Abstract
The study was carried out on four non-tidal sandy marine beaches located on the Polish part of the southern Baltic Sea coast. We applied a LIVE/DEAD™ BacLight™ Bacterial Viability Kit (Invitrogen™) method to determine the abundance of live and dead bacteriopsammon. Live psammon bacteria cells constituted 31–53% of the total number of bacteria inhabiting sand of the studied beaches. Abundance of live and dead psammon bacteria generally differed along the horizontal profile in all beaches. The maximum density of bacteria was noted in the dune and the middle part of the beach (dry zones) and the minimum in wet zones, i.e., under seawater surface and at the swash zone. Generally along the vertical profile, the highest numbers of two studied bacterial groups were noted in the surface sand layer, while with increasing sediment depth their numbers significantly decreased. The abundance of live and dead bacteria showed a distinct seasonal variation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine beaches present in all coasts constitute buffer zones between the atmosphere, land, and sea [1,2,3]. These marine coastal areas represent one of the largest ecosystems of the Earth and cover about 75% of the world’s unfrozen shorelines [4, 5]. Sandy beaches are characterised by the uncontrollable dynamic nature and their structure is determined by wind, sand, rainfall, variation in the rates of sun exposure, sea waves’ action, and water in the state of constant motion, therefore they are morphodynamically and climatically strongly differentiated [6, 7]. Marine beaches and coastal areas are also very often subject to considerable anthropogenic pressure due to their recreational function and holiday activities of millions of tourists mainly in the summer season [8, 9]. According to Velonakis et al. [2], time spent by sunbathers on the beaches is usually longer than that spent in seawater.
The permeable nature of sandy beach sediments allows for large quantities of seawater to pass through them over relatively short time. According to Brown and McLachlan [10] and Urban-Malinga et al. [9] sandy beaches can be regarded as gigantic filters through which large amounts of water are being filtered. Depending on whether a beach is dissipative or reflective between 1 and 100 m3 of seawater × m2 × v day can be flushed through the intertidal seawater via wave and tidal pumping and wave ramp [11, 12]. While seawater is being filtered, great amounts of organic compounds, mainly lipids, proteins, and carbohydrates from terrestrial, atmosphere, and marine environments are accumulated. These organic compounds are adsorbed onto the sand grain surface in the form of particulate organic matter (POM), and dissolved (DOM) organic matter [3, 9, 13]. POM and DOM are mineralized as seawater passes through sand, thus beaches play an important role in biogeochemical cycles of different nutrients, and also purification of these marine ecosystems [1, 14, 15].
Marine sandy ecosystems are inhabited by benthic prokaryote microbial community (micropsammon) and psammon bacteria constitute up to 90–99% of benthic biomass [16,17,18]. Psammon bacteria residing in interstidial spaces between sand grains live attached to surface grains as biofilm and play a key role in decomposition, biodegradation, transformation, and mineralization of organic matter accumulated during water filtration [1, 15]. According to Podgórska and Mudryk [19] and Astel et al. [3], about 70% of organic matter reaching the beach is being mineralized by metabolic and biochemical processes carried out by psammon bacteria. For this reason, these organisms function as an enormous biological filter, which plays a very important role in cleaning of such ecosystems [5, 9, 20]. Bacteria inhabiting the sand of the sea beach, which represents the border zone between land and sea, are colonized simultaneously by bacteria of terrestrial origin (limnotolerant) as well as bacteria of marine environment (halotolerant) [21]. Bacteriopsammon is characterized by high diversity. The physiological groups of bacteria colonizing the sand grains of sea beaches of the southern Baltic Sea are mainly dominated by ammonifying bacteria and uric acid hydrolyzing bacteria and the least numerous physiological group among the bacteriopsammon are sulphate-reducing bacteria [22]. Among the taxonomic composition of bacteria colonizing the sand of sea beaches in Czołpino and Sopot (southern Baltic Sea) bacteria of the genera Acinetobacter and Microccocus where predominant. Bacteria of the genera Escherichia, Vibrio, and Photobacterium were much less numerous [23].
The evaluation of the total bacterial number and their metabolic activity is thus essential in our understanding of their ecological role and their contribution to marine processes [24, 25]. Traditional direct counting technique of the total bacterial number applied by hydromicrobiologists using acridine orange and 4′,6-diamidino-2-phenylindole stains results in an overestimation as it includes also dead cells and consequently does not determine metabolically active and inactive fraction in aquatic ecosystems [26, 27]. For this reason, in recent years many different fluorescent staining techniques (6-carboxyfluorescein diacetate, 5-cyano-2, 3-ditolyl tetrazolium chloride, iodonitrotetrazolium chloride, fluorescein diacetate, SYBR® Green I and II) have been proposed to determine physiological state and metabolic activity of natural bacterial communities [25, 28]. One of the most commonly used method applied to determine metabolic activity of natural bacterial assemblages is commercially available LIVE/DEAD BacLight staining method [29, 30]. It allows discrimination between metabolic active (live) and metabolic inactive (dead) cells and is based on the cell membrane integrity and identification of a visible nucleoid region inside the bacterial cell [31, 32]. The LIVE/DEAD BacLight method is widely accepted as a rapid, simple, relatively precise counting and ecologically valuable technique of a quantitative measure of live and dead individual bacterial cells and which also provides a total count of bacteria [31, 33].
