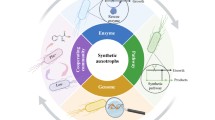
Abstract
The literature provides more and more examples of research projects that develop novel production processes based on microorganisms organized in the form of biofilms. Biofilms are aggregates of microorganisms that are attached to interfaces. These viscoelastic aggregates of cells are held together and are embedded in a matrix consisting of multiple carbohydrate polymers as well as proteins. Biofilms are characterized by a very high cell density and by a natural retentostat behavior. Both factors can contribute to high productivities and a facilitated separation of the desired end-product from the catalytic biomass. Within the biofilm matrix, stable gradients of substrates and products form, which can lead to a differentiation and adaptation of the microorganisms’ physiology to the specific process conditions. Moreover, growth in a biofilm state is often accompanied by a higher resistance and resilience towards toxic or growth inhibiting substances and factors. In this short review, we summarize how biofilms can be studied and what most promising niches for their application can be. Moreover, we highlight future research directions that will accelerate the advent of productive biofilms in biology-based production processes.






Similar content being viewed by others
References
Asimakopoulos K, Gavala HN, Skiadas IV (2018) Reactor systems for syngas fermentation processes: a review. Chem Eng J 348:732–744
Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, Desvaux M, Di Bonaventura G, Hébraud M, Jaglic Z, Kacániová M, Knochel S, Lourenco A, Mergulhao F, Meyer RL, Nychas G, Simones M, Tresse O, Sternberg C (2017) Critical review on biofilm methods. Crit Rev Microbiol 43:313–351
Beblawy S, Bursac T, Paquete C, Louro R, Clarke TA, Gescher J (2018) Extracellular reduction of solid electron acceptors by Shewanella oneidensis. Mol Microbiol 109:571–583
Bennetto HP, Delaney GM, Mason JR, Roller SD, Stirling JL and Thurston CF (1988) Applications of microbial electrochemistry. In Resources and applications of biotechnology. Palgrave Macmillan UK, London. pp. 363–374
Beyenal H and Babauta J (2013) Microsensors and microscale gradients in biofilms. Springer, Adv Biochem Eng Biotechnol, pp. 235–256.
Biffinger JC, Ray R, Little BJ, Fitzgerald LA, Ribbens M, Finkel SE, Ringeisen BR (2009) Simultaneous analysis of physiological and electrical output changes in an operating microbial fuel cell with Shewanella oneidensis. Biotechnol Bioeng 103:524–531
Blauert F, Horn H, Wagner M (2015) Time-resolved biofilm deformation measurements using optical coherence tomography. Biotechnol Bioeng 112:1893–1905
Bosire EM, Rosenbaum MA (2017) Electrochemical potential influences phenazine production, electron transfer and consequently electric current generation by Pseudomonas aeruginosa. Front Microbiol 8:892
Bouwer EJ, Crowe PB (1988) Biological processes in drinking water treatment. J Am Water Works Assoc 80:82–93
Bursac T, Gralnick JA, Gescher J (2017) Acetoin production via unbalanced fermentation in Shewanella oneidensis. Biotechnol Bioeng 114:1283–1289
Cheng K-C, Demirci A, Catchmark JM (2010) Advances in biofilm reactors for production of value-added products. Appl Microbiol Biotechnol 87:445–456
Demler M, Weuster-Botz D (2011) Reaction engineering analysis of hydrogenotrophic production of acetic acid by Acetobacterium woodii. Biotechnol Bioeng 108:470–474
Doll K, Rückel A, Kämpf P, Wende M, Weuster-Botz D (2018) Two stirred-tank bioreactors in series enable continuous production of alcohols from carbon monoxide with Clostridium carboxidivorans. Bioprocess Biosyst Eng 41:1403–1416
Flemming H-C (2002) Biofouling in water systems – cases, causes and countermeasures. Appl Microbiol Biotechnol 59:629–640
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575
Förster AH, Beblawy S, Golitsch F, Gescher J (2017) Electrode-assisted acetoin production in a metabolically engineered Escherichia coli strain. Biotechnol Biofuels 10:65
Fu C, Yue X, Shi X, Ng KK, Ng HY (2017) Membrane fouling between a membrane bioreactor and a moving bed membrane bioreactor: effects of solids retention time. Chem Eng J 309:397–408
Groher A, Weuster-Botz D (2016) Comparative reaction engineering analysis of different acetogenic bacteria for gas fermentation. J Biotechnol 228:82–94
Gross R, Schmid A, Buehler K (2012) Catalytic biofilms: a powerful concept for future bioprocesses. In: Lear G, L. G (eds) Microbial biofilms. Caister Academic Press, Norfolk, pp 193–222
Halan B, Schmid A, Buehler K (2011) Real-time solvent tolerance analysis of Pseudomonas sp. strain VLB120ΔC catalytic biofilms. Appl Environ Microbiol 77:1563–1571
Halan B, Buehler K, Schmid A (2012) Biofilms as living catalysts in continuous chemical syntheses. Trends Biotechnol 30:453–465
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the Natural environment to infectious diseases. Nat Rev Microbiol 2:95–108
Hansen SH, Kabbeck T, Radtke CP, Krause S, Krolitzki E, Peschke T, Gasmi J, Rabe KS, Wagner M, Horn H, Hubbuch J, Gescher J, Niemeyer CM, (2017) Machine-assisted cultivation and analysis of biofilms. bioRxiv 210583.
