Abstract
In this study, the effect of polyethylene glycol 8000 (PEG8000) stress on cellulase biosynthesis in Trichoderma reesei CICC2626 via calcium signaling was investigated, and a plausible mechanism by which intracellular Ca2+ regulates the transcription of cellulase genes was proposed. The results indicated that the total cellulase (filter paper-hydrolyzing activity [FPase]), endoglucanase (carboxymethyl cellulase activity [CMCase]), and β-glucosidase activities of the strain were 1.3-, 1.2-, and 1.3-fold higher than those of the control (no PEG8000 addition) at a final concentration of 1.5% (w/v) PEG8000. Moreover, the transcriptional levels of cellulase genes, protein concentrations, and biomass increased. With the synergistic use of commercial cellulase and T. reesei CICC2626 cellulase to hydrolyze alkali-pretreated rice straw, the released reducing sugar concentration reached 372.7 mg/g, and the cellulose content (22.7%, 0.32 g) was significantly lower than the initial content (62.5%, 1.88 g). Transcriptome data showed that 12 lignocellulose degradation–related genes were significantly upregulated in the presence of 1.5% PEG8000. Furthermore, the addition of Ca2+ inhibitors and deletion of crz1 (calcineurin-responsive zinc finger 1-encoding gene, which is related to the calcium signaling pathway) demonstrated that calcium signaling plays a dominant role in PEG8000-induced cellulase genes overexpression. These results revealed a link between PEG8000 induction and calcium signaling transduction in T. reesei CICC2626. Moreover, this study also provides a novel inducer for enhanced cellulase production.
Key points
• Cellulase biosynthesis in Trichoderma reesei could be enhanced by PEG8000
• PEG8000 could induce a cytosolic Ca2+ burst in Trichoderma reesei
• The activated calcium signaling was involved in cellulase biosynthesis
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most developing countries, agricultural residues are usually disposed of in landfills, which can lead to serious environmental problems such as groundwater pollution. Residues such as straw, composed mainly of cellulose, hemicellulose, and lignin, are promising feedstocks for converting biofuels and other chemicals (Douvartzides et al. 2022). However, as a component of lignocellulosic substrates, lignin prevents cellulase from binding to cellulose, resulting in the difficult hydrolysis of natural cellulose (Saini et al. 2016). High cellulase activity is required during enzymatic saccharification to effectively convert cellulose into fermentable sugars. However, the low yield of cellulases is a crucial technical bottleneck for commercial utilization (Hao et al. 2016). As one of the most prominent cellulase producers in nature, Trichoderma reesei has been used to produce cellulase because of its high growth rate and ability to produce highly stable enzymes (Xu et al. 2017).
Additionally, cellulase production requires inducers, including cellulose (Derntl et al. 2017), cellobiose (Kubicek et al. 2009), and sophorose (Mandels et al. 1962), which have long been known. Therefore, it is necessary to identify novel inducers with high cellulase yield. Polyethylene glycol (PEG) is a flexible nonionic surfactant with a glycol subunit (HOCH2CH2OH) that induces hyperosmosis in the solution (Yang et al. 2014). Hemansi et al. (2018) found that the cellulase activity in Aspergillus niger significantly increased after supplementation with PEG8000. Liu et al. (2019) reported that the cellulase activity of Bacillus velezensis A4 increased by 67.8% after adding PEG4000 (polyethylene glycol) to the medium. However, little attention has been given to the regulatory mechanisms underlying cellulase biosynthesis in PEG-exposed microbial cells.
As a secondary messenger, Ca2+ is an important intracellular signaling molecule in microorganisms (Thewes 2014). Under the condition of added organic solvents, intracellular Ca2+ levels in T. reesei were involved in cellulase biosynthesis (Chen et al. 2018; Stranks 1973). Ca2+ is the key component of the calcium signal transduction pathway (Sasanuma and Suzuki 2016). Chen et al. (2016) demonstrated that increased cytosolic Ca2+ levels could activate crz1 (a calcineurin-responsive zinc finger 1-encoding gene related to the calcium signaling pathway), then the activated Crz1 enters the nucleus and binds directly to the upstream regions of the cellulase gene cbh1. Several studies have shown that the calcineurin-Crz1 signaling cascade is ubiquitous in eukaryotes and can be activated by different external stimuli, such as Ca2+ (Soriani et al. 2008), Mn2+ (Cramer et al. 2008), caffeine (Calvo et al. 2009), and antifungal drugs (Edlind et al. 2002). However, the mechanism by which the calcium signaling pathway regulates cellulase biosynthesis in microbial cells induced by PEG8000 remains unclear.
In this study, the effect of PEG8000 on cellulase production and the underlying regulatory mechanism of T. reesei CICC2626 were investigated, the response of calcium signaling to PEG8000 was analyzed, and a potential mechanism by which PEG8000 induces cellulase production through calcium signal transduction in T. reesei was proposed. These results provide novel and valuable insights into effective methods to enhance cellulase production.
Materials and methods
Strains and growth conditions
Escherichia coli DH5α purchased from TransGen Biotech, Beijing, China, was used for plasmid amplification. T. reesei CICC2626 was purchased from China Industrial Microbial Species Conservation and Management Center (CICC). Luria–Broth (LB) medium and YPD medium (yeast extract 10 g/L; peptone 20 g/L; glucose 20 g/L) were adopted to cultivate E. coli DH5α and T. reesei, respectively. Minimal medium (MM) (KH2PO4 6 g/L; (NH4)2SO4 3 g/L; MgSO4·7H2O 1 g/L; CaCl2 1 g/L; ZnSO4·7H2O 0.01 g/L, MnSO4·6H2O 0.01 g/L, CuSO4·7H2O 0.01 g/L, pH 6.0) with 2% glucose was used to assess the effect of PEG8000 on hyphal growth. The reagents used above are purchased from Sangon Biotech Co. Ltd. (Shanghai, China).
Before the experiment, the seed solution of T. reesei was cultured in YPD medium at 30 °C and 150 rpm for 24 h. Then, mycelia of about 0.1 g were collected by centrifugation at 5000 × g and washed using 0.9% NaCl solution. The collected mycelia were inoculated to 100 mL MM medium containing 2% (w/v) Avicel (Sigma, Shanghai, China) as the sole carbon source supplemented with 0% (control), 0.5%, 1.5%, 2%, or 3% (w/v) PEG8000. The corresponding osmotic pressure was 203, 206, 229, 236, and 268 mOsm/kg was measured by FM-8P automatic freezing osmometer (Shanghai Medical Instruments Co., Shanghai, China). The culture solution was aerobically incubated at 150 rpm in a shaker with constant temperature of 30 °C. The samples were collected on the third, fourth, and fifth day after inoculation for detecting enzymatic activity, extracellular protein concentration, and biomass, respectively, or on the 36th and 48th hour for RT-qPCR analyses. To identify the effects of Ca2+ channel of plasma membrane and cytosolic Ca2+ level on cellulase biosynthesis of T. reesei CICC2626, LaCl3 (a plasma membrane Ca2+ channel blocker) or EGTA (ethylene glycol tetraacetic acid, a putative extracellular Ca2+ chelator) (Aladdin, Shanghai, China) as an Ca2+ inhibitor at a concentration of 10 mM was individually added into liquid MM supplemented with 1.5% (w/v) PEG8000 after culture of 1 day, then the culture solution was cultivated for 5 days.
