Abstract
Temperate rocky reefs often support mosaics of alternative habitats such as macroalgal forests, algal turfs and sea urchin barrens. Although the composition of epilithic microbial biofilms (EMBs) is recognized as a major determinant of macroalgal recruitment, their role in regulating the stability of alternative habitats on temperate rocky reefs remains unexplored. On shallow rocky reefs of the Island of Capraia (NW Mediterranean), we compared EMB structure among canopy stands formed by the fucoid Ericaria brachycarpa, algal turfs, and urchin barrens under ambient versus experimentally enhanced nutrient levels. The three habitats shared a core microbial community consisting of 21.6 and 25.3% of total ASVs under ambient and enhanced nutrient conditions, respectively. Although Gammaproteobacteria, Alphaproteobacteria and Flavobacteriia were the most abundant classes across habitats, multivariate analyses at the ASV level showed marked differences in EMB composition among habitats. Enhancing nutrient level had no significant effect on EMBs, although it increased their similarity between macroalgal canopy and turf habitats. At both ambient and enriched nutrient levels, ASVs mostly belonging to Proteobacteria and Bacteroidetes were more abundant in EMBs from macroalgal canopies than barrens. In contrast, ASVs belonging to the phylum of Proteobacteria and, in particular, to the families of Rhodobacteraceae and Flavobacteriaceae at ambient nutrient levels and of Rhodobacteraceae and Bacteriovoracaceae at enhanced nutrient levels were more abundant in turf than canopy habitats. Our results show that primary surfaces from alternative habitats that form mosaics on shallow rocky reefs in oligotrophic areas host distinct microbial communities that are, to some extent, resistant to moderate nutrient enhancement. Understanding the role of EMBs in generating reinforcing feedback under different nutrient loading regimes appears crucial to advance our understanding of the mechanisms underpinning the stability of habitats alternative to macroalgal forests as well as their role in regulating reverse shifts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human activities are causing unprecedented changes to terrestrial and aquatic systems, impairing their functioning and ability to deliver services globally [1, 2]. Alterations in biotic or abiotic conditions can shift ecosystems to alternative undesirable states, which are generally characterized by lower species diversity and productivity [3,4,5]. Once the transition has occurred, stabilizing feedbacks can lock the system into the degraded state [3, 5,6,7,8,9]. Thus, understanding the mechanisms generating hysteresis is key for conserving the resilience of pristine systems and for promoting reverse shifts in degraded systems.
Subtidal macroalgal forests, composed of Laminariales and Fucales, support biodiversity through the formation of habitat and are among the most productive marine systems [9,10,11], delivering key ecosystem services to humans [9, 12]. Nonetheless, the collapse of these systems has been documented along temperate coasts worldwide [10, 13,14,15]. Overgrazing by either sea urchins or fish can cause the shift in rocky reef dominance from canopy forming (i.e., macroalgal forests) to encrusting coralline macroalgae (i.e., sea urchin barrens), while excessive nutrient loading can elicit algal turf dominance [5, 6, 9, 14,15,16]. Processes that reduce the recruitment of canopy-forming macroalgae contribute to barren or algal turf stabilization [6]. Continuous scraping by sea urchins, fish or limpets and reduced supply of spores or gametes from surrounding reproductive plants underpin reduced recruitment in barren areas [6, 17,18,19]. Conversely, accumulation and trapping of sediments in the complex three-dimensional matrix formed by algal turfs reduce the settlement of spores or zygotes [10, 20,21,22]. Although the composition of epilithic microbial biofilms (EMBs) is recognized as a major determinant of macroalgal recruitment through the modification of physical and chemical characteristics of settlement surfaces [23, 24], their relevance in sustaining the stability of different states on temperate rocky reefs remains unexplored.
Brown seaweeds belonging to the genus Cystoseira sensu lato are important habitat-formers along Mediterranean rocky coasts, but they are declining throughout the basin as a consequence of human alterations of abiotic (i.e., enhanced sedimentation rates and nutrient loading) and biotic conditions (i.e., increased herbivory due to predator over-exploitation) [15, 25,26,27,28]. Indeed, even at relatively pristine sites, macroalgal canopy stands often alternate with areas occupied by algal turfs or encrusting corallines [28]. However, to the best of our knowledge, no study has attempted to compare EMBs among alternative habitats that form mosaics on rocky reefs. Although, according to the evidence from coral reefs [29,30,31], EMBs from canopy stands, algal turfs and urchin barrens can be expected to differ, we have no clue of the magnitude of these differences.
Macroalgae can condition bacterial biofilms on underlying or adjacent hard surfaces through different mechanisms. For instance, macroalgal release of dissolved organic carbon (DOC), rich in labile sugars, has been shown to promote the growth of copiotrophic bacteria on coral reefs [32] and to differ among macroalgal species [33]. Likewise, there is strong variation among macroalgal species in the type and amount of allelochemicals they produce and release in the water column [34]. Free-living microbes, exosomes, and allelochemicals can be released by macroalgae into the diffusive boundary layer (DBL) and transferred to neighboring surfaces [35,36,37]. Although large variation in the structure of epiphytic microalgal communities has been documented at a hierarchy of spatial scales [38], the composition of epiphytic bacterial communities on macroalgae is generally highly host-specific [39,40,41,42] and a core microbial community is often maintained [24, 43,44,45]. Different macroalgal species or assemblages can also cause variation in epilithic bacterial assemblages by influencing physical conditions, such as illumination and water-flow. For these reasons, the structure of epilithic biofilms can be expected to vary among areas dominated by different macroalgal assemblages. Thus, the aim of this study was to test the hypothesis that EMBs differ between canopy-dominated areas and alternative habitats (i.e., urchin barrens or algal turfs) on shallow rocky reefs.
In addition, since excessive nutrient loading due to run-off from urban and agricultural areas has been widely shown to promote macroalgal growth on both temperate and tropical reefs [8, 10] and, indeed, to be an important driver of the decline of temperate macroalgal forests [10, 16, 20], we also experimentally tested the hypothesis that variations in the epilithic microbial biofilms among alternative habitats are influenced by nutrient enrichment. Evidence from tropical reefs suggests, in fact, that nutrient levels contribute to shape the EMB composition [29, 30].
Materials and Methods
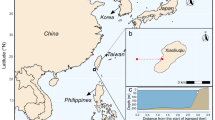
This study was carried out on shallow rocky reefs at Capraia Island in the Tuscan Archipelago (NW Mediterranean) from June 2014 to July 2016. The island can be considered pristine since, due to the very small resident population, lack of relevant agricultural activities and distance from mainland (~ 30 nautical miles), it is not exposed to inorganic and organic pollution or nutrient run-off [28]. Rocky reefs between 2 and 8 m deep are dominated by canopies stands formed by the fucoid Ericaria brachycarpa, which alternates with patches of algal turfs or urchin barrens colonized by encrusting corallines [28]. Here, algal turfs are composed of foliose (e.g., Dictyota spp., Padina pavonica), filamentous (e.g., Sphacelariales), siphonous algae (e.g., Acetabularia acetabulum, Caulerpa cylindracea), and corticated rhodophytae (e.g., Laurencia obtusa, Gastroclonium sp.).
