Abstract
Background
The filamentous fungus Trichoderma reesei Rut-C30 is one of the most vital fungi for the production of cellulases, which can be used for biofuel production from lignocellulose. Nevertheless, the mechanism of transmission of external stimuli and signals in modulating cellulase production in T. reesei Rut-C30 remains unclear. Calcium is a known second messenger regulating cellulase gene expression in T. reesei.
Results
In this study, we found that a biologically relevant extracellular Mn2+ concentration markedly stimulates cellulase production, total protein secretion, and the intracellular Mn2+ concentration of Rut-C30, a cellulase hyper-producing strain of T. reesei. Furthermore, we identified two Mn2+ transport proteins, designated as TPHO84-1 and TPHO84-2, indicating that they are upstream in the signaling pathway that leads to cellulase upregulation. We also found that Mn2+ induced a significant increase in cytosolic Ca2+ concentration, and that this increased cytosolic Ca2+ might be a key step in the Mn2+-mediated regulation of cellulase gene transcription and production. The utilization of LaCl3 to block plasma membrane Ca2+ channels, and deletion of crz1 (calcineurin-responsive zinc finger transcription factor 1) to interrupt calcium signaling, showed that Mn2+ exerts the induction of cellulase genes via calcium channels and calcium signaling. To substantiate this, we identified a Ca2+/Mn2+ P-type ATPase, TPMR1, which could play a pivotal role in Ca2+/Mn2+ homeostasis and Mn2+ induction of cellulase genes in T. reesei Rut-C30.
Conclusions
Taken together, our results revealed for the first time that Mn2+ stimulates cellulase production, and demonstrates that Mn2+ upregulates cellulase genes via calcium channels and calcium signaling. Our research also provides a direction to facilitate enhanced cellulase production by T. reesei.
Similar content being viewed by others
Background
Lignocellulosic biomass, the most abundant renewable energy source, can be hydrolyzed to sugars for bioethanol production. A common host cited for the production of cellulases and hemicellulases is the saprotrophic, filamentous fungus Trichoderma reesei, which is well known for its excellent ability to secrete a broad range of cellulases at very high levels [1,2,3,4]. Due to its ability to degrade and thrive on cellulose-containing fabrics, T. reesei has attracted attention and was consequently studied in detail [5, 6]. However, compared with the energy-efficient production of cellulases and hemicellulases, the induction and regulation of the expression of genes enoding these enzymes in T. reesei are still not completely understood. Additionally, the induction of high-level cellulase production is dependent on inducers such as cellulose, d-xylose, lactose, or sophorose [3, 7,8,9], increasing the costs for the application of produced enzymes. Due to the extensive applications of cellulases and hemicellulases, the induction and regulation of the expression of genes encoding these enzymes have drawn significant attention.
Recent studies have demonstrated that cellulase production is regulated in response to environmental stress, such as light [10, 11], organic solvents [12], and metal ions [13,14,15]. A great example was shown by Chen and co-workers, who illustrated that Ca2+ plays an important role in the production of cellulase or hemicellulase in T. reesei Rut-C30 [13]. Chen et al. [13] suggested that external Ca2+ stimulated hyphal growth, growth-independent cellulase production, and total protein secretion of T. reesei Rut-C30 through the Ca2+ (/calmodulin)–calcineurin–CRZ1 signal transduction pathway.
Intracellular accumulation of Mn2+ can interfere with calcium metabolism [16]. In Ganoderma lucidum, one of the most well-known medicinal basidiomycetes producing many bioactive compounds such as ganoderic acids, Mn2+ is thought to enhance cytosolic Ca2+ to induce ganoderic acid biosynthesis through the calcineurin signal pathway, to upregulate its biosynthetic genes at the transcriptional level [17]. In Aspergillus nidulans, high levels of Mn2+ can induce an increase in intracellular Ca2+ levels, which leads to the nuclear accumulation of CrzA [18, 19]. These observations suggest that Ca2+ and Mn2+ have relevant impacts on the cellular physiology and metabolism of various organisms.
The ability to sense and respond to Mn2+ by the production of import and efflux systems to maintain Mn2+ homeostasis is critical for cells [20,21,22,23]. Such homeostasis factors include cell surface and intracellular Mn2+ transporters that collectively guide the metal through a designated trafficking pathway [20, 22]. PMR1, a P-type ATPase ion pump, is a transporter for both Ca2+ and Mn2+ and is also a homeostasis factor, associating with delivering both Mn2+ and Ca2+ to the secretory pathway [22, 24,25,26]. Cytosolic Mn2+ accumulates in yeast cells lacking the PMR1 transporter [22]. Although many studies have reported on Mn2+ homeostasis in various organisms, the detailed mechanisms are yet unclear. First, studies have focused mainly on yeast or bacterial species, and less work has been conducted with filamentous fungi. Second, the detailed roles of Mn2+ in the biological processes of filamentous fungi remain unclear and need further studies. Third, the conjunction between Mn2+ and calcium signaling in filamentous fungi is still not clear. Therefore, it is necessary to study the mechanism of Mn2+ stimulation in filamentous fungi.
In this study, the impact of Mn2+ on the growth and protein production of T. reesei Rut-C30 was investigated. The temporal dynamics of intracellular and extracellular Mn2+ were detected. Additionally, the function of Mn2+ transport proteins in T. reesei Rut-C30 was characterized. The conjunction between Mn2+ and Ca2+ was further investigated to elucidate how Mn2+ regulates the production of cellulase via calcium signaling in T. reesei Rut-C30. These results could be used for more efficient production of cellulase by T. reesei, and provide a new approach to understand the regulatory mechanisms that respond to environmental stimuli. This research may also offer the basis for the study of Mn2+-induced signal transduction in other fungi.
Results
Effects of the addition of Mn2+ on growth and cellulase production of T. reesei
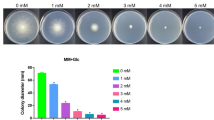
To determine how Mn2+ influences the hyphal growth, T. reesei Rut-C30 strains were cultured on MM (minimal medium) plates supplemented with different concentrations of Mn2+ (0, 1, 10, 20, and 40 mM final concentration) and 2% glucose as the sole carbon source. The mycelium length of T. reesei Rut-C30 after addition Mn2+ is shown in Fig. 1a. There was no significant difference in the hyphal growth with 1–10 mM Mn2+. However, when the concentrations of Mn2+ increased to 20 mM, the strains grew more slowly and sparsely. As shown in Fig. 1b, treatment with 20 mM Mn2+ caused a 30% reduction in the colony diameter compared with that of the untreated strains. Moreover, after treatment with 40 mM Mn2+, the treated strains showed a severe reduction in the colony diameter (53%).
Effects of Mn2+ on hyphal growth and protein production in T. reesei Rut-C30. a The hyphal growth of T. reesei Rut-C30 on plates. Mn2+ was added at a final concentration of 1, 10, 20, or 40 mM. b The measurement of colony diameter. The effects of different concentrations of Mn2+ (final concentration 0, 1, 10, 20, and 40 mM) on CMCase activity (c), pNPCase activity (d), and total protein concentrations (e) of T. reesei Rut-C30. Values are the mean ± SD of the results from three independent experiments. Asterisks indicate a significant difference compared to the untreated strain (*p < 0.05, Student’s t test)
To evaluate the effects of Mn2+ on cellulase production and total protein secretion, the same weight of precultured mycelia of T. reesei Rut-C30 was transferred to liquid MM containing 1% Avicel as the sole carbon source and different concentrations of Mn2+ (0, 1, 10, 20 and 40 mM). As shown in Fig. 1c (Additional file 8: Figure S7A), addition of Mn2+ at a final concentration of 10 mM significantly stimulated CMCase activity (representing endo-β-glucanase activity), with an increase of approximately 140% compared to the controls without addition of Mn2+. However, 1, 20, and 40 mM of Mn2+ did not evidently affect CMCase activity. As shown in Fig. 1d (Additional file 8: Figure S7B) and e, the addition of Mn2+ at a final concentration of 10–40 mM significantly stimulated pNPCase activity (representing exo-β-glucanase activity) and increased total protein concentration after 1 day of fermentation, with an increase of approximately 327 and 55%, respectively.
The above results demonstrated that 10–40 mM Mn2+ could stimulate cellulase production and total protein secretion in T. reesei Rut-C30, and that 20–40 mM Mn2+ could delay hyphal growth. The optimal concentration of Mn2+ to enhance cellulase production was 10 mM, which was selected for further research in our study. CMCase or pNPCase activity was directly used to represent cellulase activity in our study.
To further determine the effects of Mn2+ supplementation on the synthesis of cellulases or total protein secretion, the expression levels of four main cellulase genes (cbh1 encoding cellobiohydrolase I, cbh2 encoding cellobiohydrolase II, egl1 encoding endoglucanase I, and egl2 encoding endoglucanase II), and a transcriptional regulator of cellulases, xyr1, were compared by quantitative reverse-transcription PCR (RT-qPCR) after induction for 24, 48, and 72 h in cultures with 0 or 10 mM Mn2+ supplementation. The primers used to detect transcriptional changes of these genes are listed in Additional file 1: Table S1. The transcriptional levels of four main cellulase genes significantly increased by almost 2- to 3.5-fold, after 24, 48, and 72 h of induction following supplementation with 10 mM Mn2+ (see Additional file 2: Figure S1A–D). In T. reesei, XYR1 is a global transcriptional activator of cellulose and hemicellulase genes [27]. In accordance with the transcription of cbh1, cbh2 and egl1, egl2, the expression level of xyr1 was also significantly stimulated after 72 h of induction (see Additional file 2: Figure S1E). These results were consistent with the upregulation of cellulase activity through the addition of 10 mM Mn2+. However, when compared to expression of other cellulase-related genes, the delayed upregulation of xyr1 expression implied that other putative regulators may participate in Mn2+ metabolism/regulation to directly induce cellulase gene expression, besides indirect induction through xyr1.