Determination of fungal growth, enzymatic activity, protein concentration, and biomass
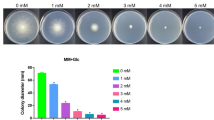
Five milliliters of sterile water was added into the YPD plate of T. reesei being cultured for 3 days, and the mycelia and spores growing on the surface of the plate were gently scraped off from the plate surface. The suspension containing mycelia and spores was filtered through four layers of sterilized gauze, and the filtrate containing spores was collected. The spore count was determined using a blood cell counting plate, and the spore suspension was diluted with sterile water to obtain a concentration of 1 × 106 CFU (colony-forming units)/mL. Next, 5 μL (5 × 103 CFU) of diluent was inoculated onto MM plates and further incubated at 30 °C for 4 days. The radius of each colony was measured using Vernier calipers.
One milliliters of the above-culture solutions supplemented with different amounts of PEG8000 was taken out and filtered through a 0.22-μm microfiltration membrane, and the filtrate as crude enzyme solution was then collected for the following test. The enzymatic activity and extracellular protein concentration in the filtrate were determined. Total cellulase (filter paper-hydrolyzing activity [FPase]) and endoglucanase (carboxymethyl cellulase activity [CMCase]) levels were determined based on the reducing sugars produced using Whatman filter paper and CMC-Na as the substrate. To test FPase activity, Whatman filter paper (1 × 5 cm) and 0.2 mL of the filter liquor were mixed in citrate buffer (pH 4.8), and incubated at 50 °C for 1 h. To detect CMCase activity, 1 mL of 2% (w/v) CMC-Na (Cowin Biotech Co. Ltd., Beijing, China) and 0.2 mL of filter liquor were mixed and incubated at 50 °C for 30 min. The amount of released reducing sugars was determined using the dinitrosalicylic acid (DNS) method (Miller 1959). β-Glucosidase activity was assayed using p-nitrophenyl β-d-glucopyranoside (p-NPG) (Aladdin, Shanghai, China) as described previously (Rajasree et al. 2013). For xylanase determination, 2 mL of 1% (w/v) xylan (Sangon Biotech Co. Ltd., Shanghai, China) and 0.5 mL crude enzyme solution were incubated at 40℃ for 1 h (Bailey et al. 1992). One unit of enzyme activity was defined as the enzyme quantity required to produce 1 μmoL glucose, pNP(p-nitrophenol), or xylose equivalents in 1 min. According to the manufacturer’s instructions, protein concentrations were determined using a total protein quantitative assay kit (Sangon Biotech, Shanghai, China).
To measure biomass, the Eppendorf tube (size 5 mL) was first dried at 40 °C in a vacuum oven till a constant weight (w1), and then 4 mL MM medium culture solution was centrifuged at 10,000 × g at 4 °C for 10 min in a dried Eppendorf tube to collect mycelia. Mycelia were washed three times with sterile water and dried at 80 °C in a vacuum oven until a constant weight (w2) was reached. The biomass in the culture solution was calculated using the following formula
Synergistic hydrolysis of alkali-pretreated rice straw
The rice straw was dried at 50 °C for 24 h, crushed in a mixer, and passed through a 20-mesh sieve. The powder of rice straw was pretreated with NaOH solution (10%, w/v) at a solid/liquid ratio of 1:15 at 80 °C for 2 h. Then, the mixture was filtered with four layers of gauze to collect the solids and rinsed with distilled water until its pH became neutral. After drying at 50 °C, the solids were used for the subsequent experiments. Composition of the rice straw was determined according to the standardized method of the National Renewable Energy Laboratory (NREL, Golden, CO, USA) (Xu et al. 2017). The composition analysis of the raw rice straw showed that the content of cellulose, hemicellulose, and lignin was 37.8, 35.2, and 15.4%, respectively. After alkaline pretreatment, it contained 62.5% cellulose, 23.4% hemicellulose, and 7.8% lignin. The yield of treated residue from rice straw was 76.1%.
Synergistic hydrolysis of alkali-pretreated rice straw by commercial cellulase from A. niger (Aladdin, Shanghai, China) and the crude enzyme (T. reesei CICC2626 cultivated solution from 0 or 1.5% PEG8000 supplement was filtered by 0.22-μm membrane) was performed in 50 mM citrate phosphate buffer (pH 4.8) in a total volume of 20 mL. During hydrolysis, the initial biomass concentration of alkali-pretreated rice straw was 15% (w/v), the enzyme loads for commercial cellulase and crude enzyme were both 15 FPU/g (filter paper activity unit /g) substrate, and the mixture was incubated at 50 °C with 200 rpm agitation for 48 h. Furthermore, commercial cellulase (30 FPU/g substrate) and crude enzyme (30 FPU/g substrate) were incubated individually with 15% (w/v) alkali-pretreated rice straw, and the reactions were performed under the same conditions. Samples were withdrawn at regular intervals, and the supernatant was collected for the reducing sugar assay.
RNA isolation and RT-qPCR
The transcriptional level of mRNA was detected through RT-qPCR. The total RNA was extracted by Trizol reagent (TaKaRa, Dalian, China). cDNA synthesis of the total RNA was performed using the PrimeScript™ RT reagent Kit with gDNA Eraser Kit (TaKaRa Dalian, China) as per the manufacturer’s instructions. RT-qPCR was conducted using an ABI StepOne thermocycler (Applied Biosystems, Foster City, CA, USA), gene transcription was then analyzed using SYBR green assays. The actin (ID: 18482341) gene was applied as internal references to normalize the gene transcription of targets genes. The sequences of the primers for RT-qPCR are shown in Supplementary file 2: Table S1. The relative gene expression levels were analyzed using the 2−ΔΔCt method (Livak 2001).
Transcriptome analysis
The cells were harvested by centrifugation from cultures grown for 36 h in liquid MM medium containing 1% Avicel as the sole carbon source with the addition of 0 (control) or 1.5% PEG8000. Total RNA was respectively extracted from the control group (repeated samples, CK1, CK2, and CK3) and the PEG8000 group (repeated samples, T1, T2, and T3). Then, the extracted RNA was sequenced by Personal Biotechnology Co., Ltd. (Shanghai, China). The sequence reads were mapped to the T. reesei reference genome (https://www.ncbi.nlm.nih.gov/genome/?term=Trichoderma%20reesei) for bioinformatic analysis. The raw whole transcriptome shotgun sequencing data (BioProject ID PRJNA761525) were deposited in the NCBI Sequence Read Archive (SRA) database. The differential analysis of gene expression was performed by DESeq software (version 1.18.0) (Zhang et al. 2014), and the differentially expressed genes were screened out according to expression fold difference log2 |fold change|> 1 and significance p-value < 0.05 (Robinson et al. 2010). To confirm RNA-seq data, five upregulated genes and five downregulated genes were randomly selected using the RNA extracted above as the template for RT-qPCR analysis. These genes and primer sequences used for verifying transcriptome data are shown in Supplementary file 2: Table S1. The information of selected genes and their differential expression levels are shown in Supplementary file 2: Table S2. All experiments were conducted in three biological and technical replications.