In June 2014, four 1.5 × 0.5 m areas of each habitat (macroalgal canopies, algal turfs, and urchin barrens) were randomly identified on large boulders at 4–6 m depth and about 10 m apart, along a 600-m stretch of coast (a total of 12 areas). Barren areas were adjacent to canopy stands. Two areas of each habitat were exposed to nutrient addition and two areas were left at ambient nutrient conditions (i.e., controls). Nutrients were elevated by means of slow-release fertilizer pellets (Osmocote®, 6 months, 17:11:10 N:P:K) contained in plastic net bags (1-mm mesh size), a common procedure on rocky reefs [46,47,48,49]. Nutrient enrichment was designed to simulate conditions comparable to those recorded in urban areas of the same region [50]. Eight mesh bags, containing about 100 g of fertilizer each, were fixed in each area assigned to nutrient enrichment by means of steel hooks anchored to the substratum. Nutrient bags were replaced every 3 months throughout the duration of the study in order to maintain nutrient release [47]. This method has been successfully used on temperate reefs, since it allows to generate a localized nutrient enrichment [47, 49,50,51]. In order to assess nutrient concentrations in experimental areas, seawater samples were collected from each area at two random times, in June and November 2015. Two samples taken approximately 3 cm above each area using a 60-ml syringe were immediately filtered (0.45 μm) and frozen for transportation to the laboratory. Nutrient concentrations were quantified by means of a continuous-flow AA3 Auto-Analyzer (Bran-Luebbe), according to Grasshoff et al. [52]. Each sample was analyzed in technical triplicates to obtain an average value. Our study area is oligotrophic [28] and the experimental nutrient enrichment was effective in enhancing nitrate, nitrite, and phosphate concentrations (Fig. 1), although to levels lower than those recorded in areas exposed to intense human activities [50, 53]. Dissolution rates of fertilizer pellets vary with temperature and on fine spatial and temporal scales according to hydrodynamic regimes, making it difficult to obtain precise estimates of nutrient concentrations through the analysis of water samples [47, 49, 54]. Thus, water sample analyses were complemented by quantifying the rate of pellet dissolution, as suggested by Carnell and Keough [48]. The weight of the fertilizer in each nutrient bag was measured at the third decimal by means of a precision scale before deployment. Upon retrieval, nutrient bags were dried in a muffle for 28 h at 60 °C, and the amount of fertilizer that had not dissolved was re-weighted in order to estimate the average nutrient release rate per day over the duration of the experiment [48]. Nutrient pellets dissolved at an average rate of 2.3 g per day per area (data averaged across 5 sampling times: October 2014 and March, June, August, and November 2015).
Since budgetary constraints prevented multiple samplings, we assessed the response of EMBs to fertilization after 2 years since the start of the experiment (i.e., in July 2016), a period of time generally sufficient to trigger changes in the benthic communities within each habitat including canopy-stands [46, 55, 56]. Using hammer and chisel, 3 chips of rock about 3 cm2 and free of erect macroalgae and sessile invertebrates were collected within each study area and were inserted into sterile 50 ml centrifuge tubes and immediately placed on ice. To harvest epilithic bacterial assemblages, chips were washed twice with 40 ml filter-sterilized artificial seawater (ASW) made with Sea Salts (Sigma S9883) to remove seawater carryover, resuspended in 40 ml ASW, and then vigorously shaken for 30 s to dislodge and suspend surface-associated bacterial communities. The resulting supernatant was then transferred to a sterile lur-lock syringe and filtered into a 0.22-µm Sterivex filter (Millipore SVGV010RS). Filter cartridges were then stored in sterile bags and immediately placed on ice prior to long-term storage at – 80 °C.
DNA was extracted from samples using a modified method adapted from Thompson et al. [57]. Sterivex filters were removed from the casing and excised using a sterile razor blade and placed in a 2-ml screw cap microfuge tube with 0.2 g of 0.1 mm zirconium beads (Biospec Products) with 750 ml cell lysis solution (PureGene Cell and Tissue Kit, Gentra Systems). Filters were bead beated at 5,000 rpm for 1 min, placed at 80 °C for 5 min, and then stored at − 20 °C until further processing. DNA extraction was initiated by first incubating samples with 4 ul Rnase at 37 °C for 30 min, followed by addition of 250 ul protein precipitation solution (PureGene Cell and Tissue Kit; Gentra Systems). Samples were then centrifuged for 3 min at 15,000 × g to remove filter debris, beads, and precipitated protein before transferring the supernatant to a clean 1.5-ml Eppendorf tube. Samples were spun again at 15,000 × g for 3 min, and the supernatant was placed into a clean 1.5-ml Eppendorf tube containing 750 ul 100% IPA and pipette mixed. Samples were centrifuged for 1 min at 15,000 × g, and precipitated DNA was washed twice with 75 and 70% ethanol solution, air dried for 30 min, resuspended in 100 ul DNA hydration solution (PureGene Cell and Tissue Kit; Gentra Systems), and stored at – 20 °C until community libraries were constructed. DNA could not be extracted from one sample taken in an urchin barren area. Amplicon libraries were prepared using methods described in [58]. Briefly, the 16S V4 region was amplified using the primers 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGTWTCTAAT) for 13 cycles, and then in Illumina, adapters and barcodes were added in subsequent steps. These libraries were sequenced using Illumina Miseq 2 × 250 paired end at the BioMicro Center (Massachusetts Institute of Technology, Cambridge, MA). The primers were removed from forward and reverse reads using a custom python script, allowing one mismatch and discarding reads without the primer. The reads were then quality filtered and truncated to a common length, allowing for up to two expected errors and truncating to 140 bases for the forward reads and 140 for the reverse. Dada2 [59] was used to infer the Amplicon Sequence Variants (ASVs) from the raw reads that have been deposited in the National Center for Biotechnology Information (NCBI) under study accession number PRJNA901976 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA901976). Pseudopooling was performed by running Dada2 twice and using all identified ASVs from the first run as prior sequences in the second, as described on the pooling documentation on the Dada2 website. This reduces the risk of false negatives with Dada2, where sequences with only one or two reads that are close to a more abundant sequence are erroneously corrected. After denoizing and merging, the ASVs were assigned using a naive Bayes classifier implemented in Dada2 against the RDP 16S rRNA gene database [60].