He Z, Mansfeld F (2009) Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ Sci 2:215–219
Herrling MP, Weisbrodt J, Kirkland CM, Williamson NH, Lackner S, Codd SL, Seymour JD, Guthausen G, Horn H (2017) NMR investigation of water diffusion in different biofilm structures. Biotechnol Bioeng 114:2857–2867
Horn H, Morgenroth E (2006) Transport of oxygen, sodium chloride, and sodium nitrate in biofilms. Chem Eng Sci 61:1347–1356
Ivleva NP, Wagner M, Horn H, Niessner R, Haisch C (2009) Towards a nondestructive chemical characterization of biofilm matrix by Raman microscopy. Anal Bioanal Chem 393:197–206
Ivleva NP, Wagner M, Horn H, Niessner R, Haisch C (2010) Raman microscopy and surface-enhanced Raman scattering (SERS) for in situ analysis of biofilms. J Biophotonics 3:548–556
Janczewski L, Trusek-Holownia A (2016) Biofilm-based membrane reactors – selected aspects of the application and microbial layer control. Desalin Water Treat 57:22909–22916
Kipf E, Koch J, Geiger B, Erben J, Richter K, Gescher J, Zengerle R, Kerzenmacher S (2013) Systematic screening of carbon-based anode materials for microbial fuel cells with Shewanella oneidensis MR-1. Bioresour Technol 146:386–392
Kipf E, Zengerle R, Gescher J, Kerzenmacher S (2014) How does the choice of anode material influence electrical performance? A comparison of two microbial fuel cell model organisms. ChemElectroChem 1:1849–1853
Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jørgensen A, Molin S, Tolker-Nielsen T (2003) Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol 48:1511–1524
Korneel Rabaey, Nico Boon, Höfte M, Verstraete W (2005) Microbial phenazine production enhances electron transfer in biofuel cells. Environ Sci Technol 39(9):3401–3408
Kracke F, Lai B, Yu S, Krömer JO (2018) Balancing cellular redox metabolism in microbial electrosynthesis and electro fermentation – a chance for metabolic engineering. Metab Eng 45:109–120
Krieg T, Sydow A, Schröder U, Schrader J, Holtmann D (2014) Reactor concepts for bioelectrochemical syntheses and energy conversion. Trends Biotechnol 32:645–655
Lackner S, Terada A, Horn H, Henze M, Smets BF (2010) Nitritation performance in membrane-aerated biofilm reactors differs from conventional biofilm systems. Water Res 44:6073–6084
Ledezma P, Greenman J, Ieropoulos I (2012) Maximising electricity production by controlling the biofilm specific growth rate in microbial fuel cells. Bioresour Technol 118:615–618
Li W-W, Sheng G-P (2011) Microbial fuel cells in power generation and extended applications. Adv Biochem Eng/Biotechn 128:165–197
Liu T, Yu Y-Y, Deng X-P, Ng CK, Cao B, Wang J-Y, Scott AR, Kjelleberg S, Song H (2015) Enhanced Shewanella biofilm promotes bioelectricity generation. Biotechnol Bioeng 112:2051–2059
Lovley DR, Nevin KP (2013) Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr Opin Biotechnol 24:385–390
Martinez CM, Alvarez LH (2018) Application of redox mediators in bioelectrochemical systems. Biotechnol Adv 36:1412–1423
Mayer A, Schädler T, Trunz S, Stelzer T, Weuster-Botz D (2018) Carbon monoxide conversion with Clostridium aceticum. Biotechnol Bioeng 115:2740–2750
Melkus G, Rolletschek H, Fuchs J, Radchuk V, Grafahrend-Belau E, Sreenivasulu N, Rutten T, Weier D, Heinzel N, Schreiber F, Altmann T, Jakob PM, Borisjuk L (2011) Dynamic 13C/1H NMR imaging uncovers sugar allocation in the living seed. Plant Biotechnol J 9:1022–1037
Muffler K, Lakatos M, Schlegel C, Strieth D, Kuhne S and Ulber R (2014) Application of biofilm bioreactors in white biotechnology. In Productive Biofilms 123–161.
Neu TR and Lawrence JR (2014) Investigation of microbial biofilm structure by laser scanning microscopy. In Productive Biofilms pp. 1–51.
Neu TR, Lawrence JR (2015) Innovative techniques, sensors, and approaches for imaging biofilms at different scales. Trends Microbiol 23:233–242
Nielsen PH, Daims H and Lemmer H (2009) FISH handbook for biological wastewater treatment : identification and quantification of microorganisms in activated sludge and biofilms by FISH, FISH Handbook for Biological Wastewater Treatment.