Construction of plasmids and transformation
To construct a crz1 (ID:18,484,666) deletion mutant, the upstream (− 1 to − 707 bp) and downstream (+ 2149 to + 2863 bp) fragments of crz1 from the genome of T. reesei CICC2626 were amplified using PrimeSTAR® Max DNA Polymerase (TaKaRa, Dalian, China). First, the upstream and downstream fragments were ligated into hygromycin cassette using overlap extension PCR method to form a fusion gene fragment (Ucrz1-hyg-Dcrz1). Subsequently, the fusion gene fragment was inserted into plasmid pEASY-Blunt Zero Cloning Vector (TransGen Biotech, Shanghai, China) to obtain the gene deletion cassette. The knockout of crz1 in T. reesei was performed using the Agrobacterium-mediated transformation method as described previously (Wang et al. 2020) (Supplementary file 1: Fig. S1a). Positive transformants on YPD plates were selected out using hygromycin B and ampicillin. Then, the putative crz1 disruption mutants (Δcrz1) generated by double crossover were verified by diagnostic PCR. The used primers Crz1-CF1, Crz1-CR1, Crz1-CF2, and Crz1-CR2 are shown in Supplementary file 1: Fig. S1b.
Determinations of cytosolic Ca2+ and ROS levels
The level of cytosolic Ca2+ in T. reesei was measured using a Ca2+ fluorescent probe (Fluo-3AM); the fluorescent intensity of the Fluo-3AM-labelled cells was detected by using a LS-55 spectrophoto fluorometer (PerkinElmer, Waltham, USA) with a 488-nm excitation wavelength and 525-nm emission wavelength. Intracellular Ca2+ green fluorescence was imaged using an inverted fluorescent microscope (Leica, Wetzlar, Germany). The endogenous reactive oxygen species (ROS) level was quantified by ROS Assay Kit as previously described (Cui et al. 2020).
Statistical analysis
Mean values ± standard deviation of triplicate measurement data was shown in this work. Duncan’s multiple-range test was used for multiple comparisons. Student’s t test was used to measure the significance of the difference between the treatment means. p < 0.05(*) or p < 0.01(**) were considered significant.
Results
Cellulase activities, extracellular protein concentration, and biomass in T. reesei CICC2626 with the addition of PEG8000
To evaluate the effect of PEG8000 on cellulase production by T. reesei, the activities of FPase, CMCase, and β-glucosidase in the MM medium were measured (Fig. 1a–c). It can be found that cellulase activities were gradually increased with an increase in PEG8000 concentration from 0 to 1.5% (corresponding to osmotic pressure 203, 206, 229 mOsm/kg) at all times. In particular, the activities of FPase (5.4 ± 0.4 U/mL), CMCase (10.9 ± 1.2 U/mL), and β-glucosidase (4.9 ± 0.5 U/mL) supplemented with 1.5% PEG8000 were 1.3-, 1.2-, and 1.3-fold those of the control group, respectively. Those indexes in the control group were 4.1 ± 0.3, 9.0 ± 0.8, and 3.7 ± 0.3 U/mL after incubation for 5 days. Because cellulases are extracellular enzymes, the extracellular protein concentration can be used to monitor cellulase production by microbial cells (Mishra et al. 2021). As shown in Fig. 1d, the changes in extracellular protein concentration were consistent with those of cellulase activity. The protein concentration reached a maximum of 0.5 ± 0.1 mg/mL with 1.5% PEG8000 treatment after incubation for 5 days, which was 1.7-fold that of the control (0.3 ± 0.1 mg/mL). However, the protein concentration significantly decreased with a further increase in PEG8000 concentration. The results indicated that cellulase production by T. reesei drastically increased with a final concentration of 1.5% PEG8000 supplementation in the MM medium.
Effects of different concentrations of PEG8000 at the final concentrations of 0, 0.5, 1.5, 2, and 3% (w/v) on the FPase activity (a), CMCase activity (b), β-glucosidase activity (c), extracellular protein concentration (d), biomass (e), and the transcription levels of cellulase genes (f) of T. reesei CICC2626. The seed solution of T. reesei CICC2626 was cultured in YPD medium for 24 h, transformed to fresh MM medium containing 2% (w/v) Avicel with 0 to 3% PEG8000, and then cultivated for 36 to 120 h. Blue bar, addition of 0% (w/v) PEG8000; purple bar, addition of 0.5% (w/v) PEG8000; green bar, addition of 1.5% (w/v) PEG8000; red bar, addition of 2% (w/v) PEG8000; pink bar, addition of 3% (w/v) PEG8000. Values are the mean ± SD of the results from three independent experiment. Asterisks indicate significant differences (*p < 0.05, **p < 0.01, Student’s t test)
The most abundantly secreted cellulases in T. reesei are cellobiohydrolases (EC 3.2.1.91) and endo-β-1,4-endoglucanases (EC 3.2.1.4) (Häkkinen et al. 2012). To clarify whether the improved cellulase activity could be attributed to the biomass or expression levels of the cellulase genes, the biomass and expression levels of the two main cellulase genes (egl1 encoding endoglucanase I, cbh1 encoding cellobiohydrolase I) were determined. As shown in Fig. 1e, the biomass of T. reesei gradually increased with a final concentration of 0–1.5% (w/v) PEG8000 supplementation in the MM medium. The maximal biomass (13.6 ± 1.2 g/L) was achieved after incubation for 4 days when 1.5% PEG8000 was added to the culture medium, which was 1.9-fold that of the control group (7.3 ± 0.9 g/L). PEG has good biocompatibility and biodegradability. In the environment, PEG does not cause pollution to soil, water, and air because it is broken down into smaller molecules by microorganisms. In the biological body, PEG can also be hydrolyzed by enzymes into harmless substances such as water and carbon dioxide, which will not cause harm to the human body (Gao et al. 2022). Some studies have shown that methanogenic bacteria can degrade PEG (Dwyer et al. 1983). Therefore, we hypothesized that PEG8000 may be decomposed in the growth process of T. reesei, thus increasing the biomass. The mycelial morphology of T. reesei grown on YPD plates containing PEG8000 is shown in Supplementary file 1: Fig. S2. There was little difference in colony radii among the 0, 0.5, and 2% groups. However, when 3% PEG8000 was added, the colony radius decreased. The maximal colony radius (3.6 ± 0.1 cm) was achieved after incubation for 4 days supplemented with 1.5% PEG8000, which was 1.1-fold that of the control group (3.2 ± 0.1 cm). In addition, the hyphal morphology of 0.5, 1.5, and 2% PEG8000 was fuller and stronger than that of 0 and 3% PEG8000, and the mycelial growth state was the worst when supplemented with 3% PEG8000. This may be owing to the high osmotic pressure caused by adding 3% PEG8000 (268 mOsm/kg), which inhibits mycelial growth. As illustrated in Fig. 1f, the transcript levels of two cellulase genes (egl1 and cbh1) displayed a sharp upregulation. In the 1.5% PEG8000 group, the transcription levels of the two genes were 4.9- and 5.6-fold higher than those in the control group. However, the transcriptional levels in cells supplemented with 3% PEG8000 were only 0.7- and 0.9-fold higher than in control. The endogenous ROS content was significantly reduced with an increase in PEG8000 concentration from 0 to 1.5%. However, ROS levels in the 3% PEG8000 treatment were observably higher than those in the 0% (Supplementary file 1: Fig. S3). These results suggest that higher PEG8000 levels may increase intracellular ROS levels, inhibiting mycelial growth and cellulase production. Based on these results, the increase in cellulase activity in T. reesei induced by PEG8000 can be ascribed to an increase in biomass and the overexpression of cellulase genes. The optimal final concentration of PEG8000 for enhancing cellulase production was 1.5%.