Statistical Analyses
Variations in the structure of epilithic microbial biofilms (EMBs) among habitats and nutrient levels were assessed by means of PERMANOVAs including the factors Habitat (fixed, with 3 levels, canopy versus turf versus barren), Nutrients (fixed, with 2 levels, ambient versus enhanced), and Area (random, 2 levels and nested within the interaction habitat × nutrients) on pairwise Euclidean distances of centered and log-ratio transformed (CLR) relative ASV abundances. Multivariate patterns were visualized in two dimensions using nonmetric multidimensional scaling (nMDS). These multivariate analyses were run using the software Primer and Permanova + v.1.0.1 (PRIMER-e). In addition, normalized pairwise distances (where 1.0 represents the maximum observed Euclidean distance) were visualized using the R package Vegan vers. 2.5–4 [61] in order to assess how nutrient addition influenced differences in microbial communities between either algal turfs or sea urchin barrens and E. brachycarpa canopies. In addition, Venn diagrams were built using the software Venny 2.1 [62] to identify the microbial core community on rocky reefs under ambient and enhanced nutrient conditions.
For each ASV, two-sided t-tests were performed to compare their relative abundance between macroalgal canopies and either turfs or urchin barrens at natural or enhanced nutrient levels using base functions in R 3.6.0 [63]. The Holm–Bonferroni method was used to deal with family-wise error rates for multiple hypothesis tests.
Results
Gammaproteobacteria, Alphaproteobacteria and Flavobacteriia were the most abundant classes across habitats at both ambient and enhanced nutrient levels and no consistent trends in variation among the treatments at this high taxonomic level were evident (Fig. 2). However, when using the highest taxonomic resolution afforded by the 16S rRNA gene tag sequence, the ASV level, compositional differences emerged. The PERMANOVA on Euclidean distances and post hoc comparisons showed that the structure of bacterial assemblages differed among habitats (Table 1) (Fig. 3). In contrast, nutrient enrichment had no significant effect on bacterial assemblages. However, bacteria in turf areas appeared more variable than those in canopy or barren areas and showed larger changes in response to nutrients (Fig. 3). Following the addition of nutrients, the microbial assemblage in turf habitats became more similar to that underneath canopies. In contrast, nutrient addition to the barren areas did not shift the microbial community toward that found in canopy-dominated areas (Fig. 4).
The three habitats shared a core microbial community consisting of 21.6 and 25.3% of ASVs under ambient and enhanced nutrient conditions, respectively (Fig. 5A and B). The addition of nutrients increased the percentage of ASVs shared between canopy- and turf-dominated habitats from 48.8 to 63.1%. In contrast, there were no major changes in the percentage of common ASVs between barrens and the other habitats at ambient versus enhanced nutrient conditions. Under ambient nutrients, the core microbial community of the rocky reef was mostly composed of the families of Candidatus_Pelagibacter (~ 5.3%), Flavobacteriaceae (~ 7.9%), Halomonadaceae (~ 8.2%), Rhodobacteraceae (~ 11.2%), Rhodospirillaceae (~ 9.2%) and Vibrionaceae (~ 9.6%), which, together, accounted for ~ 51.4% of total ASVs (Fig. 5C). The same families (Candidatus_Pelagibacter: ~ 5.3%, Flavobacteriaceae: ~ 7.9%, Halomonadaceae: ~ 4.1%, Rhodobacteraceae: ~ 10.6%, Rhodospirillaceae: ~ 6.7% and Vibrionaceae: ~ 6.4%), in addition to Bacillariophyta (~ 5.7%), were also the most represented under enhanced nutrient conditions. ASVs non-identified to family level accounted for ~ 27.2 and ~ 30.0% of the total ASV number at ambient and enhanced nutrient level, respectively.
Venn diagrams showing the percentage of total ASVs shared across the three habitats (i.e., the core microbiome) and between each pair of habitats, at A ambient versus B enhanced nutrient levels. Stacked barplots (C) illustrate the composition of the core microbiome, at the family level, separately for ambient and enhanced nutrient levels
At ambient nutrient levels, the relative abundance of 38 ASV, most belonging to the phyla of Bacteroidetes (~ 31%) and Proteobacteria (~ 58%), differed significantly between barren and canopy areas (Fig. 6A; Online Resource 1). Most of the ASVs (i.e., ~ 74%) had a greater relative abundance in canopy than barren habitats. A higher number of ASVs (i.e., 84) differed between barren and canopy areas exposed to nutrient addition (Fig. 6B; Online Resource 2). Again, most of the ASVs (~ 95%) differentiating between these two habitats had a greater abundance in canopy than barren habitats, and only seven of them were important differentiators between barren and canopy areas also at ambient nutrients. Many ASVs could not be classified at the genus or species level. In all samples, > 50% of the relative abundance was accounted for by ASVs classified to the least order, and > 50% of the relative abundance was not assignable at the genus level (Fig. S1 in Online Resource 3).
The relative abundance of 30 and 42 ASVs differed significantly between turf and canopy areas at ambient and enhanced nutrient levels, respectively (Fig. 7A and B; Online Resources 4 and 5). There was no ASV in common between the groups that differentiated between these habitats at ambient versus enhanced nutrient levels. At both ambient and enhanced nutrient levels, differences between canopy and turfs were due to a large number of ASVs (~ 73 and ~ 86% of those differing between habitats respectively) being more abundant in turf habitats. Twenty-two and 36 ASVs, mostly belonging to the phylum of Proteobacteria and, in particular, to the families of Rhodobacteraceae and Flavobacteriaceae at ambient nutrient levels and of Rhodobacteraceae and Bacteriovoracaceae at enhanced nutrient levels were more abundant in turf habitats. Only 8 ASVs, some belonging to the families of Flavobacteriaceae, Colwelliaceae, Bacteriovoracaceae, Oceanospirillales incertae sedis and GpVIII Cyanobacteria, were more abundant in canopy habitats at ambient nutrient levels. Likewise, only 6 ASVs, three of which belonged to the family of Colwelliaceae, were enriched in canopy habitats exposed to enhanced nutrient levels.
Discussion
The collapse of marine forests and their replacement by either encrusting coralline barrens or algal turfs are a global phenomenon [5, 10, 14, 64], widely documented along the coasts of the Mediterranean Sea [18, 19, 65]. Thus, understanding the mechanisms underpinning the stability of habitats alternative to forests appears key to devise sound strategies for sustaining the biodiversity and functioning of temperate rocky reefs.