Percival SL, Vuotto C, Donelli G, Lipsky BA (2015) Biofilms and wounds: an identification algorithm and potential treatment options. Adv Wound Care 4:389–397
Picioreanu C, Blauert F, Horn H, Wagner M (2018) Determination of mechanical properties of biofilms by modelling the deformation measured using optical coherence tomography. Water Res 145:588–598
Reguera G, Nevin KP, Nicoll JS, Covalla SF, Woodard TL, Lovley DR (2006) Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl Environ Microbiol 72:7345–7348
Reichert P (1995) Design techniques of a computer program for the identification of processes and the simulation of water quality in aquatic systems. Environ Softw 10:199–210
Rittmann BE (2018) Biofilms, active substrata, and me. Water Res 132:135–145
Rollefson JB, Stephen CS, Tien M, Bond DR (2011) Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J Bacteriol 193:1023–1033
Rosche B, Li XZ, Hauer B, Schmid A, Buehler K (2009) Microbial biofilms: a concept for industrial catalysis? Trends Biotechnol 27:636–643
Santoro C, Arbizzani C, Erable B, Ieropoulos I (2017) Microbial fuel cells: from fundamentals to applications. A review. J Power Sources 356:225–244
Seviour T, Derlon N, Dueholm MS, Flemming H-C, Girbal-Neuhauser E, Horn H, Kjelleberg S, Loosdrecht MCM, Lotti T, Malpei MF, Nerenberg R, Neu TR, Paul E, Yu H, Lin Y (2019) Extracellular polymeric substances of biofilms: suffering from an identity crisis. Water Res 151(1–7):1–7
Shen Y, Brown R, Wen Z (2014) Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: evaluating the mass transfer coefficient and ethanol production performance. Biochem Eng J 85:21–29
Staudt C, Horn H, Hempel D and Neu T (2003) Screening of lectins for staining lectin-specific glycoconjugates in the EPS of biofilms. In Biofilms in industry, medicine & Environmental Biotechnology. pp. 308–327.
Staudt C, Horn H, Hempel DC, Neu TR (2004) Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnol Bioeng 88:585–592
Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6:199–210
Sturm-Richter K, Golitsch F, Sturm G, Kipf E, Dittrich A, Beblawy S, Kerzenmacher S, Gescher J (2015) Unbalanced fermentation of glycerol in Escherichia coli via heterologous production of an electron transport chain and electrode interaction in microbial electrochemical cells. Bioresour Technol 186:89–96
Subramanian P, Pirbadian S, El-Naggar MY, Jensen GJ (2018) Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proc Natl Acad Sci U S A 115:3246–3255
Sun D, Chen J, Huang H, Liu W, Ye Y, Cheng S (2016) The effect of biofilm thickness on electrochemical activity of Geobacter sulfurreducens. Int J Hydrog Energy 41:16523–16528
Thorn RMS, Austin AJ, Greenman J, Wilkins JPG, Davis PJ (2009) In vitro comparison of antimicrobial activity of iodine and silver dressings against biofilms. J Wound Care 18:343–346
Timberlake DL, Strand SE, Williamson KJ (1988) Combined aerobic heterotrophic oxidation, nitrification and denitrification in a permeable-support biofilm. Water Res 22:1513–1517
van Benthum WAJ, van Loosdrecht MDM, Heijnen JJ (1997) Control of heterotrophic layer formation on nitrifying biofilms in a biofilm airlift suspension reactor. Biotechnol Bioeng 53:397–405
von Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Wagner M, Horn H (2017) Optical coherence tomography in biofilm research: a comprehensive review. Biotechnol Bioeng 114:1386–1402
Wagner M, Manz B, Volke F, Neu TR, Horn H (2010) Online assessment of biofilm development, sloughing and forced detachment in tube reactor by means of magnetic resonance microscopy. Biotechnol Bioeng 107:172–181
Wingender J, Neu TR and Flemming H-C (1999) What are bacterial extracellular polymeric substances? In Microbial extracellular polymeric substances. pp. 1–19.
Xiao Y, Zhao F (2017) Electrochemical roles of extracellular polymeric substances in biofilms. Curr Opin Electrochem 4:206–211
Yong Y-C, Yu Y-Y, Zhang X, Song H (2014) Highly active bidirectional electron transfer by a self-assembled electroactive reduced-graphene-oxide-hybridized biofilm. Angew Chem Int Ed 53:4480–4483
Zajdel TJ, Baruch M, Méhes G, Stavrinidou E, Berggren M, Maharbiz MM, Simon DT, Ajo-Franklins CM (2018) PEDOT:PSS-based multilayer bacterial-composite films for bioelectronics. Sci Rep 8:15293
Acknowledgments
Thanks to Dirk Weuster-Botz (Technische Universität München) for discussing the simulation of acetotrophic bacteria growing on membranes.
Funding
The research of Harald Horn is supported by the German Research Foundation (DFG HO 1910/16-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Edel, M., Horn, H. & Gescher, J. Biofilm systems as tools in biotechnological production. Appl Microbiol Biotechnol 103, 5095–5103 (2019). https://doi.org/10.1007/s00253-019-09869-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09869-x