Synergistic effect with commercial cellulase in the hydrolysis of alkali-pretreated rice straw
Rice straw is an abundant agricultural residue and is a promising feedstock for the production of biofuels and other chemicals due to its high cellulose and hemicellulose content. In general, the hydrolysis efficiency of rice straw can be enforced through the synergistic effect of the collected crude enzyme and the commercial cellulase (Liu et al. 2022). Here, the synergistic effect of the crude enzyme (T. reesei CICC2626 cultivated solution from 1.5% PEG8000 supplement was filtered by 0.22-μm membrane) and commercial cellulase on the degradation of alkali-pretreated rice straw was evaluated. After hydrolysis for 48 h, the reducing sugar concentration for synergy of commercial cellulase (15 FPU/g substrate) and 15 FPU/g substrate of crude enzyme was 372.7 mg/g, which was 1.1- and 1.2-fold those of commercial cellulase (332.5 mg/g) and crude enzyme (298.6 mg/g), respectively (Fig. 2a). This is because the rice straw after alkali treatment contains a large amount of hemicellulose (23.4%), which can be hydrolyzed by xylanase in crude enzyme (Supplementary file 1: Fig. S4), resulting in the exposed cellulase being easier to hydrolyze into reducing sugar by cellulase.
Synergistic hydrolysis of pretreated rice straw by crude enzyme and commercial cellulase from 1.5% PEG8000 supplement culture. a The concentration of reducing sugar after hydrolysis. b–c The percentage and weight of cellulose, hemicellulose, and lignin in alkali-pretreated rice straw residues after hydrolysis. The hydrolysis reaction was carried out with 15% (w/v) alkali-pretreated rice straw in a 20-mL system. After the hydrolysis reaction, the treated residue yield of groups commercial cellulase, crude enzyme, and commercial cellulase + crude enzyme from rice straw was 1.43, 1.45, and 1.40 g respectively. Values are the mean ± SD of the results from three independent experiments. Asterisks indicate significant differences (*p < 0.05, Student’s t test)
In addition, the contents of cellulose, hemicellulose, and lignin in alkali-pretreated rice straw residue were analyzed before and after hydrolysis. As shown in Fig. 2b and c, the content of cellulose significantly decreased after enzymatic hydrolysis, and the minimal content of cellulose (22.7%, 0.32 g) was obtained in the treatment with commercial cellulase and crude enzyme, which was significantly lower than the initial content (62.5%, 1.88 g). The obtained result shows that the mixture enzymes of commercial cellulase and crude enzyme could improve the hydrolysis efficiency of cellulose. Therefore, cellulases produced by T. reesei CICC2626 could collaborate with commercial cellulase in the biotransformation of lignocellulosic biomass. Of note, the hemicellulose content in the residues after hydrolysis by crude enzyme (23.6%, 0.34 g) was significantly lower than that of commercial enzyme (32.5%, 0.46 g); however, the cellulose content of residues hydrolyzed by crude enzyme (30.5%, 0.44 g) was slightly higher than that by commercial cellulase (28.3%, 0.40 g). This can be attributed to xylanase produced by T. reesei CICC2626 (Supplementary file 1: Fig. S4), which promoted the hydrolysis of hemicellulose. In addition, the synergistic effect of the crude enzyme (T. reesei CICC2626 cultivated solution from 0% PEG8000 supplement was filtered by 0.22-μm membrane) and commercial cellulase on the degradation of alkali-pretreated rice straw was also evaluated. As shown in Supplementary file 1: Fig. S5, the change trend of reducing sugar concentration and the percentage or weight of cellulose, hemicellulose, and lignin in alkali-pretreated rice straw residues after hydrolysis was consistent with that of 1.5% PEG8000 supplement culture. These results indicate that crude enzyme and commercial cellulase possessed clear synergistic effects on alkali-pretreated rice straw hydrolysis.
Transcriptome of T. reesei CICC2626 supplemented with PEG8000
To gain insight into the potential mechanism by which the expression of cellulase genes is regulated by PEG8000 addition, two transcriptomes of T. reesei CICC2626 cultured in media without PEG8000 as control and with 1.5% PEG8000 were compared. The transcriptome data indicated that 3850 genes showed differential expression between the two groups with a log2 |fold change|> 1 and a significant p-value < 0.05. Of the 3850 differentially expressed genes (DEGs), 2081 were upregulated and 1769 were downregulated (Supplementary file 1: Fig. S6 and Supplementary file 2: Table S2). Furthermore, functional categorization and metabolic processes of the 3850 DEGs were performed using Gene Ontology terms (https://urldefense.com/v3/__https://geneontology.org/__;!!NLFGqXoFfo8MMQ!pW8xTxVrhoJQuCYrJLGO5j6gT_5ysXjbSrX6ZF01JwmkI2Qstmc2AkywLpdYvxPvxxB8cb9C1zYR05Kwe8tpV_t8_nTf$) and KEGG (https://urldefense.com/v3/__https://www.genome.jp/kegg/__;!!NLFGqXoFfo8MMQ!pW8xTxVrhoJQuCYrJLGO5j6gT_5ysXjbSrX6ZF01JwmkI2Qstmc2AkywLpdYvxPvxxB8cb9C1zYR05Kwe8tpV6HHoSwX$) pathway enrichment analysis, respectively. Analysis using Gene Ontology (GO) terms showed that in the presence of PEG8000, most of the DEGs were related to ribosome, structural constituent of ribosome, and small molecule metabolic process (Supplementary file 2: Table S3). On the other hand, KEGG pathway enrichment analysis showed that most of the DEGs were related to pentose phosphate pathway, MAPK signaling pathway, calcium signaling pathway, and mRNA surveillance pathway with 1.5% PEG8000 treatment (Supplementary file 2: Table S4). Five upregulated and five downregulated genes were selected for RT-qPCR analysis to verify the RNA-seq data (Supplementary file 1: Fig. S7). The validated DEGs were consistent with the RNA-seq results, supporting the validity of the RNA-seq data.