A correlation between the composition of benthic organisms (i.e., algae and invertebrates) and microbial communities has been documented on coral reefs [29,30,31]. Although there is some evidence of species-specific influence of macroalgae on seawater [66, 67] and hard substrata microbial communities [68,69,70], to the best of our knowledge, this is the first study attempting to describe variation in epilithic microbial communities among the alternative habitats, i.e., macroalgal forests, urchin barrens and algal turfs, that compose mosaics on temperate rocky reefs. EMBs were mostly composed of Proteobacteria and Bacteroidetes, widely distributed and abundant bacterial phyla in the global oceans [71, 72] and reported as dominant on macroalgal surfaces, rocky substrata and marine sediments [68, 73, 74]. Nonetheless, when compared at the ASV level, EMBs from alternative habitats differed at both natural and enhanced nutrient levels, in accordance with previous studies that documented decreased similarity in microbial assemblages from higher to lower taxonomic levels [68]. Indeed, only about 22% of ASVs were common to the three habitats, suggesting that the core microbiome of this rocky reef was a small subset of the total number of ASVs recorded on the scale of the study site. Thus, the influence of small-scale habitat patchiness on Mediterranean shallow rocky reefs is not limited to eukaryotes, i.e., the associated fish and macroalgal and invertebrate assemblages [56, 75, 76], but extends to the prokaryotic compartment.
Several mechanisms can underpin EMB control by macroalgae, including the release of dissolved organic carbon and combined neutral sugars, secondary metabolites, exosomes, and free-living bacteria [35, 77,78,79,80,81,82]. In addition, changes in EMBs might be generated by macroalga-induced modification of environmental conditions such as light, water flow and sedimentation rates, and water chemistry, including pH and alkalinity [66]. Our study cannot provide a mechanistic explanation of differences in EMB composition among habitats, but together with evidence of macroalgal settlement being influenced by bacteria [23, 24, 42, 83], it suggests that epilithic microbial community control by dominant benthic macroalgae might represent a stabilizing feedback and, hence, a mechanism underpinning the stability of alternative stable states documented on temperate rocky reefs [5, 6].
Alphaproteobacteria, Gammaproteobacteria and, to a lesser extent, Flavobacteriia were dominant in EMBs, and variation in the relative abundance of ASVs belonging to these classes contributed most to differentiate between macroalgal canopies and either barrens or algal turfs. EMBs from sea urchin barrens and algal turfs were remarkably different from those occurring within macroalgal canopies. However, such differences were produced by different patterns of variation in ASV occurrence and relative abundance between canopy and either turf or barren habitats. A large proportion of ASVs differentiating EMBs between canopies and barrens had a higher relative abundance in the former habitat. In contrast, differences between forests and turfs were mostly due to turf enrichment in almost all of the differentiating ASVs. This may indicate that DOC release by macroalgae, reported to be higher in turfs [33], might have enhanced the growth of bacteria known to play a key role in algal C cycling, such as Rhodobacteraceae [84]. These patterns were even more pronounced under enhanced nutrient conditions, suggesting that release from N and P limitation, along with DOC release from macroalgae, could have fostered growth of ASVs hosted by primary surface beneath canopy and, especially, within turf-forming macroalgal assemblages.
Nonetheless, the experimental elevation of nutrient levels had no significant effect on the bacterial composition of EMBs. Our results are in contrast with those of Remple et al. [29], who documented nutrient-driven alteration in the structure of bacterial biofilms on coral reef substrata. It is, however, worth noting that enriched levels in our experiment were moderate (NO3− = 0.319 ± 0.024 μmol L−1) and one order of magnitude lower than mid or high levels generated by Remple et al. (respectively, 2.68 and 6.64 μml L−1 NO3−).
Although the multivariate analysis did not show significant effects of nutrient enrichment, the response of EMBs from algal turfs appears somewhat stronger than that from macroalgal canopies and urchin barrens. In fact, normalized pairwise distances indicate that nutrient enrichment reduced the dissimilarity in EMBs structure between canopies and algal turfs but not barrens. Indeed, the proportion of ASVs shared between canopy- and turf-dominated habitats increased by about 15% under enhanced nutrient condition. These patterns might be connected with the response to nutrient enrichment of the main macroalgal species or functional groups that form each of the three habitats and, more specifically, to changes in their biomass. Inside forests, nutrient enrichment did not cause major changes in plant size and canopy cover and in the composition of their epiphyte assemblage [55, 56]. Large-sized brown seaweeds such as Fucales and Laminariales have an efficient external uptake of N and may have, thus, reduced the influence of the experimental nutrient enrichment on EMBs [55, 85]. Likewise, in urchin barrens, nutrient enrichment was unlikely to cause a major increase in the biomass of encrusting corallines due to morphological characteristics of their prostrate, calcareous thallus. The weak response of encrusting corallines to nutrients may also explain little changes in the proportion of ASVs shared between barrens and either macroalgal canopies or turfs in response to fertilization. EMBs from barren areas appear therefore more stable than those on substrata under the influence of algal turfs.
Unfortunately, we did not assess changes in the structure of turfing assemblages, but nutrient-induced increases in cover and biomass of algal turfs have been documented globally [20]. An increment in their biomass [55] may have influenced the EMBs through a greater release of DOC. This would indicate that variations in the biomass of primary producers would underpin the differences in the structure of EMBs they host. There is also evidence indicating that the amount of DOC released varies among macroalgal species and it is particularly elevated in algal turfs [33]. Indeed, copiotrophic bacteria, such as those belonging to the classes of Flavobacteriales and Rhodobacteriales, had a greater relative abundance in EMBs from algal turfs than macroalgal canopies and contributed to differentiate these two habitats at ambient nutrient levels.
While the number of ASVs differentiating between canopies and algal turfs did not change according to nutrient levels, those that differentiated between canopies and barrens doubled under nutrient enrichment. In both cases, differences were mainly driven by ASVs, most of which (~ 77%) belonging to the classes of Alphaproteobacteria or Gammaproteobacteria having a greater relative abundance in EMBs from macroalgal canopies than barrens. However, in both cases, there were ASVs which were markedly more represented in barrens, two unannotated belonging to Alphaproteobacteria and one to the family of Saprospiraceae under ambient nutrient conditions and one belonging to the family of Rhodospirillaceae under elevated nutrients. This might suggest that EMBs that colonize barren areas could be characterized by few dominant ASVs.