Variation in intracellular and extracellular Mn2+ concentration after the addition of Mn2+
Extracellular Mn2+ can significantly augment the intracellular Mn2+ content, and subsequently affect the physiology and metabolism of G. lucidum [17]. To investigate whether enhanced cellulase production is linked to intracellular Mn2+ in T. reesei Rut-C30, the intracellular and extracellular Mn2+ concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS) during cultivation. As illustrated in Fig. 2a, the levels of intracellular and extracellular Mn2+ were almost constant in the control sample without Mn2+ addition. On the contrary, upon Mn2+ addition (10 mM final concentration), the intracellular Mn2+ concentration initially markedly increased, reaching its maximum at 24 h and then declined gradually, while the extracellular Mn2+ concentration dropped initially, reaching its minimum at 24 h and then increased gradually, suggesting that Mn2+ was transported into cells initially in response to a higher extracellular Mn2+ concentration. Subsequently, intracellular Mn2+ gradually effused into the medium from 24 h. We hypothesized that a mechanism could pump Mn2+ in and out of the cell.
Concentrations of Mn2+ in T. reesei Rut-C30 and its derivative mutants. a Filled circle, intracellular Mn2+ concentration after adding 10 mM MnCl2; blank circle, extracellular Mn2+ concentration after adding 10 mM MnCl2; filled square, intracellular Mn2+ concentration of control without MnCl2 supplement; blank square, extracellular Mn2+ concentration of control without MnCl2 supplement. b The concentrations of intracellular Mn2+ of T. reesei Rut-C30 and its derivative mutant strains were examined after cultured in medium containing 10 mM MnCl2. Values are the mean ± SD of the results from three independent experiments. Asterisks indicate significant differences from parental strain Rut-C30 (*p < 0.05, Student’s t test)
Identification of Mn2+ transport proteins TPHO84-1 and TPHO84-2
Jensen et al. [16] suggested that PHO84, a low-affinity transporter of Mn2+ from Saccharomyces cerevisiae, transports Mn2+ when cells are exposed to higher Mn2+ concentrations. In our study, Mn2+ efficiently transported into T. reesei Rut-C30 cells at an Mn2+ concentration of 10 mM (Fig. 2a). To identify the protein(s) responsible for transporting Mn2+ in T. reesei, we conducted a homology search with the protein sequence of PHO84 (GenBank: KZV08715.1) in the T. reesei genome from the JGI database (http://genome.jgi.doe.gov/Trire2/Trire2.home.html). Five high score hits were obtained with the following protein identity matches: TRE77552, 59.6%, TRE81389, 51.8%, TRE45852, 49.0%, TRE45868, 40.1%, and TRE106118, 35.2%.
We hypothesized that Mn2+ transport proteins would be highly expressed during Mn2+ addition. To assess which proteins encoded putative Mn2+ transport activity, transcriptional levels of tre77552, tre81389, tre45852, tre45868 and tre106118 were monitored by RT-qPCR with 10 mM Mn2+ compared to no Mn2+ addition at different induction times. As illustrated in Additional file 3: Figure S2, expression of these genes was significantly upregulated in the samples treated with 10 mM Mn2+. Based on their induction at higher Mn2+ concentration, all five genes might be responsible for Mn2+ transport of T. reesei Rut-C30.
To further investigate whether any of these five proteins displayed a function similar to that of PHO84, involved in transport of Mn2+ in S. cerevisiae, the five deletion mutants, Δ77552, Δ81389, Δ45852, Δ45868, and Δ106118, respectively, were collected to measure intracellular Mn2+ concentrations by ICP-MS compared to that in the parental strain. As shown in Fig. 2b, Δ77552 and Δ45868 showed a marked decrease in steady-state levels of cellular Mn2+ when compared with the Rut-C30 strain, in which a 70–80% decrease in metal accumulation was obtained. However, under the same conditions, Δ81389, Δ45852, or Δ106118 had an Mn2+ concentration similar to that in Rut-C30 strain (see Additional file 4: Figure S3). The tre77552 and tre45868 double mutant strain Δtpho84-1/2 showed lower intracellular Mn2+ concentration than each single mutant (Fig. 2b). Δ77552 and Δ45868 were also complemented by transforming vectors ptpho84-1-rc and ptpho84-2-rc into them, respectively (see Additional file 5: Figure S4). Complementation strains (tpho84-1-rc and tpho84-2-rc) were obtained to demonstrate the restoration of Mn2+ transport with intracellular Mn2+ concentrations similar to those of the parent strain Rut-C30 (Fig. 2b). The results demonstrated that the intracellular Mn2+ concentration can be increased via putative Mn2+ transport proteins TRE77552 and TRE45868 in T. reesei Rut-C30.
As predicted by SMART (http://smart.embl-heidelberg.de/), both TRE77552 and TRE45868 are membrane proteins with an 11- and 9-transmembrane domain topology, respectively. To visualize the location of TRE77552 and TRE45868, we constructed two chimeric proteins, RFP-77552 and RFP-45868, by fusing red fluorescence protein to their N-terminus (see Additional file 6: Figure S5A). The two chimeric proteins were overexpressed via cbh1 promoter, which allowed us to confirm that TRE77552 and TRE45868 are located at the mycelial surface. Additional file 6: Figure S5B shows the in vivo epifluorescence analysis of the two chimeric protein (RFP-77552 and RFP-45868) transformants, rfp-tpho84-1 and rfp-tpho84-2, respectively, depicting strong and stable fluorescent signal at the mycelial surface, as expected for plasma membrane proteins. However, overexpression of genes encoding membrane bound proteins by a strong promoter might cause some uncertainty.
Based on these findings, we considered that both tre77552 and tre45868 encode Mn2+ transports with Mn2+ transport function located at the plasma membrane, and named the two genes as tpho84-1 and tpho84-2, respectively. Intracellular Mn2+ can be transported into T. reesei Rut-C30 cells via TPHO84-1 and TPHO84-2. It is presently unclear whether tre81389, tre45852, and tre106118 are inactive in the transport of Mn2+.
Role of TPHO84-1 and TPHO84-2 in cellulase production
Δ77552 (Δtpho84-1) and Δ45868 (Δtpho84-2), and their complementation strains tpho84-1-rc and tpho84-2-rc were used to determine the effect of TPHO84-1 and TPHO84-2 in mediating the growth and cellulase production in T. reesei Rut-C30. To determine the effect of TPHO84-1 and TPHO84-2 on mediating growth, Rut-C30, Δtpho84-1, Δtpho84-2, tpho84-1-rc, tpho84-2-rc and Δtpho84-1/2 strains were cultured on minimal medium plates adding 0 or 10 mM Mn2+ and 2% glucose as the sole carbon source. The mycelium morphology is shown in Fig. 3a. There was no significant effect on the hyphal growth of Rut-C30, Δtpho84-1, Δtpho84-2, tpho84-1-rc and tpho84-2-rc strains at 0 or 10 mM Mn2+. However, the hyphal growth of the Δtpho84-1/2 transformant was slightly repressed, compared with that of the parent strain at both 0 and 10 mM Mn2+ (Fig. 3b).
TPHO84-1 and TPHO84-2 regulate cellulase production of T. reesei Rut-C30 with Mn2+ supplementation. a The hyphal growth of T. reesei Rut-C30 and its derivative mutant strains under 0 or 10 mM MnCl2. b The measurement of colony diameter. The CMCase activity (c) and pNPCase activity (d) of T. reesei Rut-C30 and its derivative mutant strains were examined after culture in medium containing 0 or 10 mM MnCl2. The transcriptional levels of cbh1 (e) and egl1 (f) in the parental strain Rut-C30 and its derivative mutant strains were analyzed after cultured in medium containing 0 or 10 mM MnCl2. Values are the mean ± SD of the results from three independent experiments. Asterisks indicate significant differences (*p < 0.05, Student’s t test)
To further test the effect of Mn2+ transport proteins on cellulase production, we investigated the effect of Rut-C30, Δtpho84-1, Δtpho84-2, tpho84-1-rc, tpho84-2-rc, and Δtpho84-1/2 strains on cellulase production after supplementation of 0 or 10 mM Mn2+. There was no obvious difference in CMCase (see Additional file 7: Figure S6A) and pNPCase (see Additional file 7: Figure S6B) activities between these six strains without Mn2+ addition. However, Δtpho84-1 and Δtpho84-2 strains showed approximately 30–40% reduction in CMCase activity, compared with the parental strain Rut-C30 with 10 mM Mn2+ addition. Furthermore, Δtpho84-1/2 strain had lower CMCase activity than each single mutant, at approximately 50% reduction compared with Rut-C30 (Fig. 3c and Additional file 8: Figure S8A). Similarly, Δtpho84-1 and Δtpho84-2 strains showed approximately 25 to 30% reduction in the pNPCase activity, compared with the parental strain Rut-C30, with 10 mM Mn2+ addition. Furthermore, the Δtpho84-1/2 strain showed lower pNPCase activity than each single mutant, and approximately 40% reduction compared with Rut-C30 (Fig. 3d and Additional file 8: Figure S8B). The cellulase production capabilities of tpho84-1-rc and tpho84-2-rc were complemented at 10 mM Mn2+ supplementation compared to parental strains Δtpho84-1 and Δtpho84-2, respectively, to a level similar to that of the original strain Rut-C30 (Fig. 3c, d and Additional file 8: Figure S8A, B).