In total, 12 lignocellulose degradation–related genes were significantly upregulated in the 1.5% PEG8000 addition group (Table 1). Three main cellobiohydrolase-encoding genes (cel7a, cel6a and cbh; IDs: 18483782, 18488147, and 18483234), six endoglucanase-encoding genes (IDs: 18483009, 18488225, 18483598, 18482842, 18483318, and 18484770; including cel61b, cel61a, cel12a, cel5a, cel7b, and egl), two β-glucosidase-encoding genes (IDs: 18483230 and18482947; including cel3b and cel1a), and an α-xylosidase gene (ID: 18487910) were upregulated with 1.5% PEG8000 addition. However, 2 endoglucanase-encoding genes (IDs: 18485669 and 18489491; including cel5b and egl2) and a β-xylosidase gene (ID: 18484697) were downregulated in the 1.5% PEG8000 addition group. In addition, there was no significant difference in the expression of cel6b (ID: 18484386; encoding endoglucanase) between the two groups. The transcriptome data further identified cellulase activities and RT-qPCR result (Fig. 1). It reveals that the presence of 1.5% PEG8000 significantly upregulated the transcription levels of the lignocellulose degradation–related genes.
Notably, all four genes in the calcium signaling pathway were upregulated by adding of PEG8000 compared to those in the control (Supplementary file 2: Table S2). Therefore, we investigated the relationship between the upregulated expression of cellulase genes and calcium signaling in the presence of PEG8000. As shown in Table 2, the gene plc-e (ID: 18484061), which encodes a phospholipase C protein that generates inositol-1,4,5-trisphosphate (IP3) to regulate calcium release from intracellular pools (Schmoll 2008), was remarkably upregulated in the 1.5% PEG8000 group compared to that in the control. In addition, three genes cam (ID: 18489174; encoding calmodulin), can (ID: 18486548; encoding calcineurin), and crz1 (ID: 18484666; encoding transcription factor Crz1 which are related to calcium signaling pathway) were upregulated in the 1.5% PEG8000 group.
The transcription levels of plc-e, cam, can, and crz1 were analyzed using RT-RT-qPCR (Supplementary file 1: Fig. S8). The RT-qPCR results were consistent with the whole-transcriptome shotgun sequencing analysis; this suggests that 1.5% PEG8000 treatment upregulated the intracellular cytosolic Ca2+ level in T. reesei CICC2626, and that calcium signaling transduction pathway might have been involved in expression regulation of these cellulase genes.
Effect of cytosolic Ca2+ level on cellulase production by T. reesei CICC2626 with PEG8000 treatment
Based on the transcriptional profiling results, it can be deduced that PEG8000 influences cytosolic Ca2+ levels. The cytosolic Ca2+ levels were measured using the Fluo-3 AM fluorescent dye method to verify this (Wang et al. 2020). To determine whether the cytosolic Ca2+ burst was induced by PEG8000-mediated cellulase biosynthesis, EGTA, a putative extracellular Ca2+ chelator, or LaCl3, a Ca2+ channel blocker of the plasma membrane, was added to the culture medium.
As shown in Fig. 3a and b, the green fluorescence intensity of hyphae treated with 1.5% PEG8000 was stronger than that of the control (without PEG8000). However, the green fluorescence intensity of mycelia treated with PEG8000 + LaCl3 or PEG8000 + EGTA was markedly decreased compared to that of mycelia exposed only to PEG8000. This result demonstrates that the cytosolic Ca2+ burst induced by PEG8000 was effectively blocked by adding LaCl3 or EGTA; this revealed that PEG8000 increased cytosolic Ca2+ levels in T. reesei CICC2626 cells.
Effects of Ca2+ inhibitors on the fluorescence intensity (a), relative fluorescent ratio (b), FPase activity (c), CMCase activity (d), β-glucosidase activity (e), extracellular protein concentration (f), and expression levels of egl1 (g) and cbh1 (h) of T. reesei CICC2626. T. reesei CICC2626 was cultured in YPD medium for 24 h, transformed to fresh MM containing 2% (w/v) Avicel with 0 or 1.5% (w/v) PEG8000 and 0 or 10 mM LaCl3/EGTA, and cultivated for 36 to 120 h. For detection, Fluo-3 AM was used, and fluorescence intensity was detected using inverted fluorescence microscopy. Blue bar, addition of 1.5% (w/v) PEG8000 in strain; red bar, addition of 0% (w/v) PEG8000 in strain. CK, 0% (w/v) PEG8000 (control) and 1.5% (w/v) PEG8000 group. Green fluorescence represents free cytosolic Ca2+. Values are mean ± standard deviation (n = 3). Different letters indicate significant differences between the columns (p < 0.05, Duncan’s multiple-range test)
As shown in Fig. 3, the cellulase activity, extracellular protein concentration, and transcriptional levels of egl1 and cbh1 in the PEG8000 + LaCl3 and PEG8000 + EGTA groups were decreased compared with those in the CK groups (PEG8000 group, control group) at the same cultivation time. Specifically, the activities of FPase, CMCase, and β-glucosidase, and protein the concentration in the PEG8000 + LaCl3 and PEG8000 + EGTA groups were significantly decreased by 73.74–87.01% on the fifth day, respectively, compared to the CK groups (only PEG8000) (Fig. 3c–f). The transcriptional levels of egl1 and cbh1 in the PEG8000 + LaCl3 or PEG8000 + EGTA groups decreased by 75.72–75.93% than those of the PEG8000 group of the CK on the 48th hour of inoculation (Fig. 3g, h). However, the biomasses of the PEG8000 + LaCl3 and PEG8000 + EGTA groups were not significantly different from that of the CK groups (PEG8000 group, control group) at the same cultivation time (Supplementary file 1: Fig. S9). These data indicated that the decrease in cellulase activity and transcriptional levels of cellulase genes was independent of biomass after adding LaCl3 and EGTA. These results demonstrated that blocking the cytosolic Ca2+ burst using Ca2+ inhibitors can significantly attenuate cellulase production in T. reesei exposed to PEG8000.
Expression of cellulase gene mediated by calcium signaling with PEG8000 treatment
To further clarify the role of calcium signaling in cellulase synthesis in the presence of PEG8000, a crz1 deletion mutant Δcrz1 of T. reesei was constructed. As shown in Fig. 4a and b, the green fluorescence intensities of the wild type (WT) and Δcrz1 strains exposed to PEG8000 were higher than those of the control (WT and Δcrz1 strain without PEG8000), which further confirmed that PEG8000 induced an increase in cytosolic Ca2+ levels. Meanwhile, the intracellular fluorescence intensities of the WT and Δcrz1 strains were almost the same in the control group and the PEG8000 groups, suggesting that PEG8000 induced an increase in the cytosolic Ca2+ level.