The influence of mono- or multi-specific bacterial assemblages on macroalgal recruitment has been examined for just a few species (e.g., Ulva, Enteromorpha, and Polysiphonia) [23] and there is no information on which bacterial taxa facilitate or hinder fucoid recruitment. Our results suggest that experimental assessment of the role of EMBs in regulating fucoid recruitment in barrens is warranted to deepen our understanding of the mechanisms that generate hysteresis in each of these alternative, stable habitats. While a great deal of research has attempted to assess how human-induced changes in microbiomes affect the phenology, physiology, and ecology of habitat-forming species [86], a smaller effort has been produced to assess the role of microbial biofilms in regulating the stability and persistence of habitats formed by alternative species. This is at odds with evidence from coral reefs that macroalga-induced modifications in the composition of epilithic microbial biofilms reduce coral recruitment, contributing to the stability of the macroalgal dominated state [29, 30, 69, 70]. Our study brings some evidence of major differences in the structure of EMBs from the alternative habitats that characterize shallow temperate rocky reefs and suggests that the characteristics of microbial biofilms that develop within algal turfs and urchin barrens may contribute to enhance their stability by limiting the settlement of canopy-forming species. Reducing herbivore pressure through the restoration of lost predatory interactions and controlling nutrient inputs from urban and agricultural settings have been identified as priorities for the conservation of marine forests and for enhancing their recovery [10, 87]. Nonetheless, these strategies may not be effective to trigger shifts back to the forested state due to hysteresis. Thus, experimental assessment of the ability of canopy-forming species to settle on surfaces colonized by EMBs that have been developed in the presence of adult conspecifics versus either algal turfs or urchin barrens appears warranted to enhance our understanding of the mechanisms that regulate shifts among alternative stable states on temperate rocky reefs. Finally, since the weak response of EMBs to fertilization could be due to the moderate nutrient enhancement generated in our study, experimental elevation of nutrients to levels matching those recorded in areas exposed to run-off from urban or agricultural areas is necessary to get a deeper insight into their effects on EMBs.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author.
References
Pecl GT, Araújo MB, Bell JD, Blanchard J, Bonebrake TC, Chen I-C, Clark TD, Colwell RK, Danielsen F, Evengård B, Falconi L, Ferrier S, Frusher S, Garcia RA, Griffis RB, Hobday AJ, Janion-Scheepers C, Jarzyna MA, Jennings S, Lenoir J, Linnetved HI, Martin VY, McCormack PC, McDonald J, Mitchell NJ, Mustonen T, Pandolfi JM, Pettorelli N, Popova E, Robinson SA, Scheffers BR, Shaw JD, Sorte CJB, Strugnell JM, Sunday JM, Tuanmu M-N, Vergés A, Villanueva C, Wernberg T, Wapstra E, Williams SE (2017) Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355:eaai9214. https://doi.org/10.1126/science.aai9214
Scheffers BR, De Meester L, Bridge TCL, Hoffmann AA, Pandolfi JM, Corlett RT, Butchart SHM, Pearce-Kelly P, Kovacs KM, Dudgeon D, Pacifici M, Rondinini C, Foden WB, Martin TG, Mora C, Bickford D, Watson JEM (2016) The broad footprint of climate change from genes to biomes to people. Science 354:aaf7671. https://doi.org/10.1126/science.aaf7671
Scheffer M, Carpenter SR (2003) Catastrophic regime shifts in ecosystems: linking theory to observation. Trends EcolEvol 18:648–656. https://doi.org/10.1016/j.tree.2003.09.002
Nystrom M, Folke C, Moberg F (2000) Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol Evol 15:413–417. https://doi.org/10.1016/s0169-5347(00)01948-0
Ling SD, Scheibling RE, Rassweiler A, Johnson CR, Shears N, Connell SD, Salomon AK, Norderhaug KM, Perez-Matus A, Hernandez JC, Clemente S, Blamey LK, Hereu B, Ballesteros E, Sala E, Garrabou J, Cebrian E, Zabala M, Fujita D, Johnson LE (2015) Global regime shift dynamics of catastrophic sea urchin overgrazing. Philos Trans R Soc Lond B Biol Sci 370:20130269. https://doi.org/10.1098/rstb.2013.0269
Filbee-Dexter K, Scheibling RE (2014) Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar Ecol Prog Ser 495:1–25. https://doi.org/10.3354/meps10573
Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS (2004) Regime shifts, resilience, and biodiversity in ecosystem management. Ann Rev Ecol Evol Syst 35:557–581. https://doi.org/10.1146/annurev.ecolsys.35.021103.105711
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933. https://doi.org/10.1126/science.1085046
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459. https://doi.org/10.1017/s0376892902000322
Strain EMA, Thomson RJ, Micheli F, Mancuso FP, Airoldi L (2014) Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob Chang Biol 20:3300–3312. https://doi.org/10.1111/gcb.12619
Pessarrodona A, Assis J, Filbee-Dexter K, Burrows MT, Gattuso JP, Duarte CM, Krause-Jensen D, Moore PJ, Smale DA, Wernberg T (2022) Global seaweed productivity. Sci Adv 8:eabn2465. https://doi.org/10.1126/sciadv.abn2465
Beaumont NJ, Austen MC, Mangi SC, Townsend M (2008) Economic valuation for the conservation of marine biodiversity. Mar Poll Bull 56:386–396. https://doi.org/10.1016/j.marpolbul.2007.11.013
Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ (2013) Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol Evol 3:4016–4038. https://doi.org/10.1002/ece3.774
Krumhansl KA, Okamoto DK, Rassweiler A, Novak M, Bolton JJ, Cavanaugh KC, Connell SD, Johnson CR, Konar B, Ling SD, Micheli F, Norderhaug KM, Pérez-Matus A, Sousa-Pinto I, Reed DC, Salomon AK, Shears NT, Wernberg T, Anderson RJ, Barrett NS, Buschmann AH, Carr MH, Caselle JE, Derrien-Courtel S, Edgar GJ, Edwards M, Estes JA, Goodwin C, Kenner MC, Kushner DJ, Moy FE, Nunn J, Steneck RS, Vásquez J, Watson J, Witman JD, Byrnes JEK (2016) Global patterns of kelp forest change over the past half-century. Proc Natl Acad Sci USA 113:13785–13790. https://doi.org/10.1073/pnas.1606102113
