Al II H, Elsheshtawy A, El-Serehy HA, Abdel-Daim MM, Fadl SE, Assar DH (2020) Collagen extract obtained from Nile tilapia (Oreochromis niloticus L.) skin accelerates wound healing in rat model via up regulating VEGF, bFGF, and α-SMA genes expression. BMC Vet Res 16(1):352. https://doi.org/10.1186/s12917-020-02566-2
Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ (2009) Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol 174(1):101–114. https://doi.org/10.2353/ajpath.2009.080599
Ganesh GV, Ramkumar KM (2020) Macrophage mediation in normal and diabetic wound healing responses. J Inflamm Rre 69(4):347–363. https://doi.org/10.1007/s00011-020-01328-y
Ge Y, Miao Y, Gur-Cohen S, Gomez N, Yang H, Nikolova M, Polak L, Hu Y, Verma A, Elemento O, Krueger JG, Fuchs E (2020) The aging skin microenvironment dictates stem cell behavior. PNAS 117(10):5339–5350. https://doi.org/10.1073/pnas.1901720117
Hu S, Bi S, Yan D, Zhou Z, Sun G, Cheng X, Chen X (2018) Preparation of composite hydroxybutyl chitosan sponge and its role in promoting wound healing. Carbohyd Polym 184:154–163. https://doi.org/10.1016/j.carbpol.2017.12.033
Khan ZA, Chakrabarti S (2006) Therapeutic targeting of endothelial dysfunction in chronic diabetic complications. Recent Pat Cardiovasc Drug Discov 1(2):167–175. https://doi.org/10.2174/157489006777442531
Khan ZA, Chan BM, Uniyal S, Barbin YP, Farhangkhoee H, Chen S, Chakrabarti S (2005) EDB fibronectin and angiogenesis – a novel mechanistic pathway. Angiogenesis 8(3):183–196. https://doi.org/10.1007/s10456-005-9017-6
Kim SY, Nair MG (2019) Macrophages in wound healing: activation and plasticity. Immunol Cell Biol 97(3):258–267. https://doi.org/10.1111/imcb.12236
Kraft S, Klemis V, Sens C, Lenhard T, Jacobi C, Samstag Y, Wabnitz G, Kirschfink M, Wallich R, Hänsch GM, Nakchbandi IA (2016) Identification and characterization of a unique role for EDB fibronectin in phagocytosis. J Mol Med 94(5):567–581. https://doi.org/10.1007/s00109-015-1373-0
Lai CS, Tu CW, Kuo HC, Sun PP, Tsai ML (2020) Type II collagen from cartilage of Acipenser baerii promotes wound healing in human dermal fibroblasts and in mouse skin. Mar Drugs 18(10). https://doi.org/10.3390/md18100511
Lemanska-Perek A, Adamik B (2019) Fibronectin and its soluble EDA-FN isoform as biomarkers for inflammation and sepsis. Adv Cli Exp Med 28(11):1561–1567. https://doi.org/10.17219/acem/104531
Lenselink EA (2015) Role of fibronectin in normal wound healing. Int Wound J 12(3):313–316. https://doi.org/10.1111/iwj.12109
Llopis-Hernández V, Cantini M, González-García C, Cheng ZA, Yang J, Tsimbouri PM, García AJ, Dalby MJ, Salmerón-Sánchez M (2016) Material-driven fibronectin assembly for high-efficiency presentation of growth factors. Sci Adv 2(8):e1600188. https://doi.org/10.1126/sciadv.1600188
Louiselle AE, Niemiec SM, Zgheib C, Liechty KW (2021) Macrophage polarization and diabetic wound healing. Trans Res 236:109–116. https://doi.org/10.1016/j.trsl.2021.05.006
Malone-Povolny MJ, Maloney SE, Schoenfisch MH (2019) Nitric oxide therapy for diabetic wound healing. Adv Healthc Mater 8(12):e1801210. https://doi.org/10.1002/adhm.201801210
Mao Y, Schwarzbauer JE (2005) Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. J Cell Sci 118(Pt 19):4427–4436. https://doi.org/10.1242/jcs.02566
Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Müller R, Livne E, Eming SA, Hubbell JA (2011) Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 3(100):100ra89 https://doi.org/10.1126/scitranslmed.3002614
Miao M, Niu Y, Xie T, Yuan B, Qing C, Lu S (2012) Diabetes-impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen 20(2):203–213. https://doi.org/10.1111/j.1524-475X.2012.00772.x
Natal C, Osés-Prieto JA, Pelacho B, Iraburu MJ, López-Zabalza MJ (2006) Regulation of apoptosis by peptides of fibronectin in human monocytes. Apoptosis 11(2):209–219. https://doi.org/10.1007/s10495-006-3761-y
Okabe Y, Medzhitov R (2016) Tissue biology perspective on macrophages. Nat Immunol 17(1):9–17. https://doi.org/10.1038/ni.3320