Effect of crz1 on the fluorescence intensity (a), relative fluorescent ratio (b), FPase activity (c), CMCase activity (d), β-glucosidase activity (e), extracellular protein concentration (f), and expression levels of egl1 (g) and cbh1 (h) of wild type and Δcrz1 strains. Wild-type and Δcrz1 strains were cultured in YPD medium for 24 h, transformed to fresh MM containing 2% (w/v) Avicel with 0% or 1.5% (w/v) PEG8000, and cultivated for 36 to 120 h. Blue bar, addition of 1.5% (w/v) PEG8000 in wild-type strain; red bar, addition of 0% (w/v) PEG8000 in wild-type strain; purple bar, addition of 1.5% (w/v) PEG8000 in Δcrz1; green bar, addition of 0% (w/v) PEG8000 in Δcrz1. Green fluorescence represents free cytosolic Ca2+. Values are mean ± standard deviation (n = 3). Asterisks indicate significant differences (*p < 0.05, Student’s t test)
As illustrated in Fig. 4c–h, cellulase activities, protein concentration, and transcriptional levels of egl1 and cbh1 in Δcrz1 + PEG8000 group or Δcrz1 group were decreased, compared to those in WT + PEG8000 group or WT at all times. In the PEG8000 group, the activities of FPase, CMCase, β-glucosidase, and extracellular protein concentration in the WT strain were 1.6-, 1.7-, and 1.7-fold, respectively, for the Δcrz1 strain on the 5th day of inoculation (Fig. 4c–f). Nevertheless, the biomass of the WT + PEG8000 or Δcrz1 + PEG8000 groups was higher than that of the WT or Δcrz1 strains without PEG8000 addition. The biomasses of the WT and Δcrz1 strains were similar in the control group and PEG8000 group (Supplementary file 1: Fig. S10); this suggests that the cellulase activities of the Δcrz1 strain treated with PEG8000 were remarkably decreased, and knockout crz1 had little effect on strain growth. This may be due to the enhanced influence of other signaling pathways (such as MAPK signaling pathway) on the growth and development of cells after the deletion of crz1 gene, and the activation of the enzyme coding genes related to the decomposition of PEG8000 to maintain the normal growth of cells. In addition, the transcription levels of egl1 and cbh1 of WT supplemented with PEG8000 were 4.1-fold and 4.6-fold than the indexes in Δcrz1 strains treated with PEG8000 at 48 h (Fig. 4g, h). These data showed that the transcription levels of the cellulase genes were downregulated in Δcrz1 strains compared to those in the WT strains treated with PEG8000. Taken together, these results demonstrated that the significant increase in levels of egl1 and cbh1 transcription induced by PEG8000, observed in the wild type, was abolished in the crz1 deletion strain. Chen et al. (2018) reported that that calcium signaling plays a dominant role in cellulase overexpression in T. reesei. Based on the results of this and previous studies, it can be considered that the calcium signaling pathway is involved in cellulase biosynthesis in T. reesei treated with PEG8000.
Discussion
High cellulase production by T. reesei requires inducers, and traditional inducers include cellulose and cellobiose (Derntl et al. 2017; Kubicek et al. 2009). PEG8000 can accelerate the elimination of amorphous cellulose and promote cellulase synthesis (Reese and Maguire 1969). Studies have shown that adding PEG can not only affect the biosynthesis of cellulase, but also reduce the adsorption capacity of cellulase to lignin so as to reduce the enzyme loading and promote the effective combination of enzyme and substrate (Luo et al. 2017). In addition, compared with organic solvent inducers such as N,N-dimethylformamide (DMF) (Chen et al. 2019), PEG has good biocompatibility and biodegradability (Gao et al. 2022), which ensures the safety of the biological process and the enzyme produced.
In this study, cellulase activities, extracellular protein concentration, biomass, and transcriptional levels of cellulase genes (egl1 and cbh1) in T. reesei CICC2626 supplemented with 1.5% PEG8000 were significantly increased higher than those in the control group (Fig. 1); this implies that PEG8000 could be a novel inducer for T. reesei cellulase production. However, when PEG8000 was increased to 3% (268 mOsm/kg), enzyme activity, mycelial growth, and biomass of the cells decreased (Fig. 1), whereas intracellular ROS levels increased (Supplementary file 1: Fig. S3). Liu et al. (2022) confirmed that increased intracellular ROS levels were detrimental to cellulase production. It can be inferred that PEG8000 can promote growth and enzyme production under appropriate addition (0.5–1.5%), but high addition (3%) will cause osmotic stress, producing ROS, and affect mycelia’s growth and enzyme production.
Previous studies have shown that mixed enzymes containing cellulase and xylanase are more efficient than cellulase alone for decomposing lignocellulosic substrates (Long et al. 2018). Our research demonstrated that the crude enzyme produced by T. reesei CICC2626 contains xylanase activity (Supplementary file 1: Fig. S4). The results also indicated that crude enzyme mixed with commercial cellulase have the potential to be applied to the hydrolysis of other agricultural and forestry wastes (Fig. 2). Similar results were obtained for the crude enzymes produced by Streptomyces DSK59 (Budihal et al. 2015).
Transcriptome data revealed that 12 lignocellulose degradation–related genes were upregulated in the 1.5% PEG8000 addition group (Table 1). This result further suggested that the enhancement of cellulase production by T. reesei CICC2626 exposed to PEG8000 could be attributed to the overexpression of cellulase genes. Extracellular stress can be sensed by mechanoreceptors in the cytoskeleton, activating intracellular signaling pathways that transfer stress signals to the nucleus, resulting in changes in gene expression and enzyme synthesis (Ingber and Physiology 1997). As shown in Table 2, the transcriptional levels of the calcium signaling pathway–related genes (plc-e, cam, can, and crz1) were significantly upregulated after treatment with 1.5% PEG8000. Studies have suggested that different external stimuli, including metal ions (Schumacher et al. 2008), temperature (Chen et al. 2012), and ethanol (Araki et al. 2009), can activate the calcineurin-Crz1 signaling cascade. All these stresses cause an increase in cytosolic Ca2+ concentration. The increase in cytosolic Ca2+ levels led to the activation of calmodulin, which dephosphorylated its target proteins, such as Crz1. The dephosphorylated Crz1 was bonded to its target promoters in the nucleus to regulate gene expression (Manoli and Espeso 2019). Moreover, Chen et al. (2019) found that the phospholipase C-encoding gene plc-e were significantly upregulated during DMF-induced production of cellulase by T. reesei Rut-C30, suggesting that calcium signaling was involved in the expression of cellulase genes. To our knowledge, there are no former reports that show higher Ca2+ concentration in the medium promotes an increase in cellulose activities. The results of our previous study showed that the concentration of Ca2+ in the medium had little effect on Bacillus subtilis cellulase activities (Liu et al. 2022). These results demonstrate that the calcium signaling pathway may participate in PEG8000-induced cellulase overexpression in T. reesei CICC2626.