Benedetti-Cecchi L, Pannacciulli F, Bulleri F, Moschella PS, Airoldi L, Relini G, Cinelli F (2001) Predicting the consequences of anthropogenic disturbance: large-scale effects of loss of canopy algae on rocky shores. Mar Ecol Prog Ser 214:137–150. https://doi.org/10.3354/meps214137
Gorman D, Russell BD, Connell SD (2009) Land-to-sea connectivity: linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts. EcolAppl 19:1114–1126. https://doi.org/10.1890/08-0831.1
Guarnieri G, Bevilacqua S, Vignes F, Fraschetti S (2014) Grazer removal and nutrient enrichment as recovery enhancers for overexploited rocky subtidal habitats. Oecologia 175:959–970. https://doi.org/10.1007/s00442-014-2944-4
Bulleri F, Benedetti-Cecchi L (2006) Mechanisms of recovery and resilience of different components of mosaics of habitats on shallow rocky reefs. Oecologia 149:482–492. https://doi.org/10.1007/s00442-006-0459-3
Piazzi L, Bulleri F, Ceccherelli G (2016) Limpets compensate sea urchin decline and enhance the stability of rocky subtidal barrens. Mar Environ Res 115:49–55. https://doi.org/10.1016/j.marenvres.2016.01.009
Gorgula SK, Connell SD (2004) Expansive covers of turf-forming algae on human-dominated coast: the relative effects of increasing nutrient and sediment loads. Mar Biol 145:613–619. https://doi.org/10.1007/s00227-004-1335-5
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. In: Gibson RN, Atkinson RJA, Gordon JDM (eds) Oceanogr Mar Biol, vol 45. Crc Press-Taylor & Francis Group, Boca Raton, pp 345–405
Irving AD, Balata D, Colosio F, Ferrando GA, Airoldi L (2009) Light, sediment, temperature, and the early life-history of the habitat-forming alga Cystoseira barbata. Mar Biol 156:1223–1231. https://doi.org/10.1007/s00227-009-1164-7
Mieszkin S, Callow ME, Callow JA (2013) Interactions between microbial biofilms and marine fouling algae: a mini review. Biofouling 29:1097–1113. https://doi.org/10.1080/08927014.2013.828712
Wahl M, Goecke F, Labes A, Dobretsov S, Weinberger F (2012) The second skin: ecological role of epibiotic biofilms on marine organisms. Front Microbiol 3:292. https://doi.org/10.3389/fmicb.2012.00292
Bulleri F, Cucco A, Dal Bello M, Maggi E, Ravaglioli C, Benedetti-Cecchi L (2018) The role of wave-exposure and human impacts in regulating the distribution of alternative habitats on NW Mediterranean rocky reefs. Estuar Coast Shelf Sci 201:114–122. https://doi.org/10.1016/j.ecss.2016.02.013
Thibaut T, Blanfuné A, Boudouresque CF, Verlaque M (2015) Decline and local extinction of Fucales in the French Riviera: the harbinger of future extinctions? Mediterr Mar Sci 16:206–224. https://doi.org/10.12681/mms.1032
Mangialajo L, Chiantore M, Cattaneo-Vietti R (2008) Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar Ecol Prog Ser 358:63–74. https://doi.org/10.3354/meps07400
Tamburello L, Benedetti-Cecchi L, Ghedini G, Alestra T, Bulleri F (2012) Variation in the structure of subtidal landscapes in the NW Mediterranean Sea. Mar Ecol Prog Ser 457:29–41. https://doi.org/10.3354/meps09703
Remple KL, Silbiger NJ, Quinlan ZA, Fox MD, Kelly LW, Donahue MJ, Nelson CE (2021) Coral reef biofilm bacterial diversity and successional trajectories are structured by reef benthic organisms and shift under chronic nutrient enrichment. Npj Biofilms and Microbiomes 7:84. https://doi.org/10.1038/s41522-021-00252-1
Kelly LW, Williams GJ, Barott KL, Carlson CA, Dinsdale EA, Edwards RA, Haas AF, Haynes M, Lim YW, McDole T, Nelson CE, Sala E, Sandin SA, Smith JE, Vermeij MJA, Youle M, Rohwer F (2014) Local genomic adaptation of coral reef-associated microbiomes to gradients of natural variability and anthropogenic stressors. Proc Natl Acad Sci USA 111:10227–10232. https://doi.org/10.1073/pnas.1403319111
Pearman JK, Aylagas E, Voolstra CR, Anlauf H, Villalobos R, Carvalho S (2019) Disentangling the complex microbial community of coral reefs using standardized Autonomous Reef Monitoring Structures (ARMS). Mol Ecol 28:3496–3507. https://doi.org/10.1111/mec.15167
Haas AF, Fairoz MFM, Kelly LW, Nelson CE, Dinsdale EA, Edwards RA, Giles S, Hatay M, Hisakawa N, Knowles B, Lim YW, Maughan H, Pantos O, Roach TNF, Sanchez SE, Silveira CB, Sandin S, Smith JE, Rohwer F (2016) Global microbialization of coral reefs. Nat Microbiol 1:16042. https://doi.org/10.1038/nmicrobiol.2016.42
Haas AF, Nelson CE, Kelly LW, Carlson CA, Rohwer F, Leichter JJ, Wyatt A, Smith JE (2011) Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. Plos One 6:e27973. https://doi.org/10.1371/journal.pone.0027973
Harlin MM (1987) Allelochemistry in marine macroalgae. Crc Critical Reviews in Plant Sciences 5:237–249. https://doi.org/10.1080/07352688709382241
Morrow KM, Bromhall K, Motti CA, Munn CB, Bourne DG (2017) Allelochemicals produced by brown macroalgae of the Lobophora genus are active against coral larvae and associated bacteria, supporting pathogenic shifts to vibrio dominance. Appl Environ Microbiol 83:e02391-e2416. https://doi.org/10.1128/aem.02391-16
Jorissen H, Skinner C, Osinga R, de Beer D, Nugues MM (2016) Evidence for water-mediated mechanisms in coral-algal interactions. Philos Trans R Soc Lond B Biol Sci 283:20161137. https://doi.org/10.1098/rspb.2016.1137
Barott KL, Rohwer FL (2012) Unseen players shape benthic competition on coral reefs. Trends Microbiol 20:621–628. https://doi.org/10.1016/j.tim.2012.08.004
Perkins RG, Williamson CJ, Brodie J, Barille L, Launeau P, Lavaud J, Yallop ML, Jesus B (2016) Microspatial variability in community structure and photophysiology of calcified macroalgal microbiomes revealed by coupling of hyperspectral and high-resolution fluorescence imaging. Sci Rep 6:22343. https://doi.org/10.1038/srep22343
Lachnit T, Meske D, Wahl M, Harder T, Schmitz R (2011) Epibacterial community patterns on marine macroalgae are host-specific but temporally variable. Environ Microbiol 13:655–665. https://doi.org/10.1111/j.1462-2920.2010.02371.x