Additionally, RT-qPCR was performed to determine the transcription levels of the important cellulase genes cbh1 and egl1 in the T. reesei Rut-C30, Δtpho84-1, Δtpho84-2, tpho84-1-rc, tpho84-2-rc, and Δtpho84-1/2 mutants. The primers used to detect transcriptional levels of these genes are listed in Additional file 1: Table S1. In agreement with the CMCase activity levels, no obvious difference in transcription levels of cbh1 and egl1 between above six strains was observed without Mn2+ addition (data not shown). However, the deletion strains Δtpho84-1, Δtpho84-2, and Δtpho84-1/2 showed a marked reduction in cellulase transcription compared with the parental strain Rut-C30 upon 10 mM Mn2+ addition at all time points examined (Fig. 3e, f). Similarly, the expression levels of the cbh1 and egl1 genes of tpho84-1-rc and tpho84-2-rc were complemented under Mn2+ supplementation, to a level similar to that of the original strain Rut-C30.
These results indicate that TPHO84-1 and TPHO84-2 participate in inducing cellulase production of T. reesei Rut-C30 only under Mn2+ addition, which is in accordance with its function as Mn2+ transport protein.
Increase in cytosolic Ca2+ level and calcium signaling after Mn2+ addition
Next, we investigated how Mn2+ addition can upregulate cellulase gene expression via Mn2+ transport proteins TPHO84-1 and TPHO84-2. Using Fluo-3/AM fluorescent dye, a dye that only emits green fluorescence after crossing the cell membrane and binding with Ca2+ [28], whose intensity represents relative amounts of free intracellular Ca2+ [29], we found that the cytosolic Ca2+ concentration was increased after Mn2+ addition. As shown in Fig. 4A, a stronger green fluorescence intensity was observed in the Rut-C30 cells under 10 mM Mn2+ supplement on the second day than that observed with the control (no Mn2+ supplement), demonstrating that Mn2+ leads to an increase in the level of cytosolic Ca2+. The fluorescence level emitted by the Ca2+-activated fluorochrome reached a 2.23-fold increase under 10 mM Mn2+ addition compared to that in the control (Fig. 4B). These results indicate that Mn2+ induced an increase in the concentration level of cytosolic Ca2+. A similar phenomenon was reported in S. cerevisiae [30].
Cytosolic Ca2+ levels and calcium signaling increase after Mn2+ addition. A The analysis of cytosolic Ca2+ levels via a Ca2+ fluorescent probe Fluo-3/AM. The T. reesei Rut-C30 and its derivative mutant strains were cultured in liquid minimal medium for 48–60 h with 0 or 10 mM final concentration of MnCl2 (0 or 10 Mn, respectively). For detection, 50 μM Fluo-3/AM was used, and the intensity was monitored using Automatic Inverted Fluorescence Microscopy. Green fluorescence represents the free cytosolic Ca2+. DIC, differential interference contrast. B Comparative fluorescence ratio analysis of Mn2+ influence on cytosolic Ca2+ levels. The y-axis represents the Ca2+ fluorescence ratio measured by CLSM and the x-axis the different strains tested. The effects of Mn2+ on the expression levels of certain calcium signaling-related genes cam (C), cna1 (D) and crz1 (E) in T. reesei Rut-C30. 0 Mn, no MnCl2 was added to the medium; 10 Mn, final concentration of 10 mM MnCl2. Values are the mean ± SD of the results from three independent experiments. Asterisks indicate significant differences from untreated strains (*p < 0.05, **p < 0.01, Student’s t test). Different letters indicate significant differences between the columns (p < 0.05, according to Duncan’s multiple-range test)
We measured the level of cytosolic Ca2+ in the Δtpho84-1, Δtpho84-2, and Δtpho84-1/2 strains with Mn2+ addition. As illustrated in Fig. 4A, B, the significant increase in intracellular Ca2+ induced by Mn2+, observed in parental strain Rut-C30, was absent in the Δtpho84-1, Δtpho84-2, and Δtpho84-1/2 strains. The augmentation of cytosolic Ca2+ levels induced by Mn2+ was blocked in the Δtpho84-1, Δtpho84-2, and Δtpho84-1/2 strains. These results suggest that rising cytosolic Ca2+ levels depend on Mn2+ transport into Rut-C30 cells via TPHO84-1 and TPHO84-2. This also indicates that Ca2+ influx in cells is closely associated with Mn2+ homeostasis.
Previous studies have demonstrated that the calcium signal transduction pathway can upregulate cellulase gene expression [13]. We investigated whether the increased levels of cytosolic Ca2+, induced by Mn2+, can trigger calcium signal transduction pathways in T. reesei. To test our hypothesis, RT-qPCR was carried out to analyze the transcriptional levels of calcium signaling-related genes, including calmodulin (cam, GenBank: ACZ26150.1), calcineurin (cna1, GenBank: EGR49476.1) [31], and calcineurin-responsive zinc finger transcription factor 1, crz1 [13], under Mn2+ addition. The primers used to detect transcription of these genes are listed in Additional file 1: Table S1. As shown in Fig. 4C–E, similar to the increase in the contents of cytosolic Ca2+, expression of these genes was significantly upregulated with Mn2+ addition. These results suggested that Mn2+ can increase the concentration of cytosolic Ca2+, thus stimulating the calcium signal transduction pathway to induce cellulase production in T. reesei Rut-C30.
Mn2+ induces cellulase production via cytosolic Ca2+
To investigate the assumption that Mn2+ induces cytosolic Ca2+ improvement and cellulase production via Ca2+ channels, we used LaCl3, a plasma membrane Ca2+ channel blocker to prevent influx of external Ca2+ [32]. Figure 5A, B shows that the Fluo-3/AM fluorescence intensity of mycelia remarkably reduced almost 60% with LaCl3 compared with no LaCl3 addition, under 10 mM Mn2+. The increased content of cytosolic Ca2+ induced by Mn2+ could be effectively attenuated by adding LaCl3 to T. reesei Rut-C30. Meanwhile, the increased expression of calcium signaling-related genes, cam, cna1, and crz1, which are induced by 10 mM Mn2+, was also effectively prevented by adding LaCl3 (data not shown).
Effects of Ca2+ channel inhibitor LaCl3 on the cytosolic Ca2+ concentration and cellulase production. A Fluorescence analysis of LaCl3 influence on cytosolic Ca2+ burst induced by Mn2+. The T. reesei Rut-C30 were cultured in liquid minimal medium for 48–60 h with 0 or 10 mM MnCl2 (0 or 10 Mn, respectively), and then treated with 0 or 5 mM LaCl3. For detection, 50 μM Fluo-3/AM was used, and the intensity was monitored using Automatic Inverted Fluorescence Microscopy. Green fluorescence represents the free cytosolic Ca2+. DIC, differential interference contrast, CK, not treated with LaCl3. B Comparative fluorescence ratio analysis of LaCl3 influence on the cytosolic Ca2+ burst induced by Mn2+. The y-axis represents the Ca2+ fluorescence ratio measured by CLSM and the x-axis the different treatments. The CMCase activity (C) and pNPCase activity (D) of T. reesei Rut-C30 were examined after culture in medium containing 0 or 10 mM MnCl2 and with (−) or without (+) 5 mM LaCl3. The expression levels of cbh1 (E) and egl1 (F) in T. reesei Rut-C30 were analyzed after culture in medium containing 0 or 10 mM MnCl2 and with (−) or without (+) 5 mM LaCl3. Values are the mean ± SD of the results from three independent experiments. Different letters indicate significant differences between the columns (p < 0.05, according to Duncan’s multiple-range test)
Ca2+ participates in regulating cellulase production in T. reesei [13]. To investigate whether a cellulase increase, induced by Mn2+, was blocked by LaCl3, we analyzed CMCase and pNPCase activities and transcription of key cellulase genes cbh1 and egl1. As shown in Fig. 5C, D (Additional file 8: Figure S9A, B), under Mn2+ supplementation and with LaCl3, the CMCase and pNPCase activities in T. reesei Rut-C30 decreased by 50 and 49%, respectively, compared with no LaCl3 addition. Meanwhile, the expression levels of cbh1 and egl1 were reduced by 60% at 72 h (Fig. 5E, F). However, there was no obvious change to CMCase activities and transcriptional levels of cbh1 and egl1 with or without LaCl3 when Mn2+ was not added (Fig. 5C–F, Additional file 8: Figure S9A, B). These results showed that LaCl3 could significantly decrease the Mn2+-induced high expression levels of key cellulase genes in T. reesei Rut-C30.