Ca2+ is an important intracellular signaling molecule that regulates primary and secondary metabolism in microorganisms (Zhang et al. 2013). Wang et al. (2020) reported that cellulase activity was positively correlated with increased cytosolic Ca2+ levels in Ganoderma lucidum. In this study, although PEG8000 induced a cytosolic Ca2+ burst, it was effectively blocked by Ca2+ inhibitors (LaCl3 and EGTA), resulting in decreased cellulase activity (Fig. 3). Crz1, a calcineurin-responsive zinc-finger transcription factor 1, has been found to play an essential role in calcium signal transduction in filamentous fungi (Sinha et al. 2021). Our work demonstrates that Ca2+-activated Crz1 participated in the regulation of cellulase gene expression following the PEG8000 treatment (Fig. 4). A similar result was found for T. reesei Rut-C30, in which cellulase activities in Δcrz1 strain were reduced (Chen et al. 2018). In addition, Chen et al. (2019) demonstrated that intracellular Ca2+ plays an important role in the production of lignocellulolytic enzymes through the calcineurin-Crz1 signaling cascade under DMF induction in T. reesei Rut-C30. Therefore, it can be concluded that calcium signaling was involved in the expression regulation of cellulase genes in T. reesei CICC2626 exposed to PEG8000.
Based on the results of this study, a plausible mechanism by which calcium signaling regulated cellulase biosynthesis in T. reesei CICC2626 induced by PEG8000 was inferred (Fig. 5). The cytosolic Ca2+ level significantly increased after the PEG8000 treatment, which caused the transcriptional upregulation of calcium signaling pathway–related genes (plc-e, cam, can, and crz1) in T. reesei. The transcription factor Crz1 is then activated, thereby promoting the overexpression of cellulase genes (egl1 and cbh1) in T. reesei treated with PEG8000. As a result, cellulase production by T. reesei is enhanced. The finding that cellulase activity decreased in the PEG8000 + LaCl3 and PEG8000 + EGTA groups and Δcrz1 strain further supports this view. These results demonstrate that the calcium signaling pathway could regulate the overexpression of cellulase genes in T. reesei. Thus, a putative regulation mechanism on cellulase biosynthesis in T. reesei CICC2626 induced by PEG8000 was proposed.
Data availability
The raw whole-transcriptome shotgun sequencing data have been deposited into the NCBI Sequence Read Archive (SRA) database under BioProject accession No. PRJNA761203.
References
Araki Y, Wu H, Kitagaki H, Akao T, Takagi H, Shimoi H (2009) Ethanol stress stimulates the Ca2+-mediated calcineurin/Crz1 pathway in Saccharomyces cerevisiae. J Biosci Bioeng 107(1):1–6. https://doi.org/10.1016/S0166-445X(03)00098-5
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270. https://doi.org/10.1016/0168-1656(92)90074-J
Budihal R, Saikumar A, Dayanand P, Sarvamangala R (2015) Enhanced production and application of acidothermophilic Streptomyces cellulase. Bioresour Technol 7:706–712. https://doi.org/10.1016/j.biortech.2015.10.098
Calvo IA, Gabrielli N, Iglesias-Baena I, Garcia-Santamarina S, Hoe KL, Kim DU, Sanso M, Zuin A, Perez P, Ayte J, Hidalgo E (2009) Genome-wide screen of genes required for caffeine tolerance in fission yeast. PLoS One 4(8):10. https://doi.org/10.1371/journal.pone.0006619
Chen L, Zou G, Wang J, Wang J, Liu R, Jiang Y, Zhao G, Zhou Z (2016) Characterization of the Ca2+-responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol Microbiol 100(3):560–575. https://doi.org/10.1111/mmi.13334
Chen YL, Konieczka JH, Springer DJ, Bowen SE, Zhang J, Silao FGS, Bungay AAC, Bigol UG, Nicolas MG, Abraham SN, Thompson DA, Regev A, Heitman J (2012) Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3: Genes Genomes Genet 2(6):675–691. https://doi.org/10.1534/g3.112.002279
Chen YM, Shen YL, Wang W, Wei DZ (2018) Mn2+ modulates the expression of cellulase genes in Trichoderma reesei Rut-C30 via calcium signaling. Biotechnol Biofuels 11:54. https://doi.org/10.1186/s13068-018-1055-6
Chen YM, WunC SYL, Ma YS, Wei DZ, Wang W (2019) N, N-Dimethylformamide induces cellulase production in the filamentous fungus Trichoderma reesei. Biotechnol Biofuels 12:36. https://doi.org/10.1186/s13068-019-1375-1
Cramer RA, Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, Heitman J, Perfect JR, Steinbach WJ (2008) Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot Cell 7(7):1085–1097. https://doi.org/10.1128/EC.00086-08
Cui J, Yu C, Zhong DB, Yu X (2020) Melatonin and calcium act synergistically to enhance the coproduction of astaxanthin and lipids in Haematococcus pluvialis under nitrogen deficiency and high light conditions. Bioresour Technol 305:123069. https://doi.org/10.1016/j.biortech.2020.123069
Derntl C, Kluger B, Bueschl C, Schuhmacher R, Mach RL, Machaigner AR (2017) Transcription factor xpp1 is a switch between primary and secondary fungal metabolism. Proc Natl Acad Sci USA 114:E560. https://doi.org/10.1073/pnas.1609348114
Douvartzides S, Charisiou ND, Wang W, Papadakis VG, Polychronopoulou K, Goula MA (2022) Catalytic fast pyrolysis of agricultural residues and dedicated energy crops for the production of high energy density transportation biofuels. part ii: catalytic research. Renew Energy 189:315–338. https://doi.org/10.1016/j.renene.2022.02.106
Dwyer DF, Tiedje JM (1983) Degradation of ethylene-glycol and polyethylene glycols by methanogenic consortia. Appl Environ Microbiol 46(1):185–190. https://doi.org/10.1128/AEM.46.1.185-190.1983
Edlind T, Smith L, Henry K, Katiyar S, Nickels J (2002) Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signaling. Mol Microbiol 46(1):257–268. https://doi.org/10.1046/j.1365-2958.2002.03165.x
Häkkinen M, Arvas M, Oja M, Aro N, Penttilä M, Saloheimo M, Pakula TM (2012) Re annotation of the CAZy genes of Trichoderma reesei and transcription in the presence of lignocellulosic substrates. Microb Cell Fact 11(1):134. https://doi.org/10.1186/1475-2859-11-134
Gao L, Chen L, Zhang T, Li P, Lu H, An R (2022) Preparation and characterization of polyethylene glycol/chitosan composite water-based wound healing lubricant. Front Bioeng Biotech 10:990249. https://doi.org/10.3389/fbioe.2022.990249
Hao LY, Wang R, Wang L, Fang KJ, Liu JQ, Men YJ (2016) The influences of enzymatic processing on physico-chemical and pigment dyeing characteristics of cotton fabrics. Cellulose 23(1):929–940. https://doi.org/10.1007/s10570-015-0804-y