Trias R, Garcia-Lledo A, Sanchez N, Lopez-Jurado JL, Hallin S, Baneras L (2012) Abundance and composition of epiphytic bacterial and archaeal ammonia oxidizers of marine red and brown macroalgae. Appl Environ Microbiol 78:318–325. https://doi.org/10.1128/aem.05904-11
Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T (2013) The seaweed holobiont: understanding seaweed-bacteria interactions. FEMS Microbiol Rev 37:462–476. https://doi.org/10.1111/1574-6976.12011
Goecke F, Labes A, Wiese J, Imhoff JF (2010) Chemical interactions between marine macroalgae and bacteria. Mar Ecol Prog Ser 409:267–299. https://doi.org/10.3354/meps08607
Marzinelli EM, Campbell AH, Valdes EZ, Verges A, Nielsen S, Wernberg T, de Bettignies T, Bennett S, Caporaso JG, Thomas T, Steinberg PD (2015) Continental-scale variation in seaweed host-associated bacterial communities is a function of host condition, not geography. Environ Microbiol 17:4078–4088. https://doi.org/10.1111/1462-2920.12972
Stabili L, Rizzo L, Pizzolante G, Alifano P, Fraschetti S (2017) Spatial distribution of the culturable bacterial community associated with the invasive alga Caulerpa cylindracea in the Mediterranean Sea. Mar EnvironRes 125:90–98. https://doi.org/10.1016/j.marenvres.2017.02.001
Barott KL, Rodriguez-Brito B, Janouskovec J, Marhaver KL, Smith JE, Keeling P, Rohwer FL (2011) Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ Microbiol 13:1192–1204. https://doi.org/10.1111/j.1462-2920.2010.02419.x
Piazzi L, Ceccherelli G (2017) Concomitance of oligotrophy and low grazing pressure is essential for the resilience of Mediterranean subtidal forests. Mar Poll Bull 123:197–204. https://doi.org/10.1016/j.marpolbul.2017.08.061
Bulleri F, Russell BD, Connell SD (2012) Context-dependency in the effects of nutrient loading and consumers on the availability of space in marine rocky environments. Plos One 7:e33825. https://doi.org/10.1371/journal.pone.0033825
Carnell PE, Keough MJ (2014) Spatially variable synergistic effects of disturbance and additional nutrients on kelp recruitment and recovery. Oecologia 175:409–416. https://doi.org/10.1007/s00442-014-2907-9
Worm B, Reusch TBH, Lotze HK (2000) In situ nutrient enrichment: methods for marine benthic ecology. Int Rev Hydrobiol 85:359–375
Balata D, Piazzi L, Nesti U, Bulleri F, Bertocci I (2010) Effects of enhanced loads of nutrients on epiphytes on leaves and rhizomes of Posidonia oceanica. J Sea Res 63:173–179. https://doi.org/10.1016/j.seares.2009.12.001
Russell BD, Connell SD (2005) A novel interaction between nutrients and grazers alters relative dominance of marine habitats. Mar EcolProg Ser 289:5–11. https://doi.org/10.3354/meps289005
Petty R (1984) Methods of seawater analysis, 2ndEdition – Grasshoff K, Ehrhardt M, Kremling K. Anal Chem 56:1220–1220
Gennaro P, Piazzi L (2011) Synergism between two anthropic impacts: Caulerpa racemosa var. cylindracea invasion and seawater nutrient enrichment. Mar Ecol Prog Ser 427:59–70. https://doi.org/10.3354/meps09053
Kriegisch N, Reeves SE, Johnson CR, Ling SD (2019) Top-down sea urchin overgrazing overwhelms bottom-up stimulation of kelp beds despite sediment enhancement. J Exp Mar Biol Ecol 514:48–58. https://doi.org/10.1016/j.jembe.2019.03.012
Tamburello L, Ravaglioli C, Mori G, Nuccio C, Bulleri F (2019) Enhanced nutrient loading and herbivory do not depress the resilience of subtidal canopy forests in Mediterranean oligotrophic waters. Mar Environ Res 149:7–17. https://doi.org/10.1016/j.marenvres.2019.05.015
Bulleri F, Pardi G, Tamburello L, Ravaglioli C (2020) Nutrient enrichment stimulates herbivory and alters epibiont assemblages at the edge but not inside subtidal macroalgal forests. Mar Biol 167:181. https://doi.org/10.1007/s00227-020-03789-5
Thompson JR, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, Polz MF (2004) Diversity and dynamics of a north Atlantic coastal Vibrio community. Appl Environ Microbiol 70:4103–4110. https://doi.org/10.1128/aem.70.7.4103-4110.2004
Martin-Platero AM, Cleary B, Kauffman K, Preheim SP, McGillicuddy DJ, Alm EJ, Polz MF (2018) High resolution time series reveals cohesive but short-lived communities in coastal plankton. Nat Comm 9:266. https://doi.org/10.1038/s41467-017-02571-4
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13:581-+. https://doi.org/10.1038/nmeth.3869
Cole JR, Wang Q, Fish JA, Chai BL, McGarrell DM, Sun YN, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM (2014) Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642. https://doi.org/10.1093/nar/gkt1244
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, et al. vegan: community ecology package. R package version 2.5–4. 2019. https://CRANR-project.org/package=vegan.
Oliveros J (2015) VENNY. An interactive tool for comparing lists with Venn diagrams. Available from: https:// bioin fogp. cnb. csic. es/ tools/ venny/index. Html
R Core 2019. R version 3.6.0: a language and environment for statistical computing. R Found Stat Comput
Filbee-Dexter K, Wernberg T (2018) Rise of turfs: a new battlefront for globally declining kelp forests. Bioscience 68:64–76. https://doi.org/10.1093/biosci/bix147
Sala E, Ballesteros E, Dendrinos P, Di Franco A, Ferretti F, Foley D, Fraschetti S, Friedlander A, Garrabou J, Guclusoy H, Guidetti P, Halpern BS, Hereu B, Karamanlidis AA, Kizilkaya Z, Macpherson E, Mangialajo L, Mariani S, Micheli F, Pais A, Riser K, Rosenberg AA, Sales M, Selkoe KA, Starr R, Tomas F, Zabala M (2012) The Structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 7:32742. https://doi.org/10.1371/journal.pone.0032742
Pfister CA, Altabet MA, Weigel BL (2019) Kelp beds and their local effects on seawater chemistry, productivity, and microbial communities. Ecology 100:e02798. https://doi.org/10.1002/ecy.2798
Clasen JL, Shurin JB (2015) Kelp forest size alters microbial community structure and function on Vancouver Island, Canada. Ecology 96:862–872. https://doi.org/10.1890/13-2147.1
Guo ZS, Wang L, Jiang ZY, Liang ZL (2022) Comparison studies of epiphytic microbial communities on four macroalgae and their rocky substrates. Mar Poll Bull 176:113435. https://doi.org/10.1016/j.marpolbul.2022.113435