These data indicated that Mn2+ induced cytosolic Ca2+ increase via the Ca2+ channel. When a Ca2+ channel blocker LaCl3 was added, the increase in cytosolic Ca2+ concentration and cellulase production induced by Mn2+ were effectively attenuated.
To investigate whether a cellulase increase, induced by Mn2+, is associated with calcium signal transduction, we constructed a crz1 deletion mutant Δcrz1 as Chen et al. [13] to block the calcium signal transduction pathway. As shown in Fig. 6a, b (Additional file 8: Figure S10A, B), the remarkable increase of CMCase and pNPCase activities induced by Mn2+, observed in parental strain Rut-C30, was effectively attenuated by deleting crz1. Similarly, the transcriptional levels of cbh1 and egl1 were markedly reduced in the Δcrz1 mutant at all time points examined (Fig. 6c, d).
Influence of CRZ1 on Mn2+-induced cellulase production. The CMCase activity (a) and pNPCase activity (b) of T. reesei Rut-C30 and Δcrz1 strains supplemented with 0 or 10 mM MnCl2. The expression levels of cbh1 (c) and egl1 (d) in T. reesei Rut-C30 and Δcrz1 strains supplemented with 0 or 10 mM MnCl2. Values are the mean ± SD of the results from three independent experiments. Asterisks indicate significant differences (*p < 0.05, Student’s t test)
Taken together, these data indicated that Mn2+ induces cellulase improvement via calcium signal transduction in T. reesei Rut-C30. The improvement of cellulase production induced by Mn2+ is effectively prevented in a crz1 mutant, blocking the calcium signal transduction pathway.
TPMR1 functions in conjunction with channels of Mn2+ and Ca2+
Mn2+/Ca2+ homeostasis exists in other fungi [33,34,35,36]. PMR1 plays an important role in Mn2+/Ca2+ homeostasis, and may act as a Ca2+/Mn2+ exchanger to balance Mn2+ via Ca2+ [17]. To clarify how Mn2+ increased cytosolic Ca2+ concentration, using Neurospora crassa PMR1 (GenBank: CAB65296.1) as the query, we searched for a PMR1 homolog (TRE119592) in the T. reesei genome, named as TPMR1.
We constructed a tpmr1 deletion mutant Δtpmr1. To investigate whether TPMR1 is responsible for pumping in Ca2+ upon 10 mM Mn2+ addition in T. reesei, we measured the level of intracellular Ca2+ in the Δtpmr1 strain with or without Mn2+ addition. As shown in Fig. 7A and B, the increase in intracellular Ca2+ induced by Mn2+ in Rut-C30 was absent in the Δtpmr1 strain. The augmentation of Ca2+ levels induced by Mn2+ was blocked in the Δtpmr1 strain. These results suggested that TPMR1 is responsible for the increase of cytosolic Ca2+ under Mn2+ addition in T. reesei Rut-C30.
Influence of TPMR1 on Mn2+-induced cytosolic Ca2+ burst and cellulase production. A Fluorescence analysis of the influence of TPMR1 on the cytosolic Ca2+ burst induced by Mn2+. The T. reesei Rut-C30 and Δtpmr1 strains were cultured in liquid minimal medium for 48–60 h with 0 or 10 mM MnCl2 (0 or 10 Mn, respectively). For detection, 50 μM Fluo-3/AM was used, and the intensity was monitored using Automatic Inverted Fluorescence Microscopy. Green fluorescence represents the free cytosolic Ca2+. DIC, differential interference contrast. B Comparative fluorescence ratio analysis of TPMR1 influence on cytosolic Ca2+ burst induced by Mn2+. The y-axis represents the Ca2+ fluorescence ratio measured by CLSM and the x-axis the different treatments. The CMCase activity (C) and pNPCase activity (D) of T. reesei Rut-C30 and Δtpmr1 strains were examined after culture in medium containing 0 or 10 mM MnCl2. The expression levels of cbh1 (E) and egl1 (F) in T. reesei Rut-C30 and Δtpmr1 strains were analyzed after culture in medium containing 0 or 10 mM MnCl2. Values are the mean ± SD of the results from three independent experiments. Asterisks indicate significant differences from untreated strains (*p < 0.05, **p < 0.01, Student’s t test). Different letters indicate significant differences between the columns (p < 0.05, according to Duncan’s multiple-range test)
To further investigate the function of TPMR1 in conjunction with Mn2+ and Ca2+ channels, the intracellular and extracellular Mn2+ concentrations in the Δtpmr1 strain with or without Mn2+ addition were measured by ICP-MS during cultivation. As illustrated in Additional file 9: Figure S12, upon Mn2+ addition (10 mM final concentration), the intracellular Mn2+ concentration initially markedly increased, reaching and maintaining a high level after 24 h. The extracellular Mn2+ concentration dropped initially, reaching its minimum from 24 to 72 h. The intracellular and extracellular Mn2+ concentrations of the Δtpmr1 strain (Additional file 9: Figure S12) are quite different from that of Rut-C30 (Fig. 2a), whose intracellular Mn2+ gradually effused into the medium. These results suggested that TPMR1 is responsible for pumping Mn2+ out of the cell.
To further test the effect of TPMR1 on cellulase production under Mn2+ addition, we compared the cellulase production in parental strain Rut-C30 and Δtpmr1 mutant. Upon the addition of Mn2+, the Δtpmr1 strain caused about 26 and 51% reductions in the CMCase and pNPCase activities, respectively, compared with that of Rut-C30 (Fig. 7C, D and Additional file 8: Figure S11A, B). Moreover, the transcriptional levels of cbh1 and egl1 observed are in agreement with the CMCase and pNPCase activity levels, which were markedly reduced in the Δtpmr1 mutant at all time points examined (Fig. 7E, F). The increase in cellulase production induced by Mn2+ was remarkably weakened in the tpmr1 deletion mutant. These results demonstrated that TPMR1 functions in conjunction with channels of Mn2+ and Ca2+ in T. reesei Rut-C30.
Discussion
Metal ions are important in regulating cellular metabolism. For example, Ca2+ plays an important role in the regulation of cellulase or hemicellulase in T. reesei [13,14,15]. Paraszkiewicz et al. [37] reported Cd2+, Zn2+, and Pb2+ as environmental stress factors, which increased the biosynthesis of fungal emulsifier in Curvularia lunata. Addition of Ca2+, Na+, and Mn2+ enhanced ganoderic acid production in Ganoderma lucidum liquid cultures, through induction of the calcineurin signal pathway [17, 29, 38]. These results suggest that metal ions have significant impact on the cellular physiology and metabolism of various organisms. In this study, we first report that Mn2+ stimulates cellulase production and total protein secretion of T. reesei Rut-C30. However, whether other metal ions or stimuli have an impact on cellulase production of T. reesei will require further evidence for confirmation.
Diverse Mn2+ transports have been identified in the yeasts, plants, and bacteria [21, 22, 39,40,41,42]. Mn2+ can enter the cell through two different routes including high- and low-affinity transporters [21, 22, 41, 42]. SMF1, a plasma membrane NRAMP (Natural Resistance-Associated Macrophage Protein) family Mn2+ transporter, is a high-affinity transporter for Mn2+, contributing to Mn2+ accumulation under extreme Mn2+ starvation conditions [16, 22, 43, 44]. PHO84, a family of phosphate/proton symporters [45], can transport Mn2+ under excess Mn2+ stress [16, 25], indicating that PHO84 is a low-affinity transporter of Mn2+, contributing to Mn2+ accumulation only when cells are exposed to higher Mn2+ concentrations. T. reesei might contain both high- and low-affinity transporters of Mn2+, similar to what has been described for the metal transport systems for iron, copper, and zinc [46,47,48,49,50]. A BLAST search of the genome sequence of T. reesei identified a gene (tre79644) similar to SMF1, and this gene was named tsmf1. There was no significant difference on Mn2+ transport and cellulase production under 10 mM Mn2+ surplus using a tsmf1 deletion strain (data not shown). The alternative PHO84 homologs remain to be identified in T. reesei Rut-C30. Five candidates including TPHO84-1 and TPHO84-2 are similar to PHO84. We proved that extracellular Mn2+ is transported into Rut-C30 cells via TPHO84-1 and TPHO84-2, which are annotated as putative low-affinity transports of Mn2+ responding to higher Mn2+ concentration. Meanwhile, there were no significant differences between the cytosolic Ca2+ concentrations for the T. reesei Rut-C30, Δtpho84-1, Δtpho84-2, and Δtpho84-1/2 mutants (see Additional file 10: Figure S13).