Parisi L, Toffoli A, Ghezzi B, Mozzoni B, Lumetti S, Macaluso GM (2020) A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn Dent Sci Rev 56(1):50–55. https://doi.org/10.1016/j.jdsr.2019.11.002
Patten J, Wang K (2021) Fibronectin in development and wound healing. Adv Drug delivRev 170:353–368. https://doi.org/10.1016/j.addr.2020.09.005
Reilly DM, Lozano J (2021) Skin collagen through the lifestages: importance for skin health and beauty. Plast Aesth Res 8(2). https://doi.org/10.20517/2347-9264.2020.153
Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P (2010) Wound healing in the 21st century. J Am Acad Dermatol 63(5):866–881. https://doi.org/10.1016/j.jaad.2009.10.048
Singh P, Carraher C, Schwarzbauer JE (2010) Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Bi 26:397–419. https://doi.org/10.1146/annurev-cellbio-100109-104020
Singla R, Soni S, Patial V, Kulurkar PM, Kumari A, S M, Padwad YS, Yadav SK, (2017) In vivo diabetic wound healing potential of nanobiocomposites containing bamboo cellulose nanocrystals impregnated with silver nanoparticles. Int J Biol Macromol 105(Pt 1):45–55. https://doi.org/10.1016/j.ijbiomac.2017.06.109
Sun L, Li L, Wang Y, Li M, Xu S, Zhang C (2022) A collagen-based bi-layered composite dressing for accelerated wound healing. J Tissue Viability 31(1):180–189. https://doi.org/10.1016/j.jtv.2021.09.003
To WS, Midwood KS (2011) Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 4:21. https://doi.org/10.1186/1755-1536-4-21
Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen HJ, Boshuizen MC, Ahmed M, Hoeksema MA, de Vos AF, de Winther MP (2016) Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep 17(3):684–696. https://doi.org/10.1016/j.celrep.2016.09.008
Vogel V (2018) Unraveling the mechanobiology of extracellular matrix. Annu Rev Physiol 80:353–387. https://doi.org/10.1146/annurev-physiol-021317-121312
Wu YS, Chen SN (2016) Extracted triterpenes from Antrodia cinnamomea reduce the inflammation to promote the wound healing via the STZ inducing hyperglycemia-diabetes mice model. Front Pharmacol 7:154. https://doi.org/10.3389/fphar.2016.00154
Yang L, Wu H, Lu L, He Q, Xi B, Yu H, Luo R, Wang Y, Zhang X (2021) A tailored extracellular matrix (ECM) - mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type III collagen. Biomaterials 276:121055. https://doi.org/10.1016/j.biomaterials.2021.121055
Yeung DA, Kelly NH (2021) The role of collagen-based biomaterials in chronic wound healing and sports medicine applications. Bioengineering 8(1). https://doi.org/10.3390/bioengineering8010008
Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX (2017) Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 122:34–47. https://doi.org/10.1016/j.biomaterials.2017.01.011
Acknowledgements
The authors would like to acknowledge the faculty and staff at the Biopharmaceutical R&D Center of Jinan University.
Funding
This research was funded by the Guangzhou Science and Technology Plan Project (202103030003) and the School Enterprise Cooperation Industry-University Research Project (E-SKY-YFZX-2020–012).
Author information
Authors and Affiliations
Contributions
QX, DS, and YH contributed to the study conception and design; XL and XM contributed to the acquisition of data and the conduct of the study; JC, QZ, and WC contributed to the material; KY and JC wrote the manuscript; QX modified the manuscript. All the authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethical approval
The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Jinan University (ethical review no. 20200826–11). All experiments were conducted according to the guidelines for animal care and use of China, and they were approved by the animal ethics committee of the Chinese Academy of Medical Sciences.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Mao, X., Cong, J. et al. Recombinantly expressed rhFEB remodeled the skin defect of db/db mice. Appl Microbiol Biotechnol 108, 183 (2024). https://doi.org/10.1007/s00253-024-13021-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13021-9