Hemansi Gupta R, Kuhad RC, Saini JK (2018) Cost effective production of complete cellulase system by newly isolated Aspergillus niger RCKH-3 for efficient enzymatic saccharification: Medium engineering by overall evaluation criteria approach (OEC). Biochem Eng J 132:182–190. https://doi.org/10.1016/j.bej.2018.01.019
Ingber D, Physiology EJ (1997) Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59(1):575–575. https://doi.org/10.1146/ANNUREV.PHYSIOL.59.1.575
Kubicek CP, Mikus M, Schuster A, Schmoll M, Seiboth B (2009) Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol Biofuels 2:19. https://doi.org/10.1186/1754-6834-2-19
Liu Y, Guo H, Gu J, Qin WJPB (2019) Optimize purification of a cellulase from Bacillus velezensis A4 by aqueous two-phase system (ATPS) using response surface methodology. Process Biochem 87:196–203. https://doi.org/10.1016/j.procbio.2019.08.017
Liu S, Gao YW, Quan L, Yang M, Wang YZ, Hou CJ (2022) Improvement of lignocellulolytic enzyme production mediated by calcium signaling in Bacillus subtilis Z2 under graphene oxide stress. Appl Environ Microbiol 88(19). https://doi.org/10.1128/aem.00960-22
Long L, Tian D, Zhai R, Li X, Zhang Y, Hu J (2018) Thermostable xylanase-aided two-stage hydrolysis approach enhances sugar release of pretreated lignocellulosic biomass. Bioresour Technol 257:334–338. https://doi.org/10.1016/j.biortech.2018.02.104
Lou H, Zeng M, Hu Q, Cai C, Lin X, Qiu X, Yang D, Pang Y (2017) Nonionic surfactants enhanced enzymatic hydrolysis of cellulose by reducing cellulase deactivation caused by shear force and air-liquid interface. Bioresour Technol 249(1):1–8. https://doi.org/10.1016/j.biortech.2017.07.066
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods: Companion Methods Enzymol 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Manoli M-T, Espeso EA (2019) Modulation of calcineurin activity in Aspergillus nidulans: the roles of high magnesium concentrations and of transcriptional factor CrzA. Mol Microbiol 111(5):1283–1301. https://doi.org/10.1111/MMI.14221
Mandels M, Parrish FW, Reese ET (1962) Sophorose as an inducer of cellulase in Trichoderma viride. J Bacteriol 83:400. https://doi.org/10.1128/JB.83.2.400-408
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030/
Mishra CSK, Samal S, Sishu NK, Subhadarshini A, Naik P (2021) Antioxidants in organic manures can facilitate exoenzyme activities in herbicide treated farm soil. Chem Ecol 37(4):342–351. https://doi.org/10.1080/02757540.2021.1888936
Rajasree KP, Mathew GM, Pandey A, Sukumaran RK (2013) Highly glucose tolerant β-glucosidase from Aspergillus unguis: NII 08123 for enhanced hydrolysis of biomass. J Ind Microbiol Biotechnol 40(9):967–975. https://doi.org/10.1007/s10295-013-1291-5
Reese E, Maguire A (1969) Surfactants as stimulants of enzyme production by microorganisms. Appl Microbiol Biotechnol 17(2):242–245. https://doi.org/10.1128/AM.17.2.242-245.1969
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. https://doi.org/10.1093/bioinformatics/btp616
Saini JK, Patel AK, Adsul M (2016) Cellulase adsorption on lignin: a roadblock for economic hydrolysis of biomass. Renew Energy 98:29–42. https://doi.org/10.1016/j.renene.2016.03.089
Sasanuma I, Suzuki T (2016) Effect of calcium on cell-wall degrading enzymes of Botrytis cinerea. Biosci Biotechnol Biochem 80(9):1730–1736. https://doi.org/10.1080/09168451.2016.1146064
Schmoll M (2008) The information highways of a biotechnological workhorse - signal transduction in Hypocrea jecorina. BMC Genomics 9:430. https://doi.org/10.1186/1471-2164-9-430
Schumacher J, de Larrinoa IF, Tudzynski B (2008) Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot Cell 7(4):584–601. https://doi.org/10.1128/EC.00426-07
Soriani FM, Malavazi I, Ferreira MED, Savoldi M, Kress MRV, Goldman MHD, Loss O, Bignell E, Goldman GH (2008) Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol Microbiol 67(6):1274–1291. https://doi.org/10.1111/j.1365-2958.2008.06122.x
Sinha M, Shree A, Singh K, Kumar K, Singh SK, Kumar V, Verma PK (2021) Modulation of fungal virulence through CRZ1 regulated F-BAR-dependent actin remodeling and endocytosis in chickpea infecting phytopathogen Ascochyta rabiei. PLoS Genet 17(5):33. https://doi.org/10.1371/JOURNAL.PGEN.1009137
Stranks DW (1973) Influence of phenethyl alcohol and other organic-solvents on cellulase production. Can J Microbiol 19(12):1523–1526. https://doi.org/10.1139/m73-250
Thewes S (2014) Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot Cell 13:694–705. https://doi.org/10.1128/EC.00038-14
Wang S, Han J, Xia J, Hu Y, Shi L, Ren A, Zhu J, Zhao M (2020) Overexpression of nicotinamide mononucleotide adenylyltransferase (nmnat) increases the growth rate, Ca2+ concentration and cellulase production in Ganoderma lucidum. Appl Microbiol Biotechnol 104(16):7079–7091. https://doi.org/10.1007/s00253-020-10763-0
Xu J, Gao Z, Wu B, He B (2017) Lactose-inducted production of a complete lignocellulolytic enzyme system by a novel bacterium Bacillus sp BS-5 and its application for saccharification of alkali-pretreated corn cob. Cellulose 24(5):2059–2070. https://doi.org/10.1007/s10570-017-1247-4
Yang H, Dung C, Xu X, Wang Y, Han Z (2014) Effects of NaCl and iso-osmotic polyethylene glycol stress on Na+/H+ antiport activity of three Malus species with different salt tolerance. J Integr Agric 13(6):1276–1283. https://doi.org/10.1016/S2095-3119(13)60627-9
Zhang T, Xu Q, Sun X, Li H (2013) The calcineurin-responsive transcription factor Crz1 is required for conidation, full virulence and DMI resistance in Penicillium digitatum. Microbiol Res 168(4):211–222. https://doi.org/10.1016/j.micres.2012.11.006
Zhang ZH, Jhaveri DJ, Marshall VM, Bauer C, Edson J, Narayanan K, Robinson GJ, Lundberg AE, Bartlett PF, Wray NR, Zhao QY (2014) A comparative study of techniques for differential expression analysis on RNA-Seq Data. PLoS One 9(8):e103207. https://doi.org/10.1371/journal.pone.0103207
Funding
The work was financially supported by the National Key R&D Program of the Ministry of Science and Technology of China (No. 2022YFC2106203) and by the Fundamental Research Funds for the Central Universities (No. 2023CDJXY-051).
Author information
Authors and Affiliations
Contributions
SL and LQ conceived and designed research, wrote the paper. MY analyzed data. YW performed research. SL and DW contributed new reagents or analytical tools. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Quan, L., Yang, M. et al. Regulation of cellulase production via calcium signaling in Trichoderma reesei under PEG8000 stress. Appl Microbiol Biotechnol 108, 178 (2024). https://doi.org/10.1007/s00253-023-12901-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12901-w