Calcium is widely used as a second messenger in prokaryotic and eukaryotic cells. It is known that a cytosolic Ca2+ burst and further induced calcium signaling regulate cellular responses when exposed to different external stimuli [51, 52]. For instance, Na+ induction enhances cytosolic Ca2+ to induce the ganoderic acid biosynthesis through calcineurin signal pathway, to upregulate its biosynthetic genes at the transcriptional level in Ganoderma lucidum [29]. High temperatures are known to trigger the generation of cytosolic Ca2+ in plants [53]. Alkaline pH triggers an immediate calcium burst in Candida albicans [54]. Our results indicated that Mn2+ induces a significantly increased cytosolic Ca2+ level in T. reesei Rut-C30 (Fig. 4A, B). Additionally, in our experiments, inhibition of cytosolic Ca2+ level by LaCl3 effectively attenuated the cellulase increase induced by Mn2+ (Fig. 5C–E). The results showed that 10 mM Mn2+ stress led to a cytosolic Ca2+ burst in T. reesei Rut-C30. An earlier study demonstrated that Ca2+ (/calmodulin)–calcineurin–CRZ1 signaling could induce cellulase production at the transcription level by Ca2+ stimulation [13]. In our study, Mn2+ could also activate the expression of Ca2+ (/calmodulin)–calcineurin–CRZ1 signaling-related genes in T. reesei Rut-C30 (Fig. 4C–E). Furthermore, CRZ1 participated in regulating cellulase production in Mn2+-induced strains (Fig. 6a–d). The results presented here indicate that the regulation of cellulase gene expression and production by Mn2+ are dependent on cytosolic Ca2+ burst and further induce calcium signaling.
PMR1, encoding the Ca2+/Mn2+ P-type ATPase, is required to either scavenge trace amounts of Mn2+ and Ca2+ from the medium or maintain sufficient levels of Mn2+ and Ca2+ in an intracellular compartment [22, 24,25,26, 33]. Bowman et al. [33] reported that a pmr1 deletion strain accumulates 80% lesser Ca2+ than the wild type. Under higher levels of intracellular Mn2+, extracellular Ca2+ might transport into the cells through PMR1 to increase intracellular Ca2+ and then trigger calcium signaling to regulate cellular responses [35]. Our work found that the increase in Ca2+ levels induced by Mn2+ treatment was blocked in the tpmr1 deletion strain (Fig. 7A, B) and that the improvement of cellulase production induced by Mn2+ was remarkably weakened in the tpmr1 deletion mutant. These results demonstrated that TPMR1 functions as a channel of Mn2+ and Ca2+ in T. reesei Rut-C30. Cytosolic Ca2+ concentration was enhanced through TPMR1 under Mn2+ addition, thus inducing calcium signaling to upregulate cellulase genes. Additionally, PMR1 provides a major route for cellular sequestration of Mn2+ by pumping excess Mn2+ into the Golgi, from where the metal may exit the cell via the secretory pathway vesicles that merge with the cell surface and release the Mn2+ contents back into the extracellular environment [20, 22, 24, 34]. These data correlate well with our data indicating that intracellular Mn2+ gradually effused to the medium from 24 h (Fig. 2a). However, in the Δtpmr1 mutant, the intracellular Mn2+ concentration maintained its high level after 24 h, and the extracellular Mn2+ concentration reached its minimum from 24 to 72 h. These results implied that TPMR1 participates in pumping excess Mn2+ into the Golgi, and then releases it extracellularly, meanwhile accumulating cytosolic Ca2+ in T. reesei Rut-C30. However, the detailed role of TPMR1 in T. reesei needs further research.
We found that 10 mM Mn2+ could also stimulate cellulase production and increase total protein secretion from the T. reesei wild-type strain QM6a and the mutant strain Qm9414 (data not shown). However, the results from Rut-C30 may not be identical to those of the wild-type strain, because each strain likely has its own unique regulatory mechanism.
Conclusions
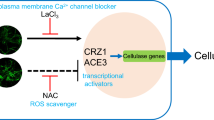
In summary, the putative mechanism of the extracellular Mn2+-induced stimulation of cellulase production was characterized in T. reesei Rut-C30 (Fig. 8). Mn2+ induces a significantly increased cytosolic Ca2+ level and triggers Ca2+-CRZ1 signaling to induce cellulase production at the transcription level. Moreover, we identified two Mn2+ transport proteins in T. reesei Rut-C30, and named TPHO84-1 and TPHO84-2. Furthermore, TPMR1 acts as a link between channels in Mn2+ and Ca2+ homeostasis in T. reesei. This study provides a successful approach to produce a higher yield of cellulase and to develop industrially applicable T. reesei strains, which is important for biofuel production from lignocelluloics. This study also provides a molecular basis for understanding the regulatory mechanism of divalent metal ions on the cellular metabolism of fungi.
A mechanistic model of the Mn2+ stimulation of cellulase production in T. reesei. After addition of 10 mM MnCl2, the intracellular Mn2+ content increases via Mn2+ transport proteins Tpho84-1 and Tpho84-2. Intracellular Mn2+ promotes a cytosolic Ca2+ burst that is required for cellulase gene transcription via Ca2+ signaling. After using LaCl3 (plasma membrane Ca2+ channels blocker), we suggest that Ca2+ channels are responsible for the cytosolic Ca2+ burst and cellulase production induced by Mn2+. Furthermore, TPMR1 is one of the links between the channels of Mn2+ and Ca2+, which may function as a Mn2+/Ca2+ exchanger to regulate Mn2+ and Ca2+ homeostasis under Mn2+ stress. Mn2+ could also employ other as-yet-unidentified pathways to regulate cellulase production. The solid arrows indicate data supported by our own experiments; dashed arrows indicate undefined regulation
Methods
Strains and growth conditions
Escherichia coli DH5α was used for plasmid amplification. Agrobacterium tumefaciens strain AGL-1 was used as a T-DNA donor for fungal transformation [55]. T. reesei Rut-C30 (ATCC 56765) was used throughout the study and as the host for genetic transformation. Luria–Broth (LB) was used for culture of E. coli and A. tumefaciens. Mandels’ medium [56] was used for the general fungal culture. All strains were maintained on potato dextrose agar (PDA) plates at 28 °C. The fungal strains constructed in this study are summarized in Table 1. All strains were cultured in the dark.
Minimal medium (MM, (NH4)2SO4 5 g/l; Urea 0.3 g/l; KH2PO4 15 g/l; CaCl2 0.6 g/l; MgSO4 0.6 g/l; FeSO4·7H2O 5 mg/l; ZnSO4·7H2O 1.4 mg/l; CoCl2·6H2O 2 mg/l; pH 5.5) with 2% glucose was used to assess the effect of Mn2+ on hyphal growth. To analyze the effects of Mn2+ on cellulase activity, protein concentration, and gene expression levels, medium replacement experiments were performed. After growth in Mandels’ medium with 2% glucose for ~ 32 h at 28 °C and 220 rpm, approximately 0.1 g of mycelia were collected and washed thoroughly using 0.85% NaCl, then transferred to 100 ml MM containing 1% (w/v) Avicel (PH-101, Sigma-Aldrich) with the addition of MnCl2 to final concentrations of 1, 10, 20, and 40 mM. Strains were induced for 1–5 days before being subjected to testing for enzymatic activity, protein concentration, or induced for 24, 48, or 72 h before being subjected to RNA extraction and RT-qPCR analyses, respectively. To assess the effect of plasma membrane Ca2+ channels on the regulation of cellulase production of T. reesei Rut-C30, 5 mM (final concentration) LaCl3 (Aladdin, Shanghai, China) was added after 1 day of culture in MM.
Fungal growth, enzymatic activity, protein concentration, and biomass assays
For fungal hyphal growth assays, conidia were collected and diluted to 107 ml−1 in sterile water. An equal volume of the solution (2 μl) was inoculated onto the center of the MM plates as described above, and was grown for 3–5 days at 28 °C.
For enzymatic activity, protein concentration, and biomass assays, 1 ml of culture liquid was collected and subjected to 0.45-μm filtration. The culture supernatants were subjected to cellulase activity and protein concentration analysis. The mycelia were subjected to biomass measurement. Fungal CMCase and pNPCase activities were measured according to the method described by Wang et al. [57]. Protein concentrations were determined using the Bradford Protein Assay Kit (Generay, Shanghai, China). Biomass concentration was indirectly measured by calculating the amount of total intracellular proteins, with some modification [58]. Briefly, harvested mycelia were suspended in 1 ml 1 M NaOH in a reaction tube and the mixture was incubated for 2 h and frequently vortexed. Total protein was collected via centrifugation at 14,000×g at 4 °C for 10 min. Total protein concentration was determined by the Modified Lowry Protein Assay Kit (Sangon Biotech, Shanghai, China). The final protein content was furthermore corrected using a set of substrate controls where no inoculum was added to the medium. The biomass dry weight was then calculated assuming an average content of 0.32 g intracellular protein per gram of dry cell mass.
RNA isolation and quantitative real-time reverse-transcription polymerase chain reaction (RT-qPCR)
The levels of gene-specific mRNA were assessed using RT-qPCR, according to our previous study, with some modification [59]. In brief, the total RNA of 50 mg fresh weight cells was extracted using a FastRNA Pro Red Kit (MPbio, Irvine, CA, USA), according to the manufacturer’s instructions. Synthesis of cDNA from total RNA was performed using the PrimeScript RT Reagent Kit with gDNA eraser (TaKaRa, Japan) as per the manufacturer’s instructions. For RT-qPCR, the TransStart TipTop Green qPCR SuperMix (TransGen, Shanghai, China) was used with 200 nM of forward and reverse primers (see Additional file 1: Table S1). Gene transcription was analyzed using SYBR green assays. Transcription levels of target genes were normalized to that of the sar1 gene [60]. Thermocycling was performed in an ABI StepOne Plus thermocycler (Applied Biosystems, Foster City, CA, USA).