Bulleri F, Thiault L, Mills SC, Nugues MM, Eckert EM, Corno G, Claudet J (2018) Erect macroalgae influence epilithic bacterial assemblages and reduce coral recruitment. Mar Ecol Prog Ser 597:65–77. https://doi.org/10.3354/meps12583
Vermeij MJA, Smith JE, Smith CM, Thurber RV, Sandin SA (2009) Survival and settlement success of coral planulae: independent and synergistic effects of macroalgae and microbes. Oecologia 159:325–336. https://doi.org/10.1007/s00442-008-1223-7
Fernandez-Gomez B, Richter M, Schuler M, Pinhassi J, Acinas SG, Gonzalez JM, Pedros-Alio C (2013) Ecology of marine Bacteroidetes: a comparative genomics approach. Isme J 7:1026–1037. https://doi.org/10.1038/ismej.2012.169
Lima-Mendez G, Faust K, Henry N, Decelle J, Colin S, Carcillo F, Chaffron S, Ignacio-Espinosa JC, Roux S, Vincent F, Bittner L, Darzi Y, Wang J, Audic S, Berline L, Bontempi G, Cabello AM, Coppola L, Cornejo-Castillo FM, d’Ovidio F, De Meester L, Ferrera I, Garet-Delmas MJ, Guidi L, Lara E, Pesant S, Royo-Llonch M, Salazar G, Sanchez P, Sebastian M, Souffreau C, Dimier C, Picheral M, Searson S, Kandels-Lewis S, Gorsky G, Not F, Ogata H, Speich S, Stemmann L, Weissenbach J, Wincker P, Acinas SG, Sunagawa S, Bork P, Sullivan MB, Karsenti E, Bowler C, de Vargas C, Raes J, Boss E, De Vargas C, Follows M, Grimsley N, Hingamp P, Iudicone D, Jaillon O, Karp-Boss L, Krzic U, Reynaud EG, Sardet C, Sieracki M, Velayoudon D, Tara Oceans C (2015) Determinants of community structure in the global plankton interactome. Science 348:1262073. https://doi.org/10.1126/science.1262073
Huang D, Zhang ZY, Sun MM, Feng ZY, Ye M (2021) Characterization and ecological function of bacterial communities in seabed sediments of the southwestern Yellow Sea and northwestern East China Sea Western Pacific. Sci Tot Environ 761:143233. https://doi.org/10.1016/j.scitotenv.2020.143233
Brunet M, de Bettignies F, Le Duff N, Tanguy G, Davoult D, Leblanc C, Gobet A, Thomas F (2021) Accumulation of detached kelp biomass in a subtidal temperate coastal ecosystem induces succession of epiphytic and sediment bacterial communities. Environ Microbiol 23:1638–1655. https://doi.org/10.1111/1462-2920.15389
Bianchelli S, Buschi E, Danovaro R, Pusceddu A (2016) Biodiversity loss and turnover in alternative states in the Mediterranean Sea: a case study on meiofauna. Sci Rep 6:34544. https://doi.org/10.1038/srep34544
Piazzi L, Bonaviri C, Castelli A, Ceccherelli G, Costa G, Curini-Galletti M, Langeneck J, Manconi R, Montefalcone M, Pipitone C, Rosso A, Pinna S (2018) Biodiversity in canopy-forming algae: structure and spatial variability of the Mediterranean Cystoseira assemblages. Estuar Coast Shelf Sci 207:132–141. https://doi.org/10.1016/j.ecss.2018.04.001
Maximilien R, de Nys R, Holmstrom C, Gram L, Givskov M, Crass K, Kjelleberg S, Steinberg PD (1998) Chemical mediation of bacterial surface colonisation by secondary metabolites from the red alga Delisea pulchra. Aquat Microb Ecol 15:233–246. https://doi.org/10.3354/ame015233
Pezzolesi L, Accoroni S, Rindi F, Samori C, Totti C, Pistocchi R (2021) Survey of the allelopathic potential of Mediterranean macroalgae: production of long-chain polyunsaturated aldehydes (PUAs). Phytochemistry 189:112826. https://doi.org/10.1016/j.phytochem.2021.112826
Nelson CE, Goldberg SJ, Kelly LW, Haas AF, Smith JE, Rohwer F, Carlson CA (2013) Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. Isme J 7:962–979. https://doi.org/10.1038/ismej.2012.161
Hay ME (1996) Marine chemical ecology: what’s known and what’s next? J Exp Mar Biol Ecol 200:103–134. https://doi.org/10.1016/s0022-0981(96)02659-7
Sneed JM, Pohnert G (2011) The green alga Dicytosphaeria ocellata and its organic extracts alter natural bacterial biofilm communities. Biofouling 27:347–356. https://doi.org/10.1080/08927014.2011.576317
Sneed JM, Ritson-Williams R, Paul VJ (2015) Crustose coralline algal species host distinct bacterial assemblages on their surfaces. Isme J 9:2527–2536. https://doi.org/10.1038/ismej.2015.67
Egan S, James S, Holmstrom C, Kjelleberg S (2001) Inhibition of algal spore germination by the marine bacterium Pseudoalteromonas tunicata. FEMS Microbiol Ecol 35:67–73. https://doi.org/10.1111/j.1574-6941.2001.tb00789.x
Michael V, Frank O, Bartling P, Scheuner C, Goker M, Brinkmann H, Petersen J (2016) Biofilm plasmids with a rhamnose operon are widely distributed determinants of the ‘swim-or-stick’ lifestyle in roseobacters. Isme J 10:2498–2513. https://doi.org/10.1038/ismej.2016.30
Pedersen MF, Borum J (1997) Nutrient control of estuarine macroalgae: growth strategy and the balance between nitrogen requirements and uptake. Mar Ecol Prog Ser 161:155–163. https://doi.org/10.3354/meps161155
Trevathan-Tackett SM, Sherman CDH, Huggett MJ, Campbell AH, Laverock B, Hurtado-McCormick V, Seymour JR, Firl A, Messer LF, Ainsworth TD, Negandhi KL, Daffonchio D, Egan S, Engelen AH, Fusi M, Thomas T, Vann L, Hernandez-Agreda A, Gan HM, Marzinelli EM, Steinberg PD, Hardtke L, Macreadie PI (2019) A horizon scan of priorities for coastal marine microbiome research. Nat EcolEvol 3:1509–1520. https://doi.org/10.1038/s41559-019-0999-7
Guidetti P (2006) Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol Appl 16:963–976. https://doi.org/10.1890/1051-0761(2006)016[0963:mrrlpi]2.0.co;2
Acknowledgements
We sincerely thank two anonymous reviewers for insightful comments and constructive criticism.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This study was funded by the MIT and the University of Pisa through the MIT-UNIPI project From Micro to Macro: The Role of Microbial Biofilms in Regulating the Fitness and Competitive Ability of Subtidal Habitat-Forming Macroalgae granted to FB and MP.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsherbini, J., Corzett, C., Ravaglioli, C. et al. Epilithic Bacterial Assemblages on Subtidal Rocky Reefs: Variation Among Alternative Habitats at Ambient and Enhanced Nutrient Levels. Microb Ecol 86, 1552–1564 (2023). https://doi.org/10.1007/s00248-023-02174-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02174-1