Determination of extracellular and intracellular Mn2+ concentration
Extracellular and intracellular Mn2+ concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS), as described for Ganoderma lucidum by Xu [17]. Five milliliter of culture liquid was collected and subjected to filtration. The culture supernatant and mycelium were subjected to extracellular and intracellular Mn2+ concentrations, respectively. The supernatant of the cultures was filtered through 0.22-μm membranes and then diluted with 1% HNO3 for measuring the extracellular Mn2+ concentration. Mycelia were washed in distilled water to remove any nonspecifically bound Mn2+, and digested with 1 ml 68% HNO3. The mixture was collected via centrifugation at 12,000×g for 5 min. The supernatant was then filtered through a 0.22-μm membrane and diluted with 1% HNO3 for measuring intracellular Mn2+ concentration. The final intracellular Mn2+ concentration was shown as micromole per gram biomass.
Construction of plasmids and strains
To construct a tpho84-1 deletion mutant, the upstream (− 1 to − 762 bp) and downstream (+ 1917 to + 2681 bp) fragments of tpho84-1 were generated from the genome of T. reesei Rut-C30 using KOD-Plus-Neo (TOYOBO, Japan). All primers are indicated in Additional file 1: Table S1. First, the upstream fragment was ligated into the PacI- and XbaI-linearized LML2.0 [61] using the ClonExpressTM II One Step Cloning Kit (Vazyme, Nanjing, China) to form pFtpho84-1. Subsequently, the downstream fragment was inserted into SwaI-linearized pFtpho84-1 to form the binary vector pDtpho84-1 (see Additional file 5: Figure S4A) for the knockout of tpho84-1 in Rut-C30 using Agrobacterium-mediated transformation [59]. Strains were selected using hygromycin B and cefotaxime on Mandels’ medium. Then, the marker was excised using the method described by Zhang [61]. The putative tpho84-1 disruption mutants (Δtpho84-1/Δ77552) generated by double crossover were verified by diagnostic PCR using the primers tpho84-1-CF and tpho84-1-CR and tpho84-1-OF and tpho84-1-OR (see Additional file 5: Figure S4E).
Similarly, the 706-bp upstream and 710-bp downstream regions of tpho84-2 were amplified and then inserted into LML2.0 as described above to generate the binary vector pDtpho84-2 (see Additional file 5: Figure S4B). After transformation and selection, the putative tpho84-2 deletion mutant (Δtpho84-2/Δ45868) was verified by diagnostic PCR using the primers tpho84-2-CF and tpho84-2-CR, and tpho84-2-OF and tpho84-2-OR (see Additional file 5: Figure S4E). The tre81389, tre45852, tre106118, crz1, and tpmr1 disruption mutants (Δ81389, Δ45852, Δ106118, Δcrz1, and Δtpmr1) were constructed similarly (see Additional file 11: Figure S14).
To construct a tpho84-1/2 deletion mutant, the pDtpho84-2 cassette was transformed into Δtpho84-1 strain using Agrobacterium-mediated transformation. The tpho84-1/2 double deletion mutant (Δtpho84-1/2) was verified by diagnostic PCR.
For tpho84-1 and tpho84-2 re-complementation, 3916- and 4087-bp DNA fragments containing total tpho84-1 and tpho84-2 expression cassette were amplified from the Rut-C30 genome using tpho84-1-rc-1/tpho84-1-rc-2 and tpho84-2-rc-1/tpho84-2-rc-2, and then inserted into SwaI-linearized LML2.0 to generate vector ptpho84-1-rc and ptpho84-2-rc, respectively. Ptpho84-1-rc and ptpho84-2-rc were then transferred to the tpho84-1 and tpho84-2 deletion strain by Agrobacterium-mediated transformation (see Additional file 5: Figure S4C, D). tpho84-1 and tpho84-2 re-complementation strains (tpho84-1-rc and tpho84-2-rc) were selected and verified by PCR using tpho84-1-OF and tpho84-1-OR, and tpho84-2-OF and tpho84-2-OR (see Additional file 5: Figure S4F).
For the construction of N-terminal RFP-tagged translational fusion of tpho84-1 under the control of the cbh1 promoter, the upstream (− 1 to − 1038 bp) and downstream (+ 1 to + 1084 bp) fragments of tpho84-1 were generated from the genome of T. reesei Rut-C30 using KOD-Plus-Neo (TOYOBO, Japan). The promoter of cbh1 (P cbh1 ) was obtained by PCR from the genome of T. reesei Rut-C30. Red fluorescent protein (rfp) was obtained by PCR using the plasmid pDsRed2-N1 (Clontech) as the template. First, the upstream fragment was ligated into the PacI- and XbaI-linearized LML2.0, using the ClonExpressTM II One Step Cloning Kit (Vazyme, Nanjing, China) to form pFrtpho84-1. Subsequently, the P cbh1 , rfp, and downstream fragments were inserted into SwaI-linearized Frtpho84-1 to form the binary vector pRFP-TPHO84-1 for subcellular location of tpho84-1 using Agrobacterium-mediated transformation. Subsequently, the marker was excised following the method of Zhang [61]. The putative rfp-tpho84-1 mutants (rfp-tpho84-1) generated by double crossover were verified by diagnostic PCR using the primers rfp-tpho84-1-CF and rfp-tpho84-1-CR (see Additional file 12: Figure S15).
Similarly, the 997-bp upstream and 797-bp downstream regions of tpho84-2 were amplified and then inserted into LML2.0 as described above to generate the binary vector pRFP-TPHO84-2 (see Additional file 6: Figure S5A). After transformation and selection, the putative rfp-tpho84-2 mutants (rfp-tpho84-2) was verified by diagnostic PCR using the primers rfp-tpho84-2-CF and rfp-tpho84-2-CR (see Additional file 12: Figure S15).
The genome sequence of T. reesei is available at the US Department of Energy (DOE) Joint Genome Institute (http://genome.jgi-psf.org/Trire2/Trire2.home.html).
Fluorescence microscopy
To localize RFP-TPHO84-1/2 fusion proteins using microscopy, the rfp-tpho84-1 and rfp-tpho84-2 strains were inoculated into Mandels’ medium and grown for 48–60 h. The mycelia were then observed using an S Plan Fluor ELWD 100×, 1.3 numerical aperture (NA) objective on a Laser Scanning Confocal Microscope (A1R, Nikon, Japan) comprising a Texas Red filter (500–620 nm band-pass excitation filter and emission filter of 670 nm). Images were processed using the NIS elements software (Nikon).
Free cytosolic Ca2+ labeling and detection
Fluo-3/AM (Sigma) was used as a Ca2+-specific probe to assess the level of cytoplasmic Ca2+ in T. reesei Rut-C30 according to the manufacturer’s protocol. Fluo-3/AM (50 μM final concentration) was loaded into cells by incubation at 37 °C for 30 min, and the cells were then washed three times with phosphate-buffered saline. Images of Ca2+ green fluorescence were observed using an S Plan Fluor ELWD 20×, 0.5 numerical aperture (NA) objective and a digital sight camera on an Eclipse Ti inverted microscope system (Ti-E, Nikon, Japan), comprising an FITC filter (420–490 nm band-pass excitation filter, and emission filter of 535 nm). The intensity of green fluorescence was quantified using NIS-Elements F package software. To eliminate the contribution of background fluorescence, cells without Fluo-3/AM labeling were also imaged under identical conditions.
Statistical analysis
All experimental data shown in this paper were carried out at least three times with identical or similar results. For every experiment, three biological replicates were performed with three technical replicates. The error bars indicate the standard deviation (SD) from the mean of triplicates. Student’s t test was used to compare two samples. Duncan’s multiple-range test was used for multiple comparisons. p < 0.05 was considered to be significant.
Abbreviations
- crz1 :
-
calcineurin-responsive zinc finger transcription factor 1
- CMCase:
-
endo-β-glucanase activity
- pNPCase:
-
exo-β-glucanase activity
- qRT-PCR:
-
quantitative reverse-transcription PCR
- ICP-MS:
-
inductively coupled plasma mass spectrometry
- NRAMP:
-
Natural Resistance-Associated Macrophage Protein
References
Cherry JR, Fidantsef AL. Directed evolution of industrial enzymes: an update. Curr Opin Biotechnol. 2003;14:438–43.
Jun H, Kieselbach T, Jönsson LJ. Enzyme production by filamentous fungi: analysis of the secretome of Trichoderma reesei grown on unconventional carbon source. Microb Cell Fact. 2011;10:68.
Kubicek CP, Mikus M, Schuster A, Schmoll M, Seiboth B. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnol Biofuels. 2009;2:19.
Schuster A, Schmoll M. Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol. 2010;87:787–99.
Bischof RH, Ramoni J, Seiboth B. Cellulases and beyond: the first 70 years of the enzyme producer Trichoderma reesei. Microb Cell Fact. 2016;15:106.
Ramoni J, Marchetti-Deschmann M, Seidl-Seiboth V, Seiboth B. Trichoderma reesei xylanase 5 is defective in the reference strain QM6a but functional alleles are present in other wild-type strains. Appl Microbiol Biotechnol. 2017;101:4139–49.
Dashtban M, Buchkowski R, Qin W. Effect of different carbon sources on cellulase production by Hypocrea jecorina (Trichoderma reesei) strains. Int J Biochem Mol Biol. 2011;2:274–86.
Jourdier E, Cohen C, Poughon L, Larroche C, Monot F, Ben Chaabane F. Cellulase activity mapping of Trichoderma reesei cultivated in sugar mixtures under fed-batch conditions. Biotechnol Biofuels. 2013;6:79.
Stricker AR, Mach RL, de Graaff LH. Regulation of transcription of cellulases- and hemicellulases-encoding genes in Aspergillus niger and Hypocrea jecorina (Trichoderma reesei). Appl Microbiol Biotechnol. 2008;78:211–20.
Seibel C, Gremel G, Silva RD, Schuster A, Kubicek CP, Schmoll M. Light-dependent roles of the G-protein α subunit GNA1 of Hypocrea jecorina (anamorph Trichoderma reesei). BMC Biol. 2009;7:58.
Tisch D, Kubicek CP, Schmoll M. New insights into the mechanism of light modulated signaling by heterotrimeric G-proteins: ENVOY acts on gna1 and gna3 and adjusts cAMP levels in Trichoderma reesei (Hypocrea jecorina). Fungal Genet Bio. 2011;48:631–40.
Stranks DW. Influence of phenethyl alcohol and other organic solvents on cellulase production. Can J Microbiol. 1973;19:1523–6.
Chen L, Zou G, Wang J, Wang J, Liu R, Jiang Y, Zhao G, Zhou Z. Characterization of the Ca2+-responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol Microbiol. 2016;100:560–75.
Mach RL, Zeilinger S, Kristufek D, Kubicek CP. Ca2+–calmodulin antagonists interfere with xylanase formation and secretion in Trichoderma reesei. Biochim Biophys Acta. 1998;1403:281–9.
Mandels M, Reese ET. Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol. 1957;73:269–78.
Jensen LT, Ajua-Alemanji M, Culotta VC. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J Biol Chem. 2003;278:42036–40.
Xu YN, Xia XX, Zhong JJ. Induction of ganoderic acid biosynthesis by Mn2+ in static liquid cultivation of Ganoderma lucidum. Biotechnol Bioeng. 2014;111:2358–65.
Hernández-Ortiz P, Espeso EA. Phospho-regulation and nucleocytoplasmic trafficking of CrzA in response to calcium and alkaline-pH stress in Aspergillus nidulans. Mol Microbiol. 2013;89:532–51.
Hernández-Ortiz P, Espeso EA. Spatiotemporal dynamics of the calcineurin target CrzA. Cell Signal. 2017;29:168–80.
Culotta VC, Yang M, Hall MD. Manganese transport and trafficking: lessons learned from Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:1159–65.
Huang XJ, Shin JH, Pinochet-Barros A, Su TT, Helmann JD. Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol Microbiol. 2017;103:253–68.
Luk E, Jensen LT, Culotta VC. The many highways for intracellular trafficking of metals. J Biol Inorg Chem. 2003;8:803–9.
Pittman JK. Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol. 2005;167:733–42.
Mandal D, Woolf TB, Rao R. Manganese selectivity of Pmr1, the yeast secretory pathway ion pump, is defined by residue Gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J Biol Chem. 2000;275:23933–8.
Ofiteru AM, Ruta LL, Rotaru C, Dumitru I, Ene CD, Neagoe A, Farcasanu IC. Overexpression of the PHO84 gene causes heavy metal accumulation and induces Ire1p-dependent unfolded protein response in Saccharomyces cerevisiae cells. Appl Microbiol Biotechnol. 2012;94:425–35.
Antebi A, Fink GR. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3:633–54.
Mach-Aigner AR, Pucher ME, Steiger MG, Bauer GE, Preis SJ, Mach RL. Transcriptional regulation of xyr1, encoding the main regulator of the xylanolytic and cellulolytic enzyme system in Hypocrea jecorina. Appl Environ Microb. 2008;74:6554–62.
Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev. 1999;79:1089–125.
Xu YN, Xia XX, Zhong JJ. Induced effect of Na plus on ganoderic acid biosynthesis in static liquid culture of Ganoderma lucidum via calcineurin signal transduction. Biotechnol Bioeng. 2013;110:1913–23.
Feske S, Rao A, Hogan PG. The Ca2+–calcineurin–NFAT signalling pathway. In: Krebs J, Michalak M, editors. New comprehensive biochemistry. Calcium: a matter of life or death. New York: Elsevier; 2007. p. 365–401.
Martinez D, Berka RM, Henrissat B, Saloheimo M, Arvas M, Baker SE, Chapman J, Chertkov O, Coutinho PM, Cullen D, et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat Biotechnol. 2008;26:553–60.
Lang RJ, Hashitani H, Tonta MA, Suzuki H, Parkington HC. Role of Ca2+ entry and Ca2+ stores in atypical smooth muscle cell autorhythmicity in the mouse renal pelvis. Br J Pharmacol. 2007;152:1248–59.
Bowman BJ, Abreu S, Johl JK, Bowman EJ. The pmr gene, encoding a Ca2+-ATPase, is required for calcium and manganese homeostasis and normal development of hyphae and conidia in Neurospora crassa. Eukaryot Cell. 2012;11:1362–70.
Dürr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9:1149–62.
Maeda T, Sugiura R, Kita A, Saito M, Deng L, He Y, Yabin L, Fujita Y, Takegawa K, Shuntoh H, et al. Pmr1, a P-type ATPase, and Pdt1, an Nramp homologue, cooperatively regulate cell morphogenesis in fission yeast: the importance of Mn2+ homeostasis. Genes Cells. 2004;9:71–82.
Mandal D, Rulli SJ, Rao R. Packing interactions between transmembrane helices alter ion selectivity of the yeast Golgi Ca2+/Mn2+-ATPase PMR1. J Biol Chem. 2003;278:35292–8.
Paraszkiewicz K, Frycie A, Slaba M, Dlugonski J. Enhancement of emulsifier production by Curvularia lunata in cadmium, zinc and lead presence. Biometals. 2007;20:797–805.
Xu YN, Zhong JJ. Impacts of calcium signal transduction on the fermentation production of antitumor ganoderic acids by medicinal mushroom Ganoderma lucidum. Biotechnol Adv. 2012;30:1301–8.
Jakubovics NS, Jenkinson HF. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiol. 2001;147:1709–18.
Ouyang ZM, He M, Oman T, Yang XF, Norgard MV. A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc Natl Acad Sci USA. 2009;106:3449–54.
Peris-Peris C, Serra-Cardona A, Sánchez-Sanuy F, Campo S, Ariño J, San Segundo B. Two NRAMP6 isoforms function as iron and manganese transporters and contribute to disease resistance in Rice. Mol Plant Microbe. 2017;30:385–98.
Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol. 2009;72:12–25.
Cohen A, Nelson H, Nelson N. The family of SMF metal ion transporters in yeast cells. J Biol Chem. 2000;275:33388–94.
Luk EEC, Culotta VC. Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J Biol Chem. 2001;276:47556–62.
Wykoff DD, O’Shea EK. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics. 2001;159:1491–9.
Dancis A, Haile D, Yuan DS, Klausner RD. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem. 1994;269:25660–7.
Dix DR, Bridgham JT, Broderius MA, Byersdorfer CA, Eide DJ. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J Biol Chem. 1994;269:26092–9.
Hassett R, Dix DR, Eide DJ, Kosman DJ. The Fe(II) permease Fet4p functions as a low affinity copper transporter and supports normal copper trafficking in Saccharomyces cerevisiae. Biochem J. 2000;351:477–84.
Zhao H, Eide D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem. 1996;271:23203–10.
Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci USA. 1996;93:2454–8.
Ariño J, Ramos J, Sychrová H. Alkali metal cation transport and homeostasis in yeasts. Microbiol Mol Biol Rev. 2010;74:95–120.
Thewes S. Calcineurin–Crz1 signaling in lower eukaryotes. Eukaryot Cell. 2014;13:694–705.
Saidi Y, Finka A, Goloubinoff P. Heat perception and signalling in plants: a tortuous path to thermotolerance. New Phytol. 2011;190:556–65.
Wang H, Liang Y, Zhang B, Zheng W, Xing L, Li M. Alkaline stress triggers an immediate calcium fluctuation in Candida albicans mediated by Rim101p and Crz1p transcription factors. FEMS Yeast Res. 2011;11:430–9.
Michielse CB, Hooykaas PJJ, van der Hondel CAMJJ, Ram AFJ. Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nat Protoc. 2008;3:1671–8.
Mandels M, Weber J. The production of cellulases. Cellulases and their applications. Washington, DC: American Chemical Society; 1969. p. 391–414.
Wang MY, Zhao QS, Yang JH, Jiang BJ, Wang FZ, Liu KM, Fang X. A mitogen-activated protein kinase Tmk3 participates in high osmolarity resistance, cell wall integrity maintenance and cellulase production regulation in Trichoderma reesei. PLoS ONE. 2013;8:e72189.
Bischof R, Fourtis L, Limbeck A, Gamauf C, Seiboth B, Kubicek CP. Comparative analysis of the Trichoderma reesei transcriptome during growth on the cellulase inducing substrates wheat straw and lactose. Biotechnol Biofuels. 2013;6:127.
Zhang G, Liu P, Wei W, Wang X, Wei D, Wang W. A light-switchable bidirectional expression system in filamentous fungus Trichoderma reesei. J Biotechnol. 2016;240:85–93.
Steiger MG, Mach RL, Mach-Aigner AR. An accurate normalization strategy for RT-qPCR in Hypocrea jecorina (Trichoderma reesei). J Biotechnol. 2010;145:30–7.
Zhang L, Zhao X, Zhang G, Zhang J, Wang X, Zhang S, Wang W, Wei D. Light-inducible genetic engineering and control of non-homologous end-joining in industrial eukaryotic microorganisms: LML 3.0 and OFN 1.0. Sci Rep. 2016;6:20761.
Authors’ contributions
WW initiated, designed, and coordinated the study and reviewed the manuscript. YC planned and carried out experiments and measurements, and interpreted experimental data. DW and WW supported the research funding. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and in Additional files 1 and 2].
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China [No. C010302-31500066], and the Fundamental Research Funds for the Central Universities [No. 222201714053].
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
Primers used in this study.
Additional file 2: Figure S1.
Influence of Mn2+ on the transcriptional levels of cellulase-encoding genes in T. reesei Rut-C30. A–E The effects of Mn2+ on the transcriptional levels of cbh1 (A), cbh2 (B), xyr1 (C), egl1 (D) and egl2 (E). 0 Mn, no Mn2+ was added to the medium; 10 Mn, final concentration of 10 mM. Three independent experiments with three biological replicates each were performed. Values are the means ± SD of the results from three independent experiments. Asterisks indicate significant differences from untreated strains (*p< 0.05, **p < 0.01, Student’s t test).
Additional file 3: Figure S2.
Influence of Mn2+ addition on the transcriptional levels of putative Mn2+ transport-encoding genes. A–E The effect of Mn2+ on the transcriptional levels of tre77552 (A), tre81389 (B), tre45852 (C), tre45868 (D), and tre106118 (E). 0 Mn, no Mn2+ was added to the medium; 10 Mn, final concentration of 10 mM. Values are the means ± SD of the results from three independent experiments. Asterisks indicate significant differences from untreated strains (*p< 0.05, Student’s t test).
Additional file 4: Figure S3.
Concentrations of Mn2+ in T. reesei Rut-C30 and its derivative mutants. The concentrations of intracellular Mn2+ of T. reesei Rut-C30 and its derivative mutant strains were examined after cultured in medium containing 10 mM MnCl2. Values are the means ± SD of the results from three independent experiments. Asterisks indicate significant differences from parental strain Rut-C30 (*p< 0.05, Student’s t test).
Additional file 5: Figure S4.
Construction and verification of Δtpho84-1, Δtpho84-2, tpho84-1-rc, tpho84-2-rc mutants. (A) Schematic representation of the tpho84-1 locus from Rut-C30 and Δtpho84-1 mutant. The binding sites of primers on the genome of Rut-C30 and Δtpho84-1, and the expected sizes of the products in PCR verification are given. The region from +1 to +1916 bp relative to the translation start site of tpho84-1 (grey box) was replaced with the hygromycin resistance expression cassette (hygromycin, black box). (B) Schematic representation of the tpho84-2 locus from Rut-C30 and Δtpho84-2 mutant. The binding sites of primers on the genome of Rut-C30 and Δtpho84-2 and the expected sizes of the PCR products are given. The region from +1 to +2087 bp relative to the translation start site of tpho84-2 (grey box) was replaced with the hygromycin resistance expression cassette (hygromycin, black box). (C) Schematic representation of the P-tpho84-1-tpho84-1-T-tpho84-1 cassette in tpho84-1-rc strains. The primer pairs indicated were used in the verification of the expression cassette. P-tpho84-1, the possible promoter of tpho84-1; T-tpho84-1, the possible terminator of tpho84-1. (D) Schematic representation of the P-tpho84-2-tpho84-2-T-tpho84-2 cassette in tpho84-2-rc strains. The primer pairs indicated were used in the verification of the expression cassette. P-tpho84-2, the possible promoter of tpho84-2; T-tpho84-2, the possible terminator of tpho84-2. (E) PCR verification of the Δtpho84-1 and Δtpho84-2 mutants. Lane M, DNA molecular mass maker. PCR amplification results of the Dtpho84-1-F were obtained using tpho84-1-CF with D70-4 and Dtpho84-1-R were obtained using HG3.6 with tpho84-1-CR (see Additional file 1: Table S1). PCR amplification results of the Dtpho84-2-F were obtained using tpho84-2-CF with D70-4 and Dtpho84-2-R were obtained using HG3.6 with tpho84-2-CR. (F) PCR verification of the tpho84-1-rc and tpho84-2-rc strains. Lane M, DNA molecular mass maker. PCR amplification results of the tpho84-1-rc were obtained using tpho84-1-OF with tpho84-1-OR and tpho84-2-rc was obtained using tpho84-2-OF with tpho84-2-OR. The genome of the Δtpho84-1 and Δtpho84-2 mutants were used as a negative control.
Additional file 6: Figure S5.
Subcellular localization of TPHO84-1 and TPHO84-2. Schematic representation of the plasmids used for expression of RFP-TPHO84-1 and RFP-TPHO84-2. RFP was fused to the N-terminal of TPHO84-1 and TPHO84-2 and expressed under the control of the cbh1 promoter. B. In vivo epifluorescence analysis of rfp-tpho84-1 and rfp-tpho84-2 transformants. The transformants were cultured in liquid Mandels’ medium for 48–60 h and observed using Laser Scanning Confocal Microscopy.
Additional file 7: Figure S6.
Cellulase activities of T. reesei Rut-C30 and its derivative mutant strains. The activities were examined after cultured in medium containing 0 mM Mn2+. (A) CMCase activities, (B) pNPCase activities. Values are the means ± SD of the results from three independent experiments.
Additional file 8.
Data for corresponding cellulase activity per biomass. The CMCase/biomass activity and pNPCase/biomass activity of T. reesei strains were examined. For every experiment, three biological replicates were performed with three technical replicates each. Values are the means ± SD of the results from three independent experiments. Asterisks indicate significant differences (*p< 0.05, Student’s t test).
Additional file 9: Figure S12.
Concentrations of Mn2+ in Δtpmr1 strain. The concentrations of extracellular and intracellular Mn2+ of the Δtpmr1 strain was examined after culturing in minimal medium containing 0 or 10 mM MnCl2. Filled circle, intracellular Mn2+ concentration after adding 10 mM MnCl2; blank circle, extracellular Mn2+ concentration after adding 10 mM MnCl2; filled square, intracellular Mn2+ concentration of control without MnCl2 supplementation; blank square, extracellular Mn2+ concentration of control without MnCl2 supplementation. Values are the means ± SD of the results from three independent experiments.
Additional file 10: Figure S13.
Cytosolic Ca2+ levels increase after Ca2+ addition. A. The analysis of cytosolic Ca2+ levels via a Ca2+ fluorescent probe Fluo-3/AM. The T. reesei Rut-C30 and its derivative mutant strains were cultured in liquid minimal medium (initial 5.4 mM Ca2+) for 48–60 h with extra 0 or 10 mM CaCl2 supplementation (low Ca or high Ca, respectively). For detection, 50 μM Fluo-3/AM was used, and the intensity was monitored using Automatic Inverted Fluorescence Microscopy. Green fluorescence represents the free cytosolic Ca2+. DIC, differential interference contrast. B. Comparative fluorescence ratio analysis of Ca2+ influence on cytosolic Ca2+ levels. The y-axis represents the Ca2+ fluorescence ratio measured by CLSM, and the x-axis represents the different strains tested.
Additional file 11: Figure S14.
Verification of Δ81389, Δ45852, Δ106118, Δcrz1, and Δtpmr1 mutants. Lane M, DNA molecular mass maker. PCR amplification results of the F were obtained using t81389-, t45852-, t106118-, tcrz1-, and ttpmr1-CF, respectively, with D70-4, and R were obtained using HG3.6 with t81389-, t45852-, t106118-, tcrz1-, and tpmr1-CR, respectively. PCR amplification using primer pairs t81389-, t45852-, t106118-, tcrz1-, and tpmr1-OF/OR, respectively, was performed as a negative confirm for gene deletion.
Additional file 12: Figure S15.
PCR Verification of rfp-tpho84-1, rfp-tpho84-2 mutants. Lane M, DNA molecular mass marker. PCR amplification results of the rfp-tpho84-1-F were obtained using rfp-tpho84-1-CF with D70-4 and rfp-tpho84-1-R were obtained using HG3.6 with rfp-tpho84-1-CR (Additional file 1: Table S1). PCR amplification results of the rfp-tpho84-2-F were obtained using rfp-tpho84-2-CF with D70-4 and rfp-tpho84-2-R were obtained using HG3.6 ith rfp-tpho84-2-CR.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, Y., Shen, Y., Wang, W. et al. Mn2+ modulates the expression of cellulase genes in Trichoderma reesei Rut-C30 via calcium signaling. Biotechnol Biofuels 11, 54 (2018). https://doi.org/10.1186/s13068-018-1055-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-018-1055-6