To our best knowledge, the current literature provides no example of the studies on the abundance of live and dead bacteria inhabiting sand of marine beaches. Therefore, with the LIVE/DEAD BacLight method, we aimed to provide reliable information on the seasonal number of live and dead bacteria and their spatial distribution in the sand of four beaches located in the Polish part of the southern Baltic Sea.
Material and Methods
Study Area
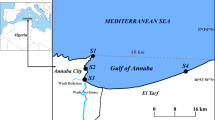
The study was carried out on four non-tidal sandy marine beaches located in Czołpino (54°43′N, 17°14′E), Darłowo (54° 25′N, 16° 24′E), Rowy (54°40′N, 17°3′E), and Ustka (54° 35′N, 16° 51′E), (southern Baltic Sea) (Fig. 1) and represent a dissipative beach type with longshore bars and troughs. They are classified as exposed and have an approximately slope of 7–9° while the width of the beach is about 45–85 m [20, 34]. In general, quartz sand of exposed beaches is fine and medium-grained, and about 90% of the sand grain size is between 0.125 and 0.250 mm [20]. The studied beaches, particularly in autumn and winter, are exposed to strong winds that generate high waves, which cause strong erosion onshore. As a result, the seashore along the studied beaches is heavily destroyed and the coastline retreats on average 0.10 m each year [35].
Location of the study beches (Czołpino, Darłowo, Rowy, Ustka) on north Poland coast of the Baltic Sea (https://d-maps.com/)
The studied beaches differ in their exploitation by recreation function and thus the level of anthropogenic pressure [3]. Moreover, the strength of anthropogenic impact on the studied beaches decreases in the following order: Ustka (very strong) > Darłowo (strong) > Rowy (moderate) > Czołpino (low). The Ustka and Darłowo beaches with their surf zone are one of the most picturesque, very popular bathing beaches and recreational areas in Poland. Polish and foreign tourists as well as local inhabitants intensively visit (about 40 to 500 thousand annually) these beaches, and they are usually very crowded mainly during the summer months. The study of Bigus et al. [36] also indicates that the analyzed beaches differing significantly in the influence of anthropopression are characterized by different levels of chemical parameters. The beach in Ustka had higher values of organic matter (4.53–34.45 mg × g−1) and organic carbon (93.06–20.46 mg × g−1) than the beach in Czołpino (organic matter 3.33–21.06 mg × g−1, organic carbon 0.92–19.68 mg × g−1). Also, the concentrations of heavy metals on the studied beaches indicate clear differences in the influence of anthropopressure on sandy beach sediments of a highly urbanized beach Ustka (Pb — 49.05 mg/kg, Mo — 70.95 mg/kg, Al — 106.87 mg/kg) and a beach Czołpino in a area under legal protection (Pb — 1.67 mg/kg, Mo — 25.89 mg/kg, Al — 76.30 mg/kg) [20]. The beach in Ustka is a municipal beach situated in the vicinity of a sea port and covers the area of 0.3 km2; it is dominated by fishing and tourist cruises. The beach in Rowy is used for recreational purposes to a much lesser extent than the beaches in Ustka and Darłowo. It is located in a very small resort and is one of the most popular nudist beaches in Poland, which significantly limits its general availability for recreational purposes. The beach in Czołpino is located in the Słowiński National Park, which is registered in the World Network of Biosphere Reserves. Characteristic protected elements within the Park are sandbars with moving dunes — unique in Europe. Dunes moving with the speed of 5–30 m per year are covered by saltgrass species such as: Cakile maritima ssp. baltica, Honckenya peploides, Calammophila baltica and Linaria odora. Due to the location in the national park, this beach is very rarely visited by tourists. This region is one of the least polluted in the Polish coastal zone, and as such, it meets one of the selection criteria of marine protected areas [20].
Sampling Procedure
Sand samples were taken from four beaches in spring, summer, and autumn in 2017. In each of the studied beaches, a transect was marked along a profile perpendicular to the shoreline, and four sampling sites were located along this transect (Fig. 2):
-
Site 1– in the sea, approximately 1–1.5 m from the waterline into the open water at a depth of about 0.5–1 m;
-
Site 2 – at the waterline, at the boundary between the beach and the sea;
-
Site 3 – halfway up the beach;
-
Site 4 – in a sheltered place among dunes.
Sand core samples, three per site, were taken with a Morduchaj-Boltowski sampler (length — 30 cm, inner diameter — 15 cm). Already in the field, 15 cm long sand cores were divided horizontally into three sections: 0—5 cm, 6—10 cm, and 11—15 cm. Sand samples were collected in sterile plastic boxes, which were put into containers with ice, in which temperature did not exceed 4 °C. All samples were transported to the laboratory as soon as possible, and then stored at − 60 °C which ensures high viability of microorganisms [37] for further bacteriological analyses.
Bacteriological Assay
According to Quéric et al. [38], Ríos et al. [39], and Perliński and Mudryk [29], to estimate the total number of bacteriopsammon (TBN) inhabiting the sand of the studied beaches, and to distinguish between live and dead bacteria, we used the LIVE/DEAD (L/D) BacLight viability kit (L-13152, Molecular Probes, Eugene, OR, USA). In order to determine bacteriological parameters, sand samples were thawed. Then, 5 g of sand was added to 45 ml−1 of sterile 8‰ artificial seawater and the sample was sonicated for 60 s in a sonicator (Bandelin Sonoplus HD 2070, 70 W, 20 kHz) in order to desorb bacteria normally stuck to sand grains. A study by Joyce et al. [40] indicated that the short-term sonification of ultrasound used in this study did not significantly affect bacterial cell viability. After sonification, 1 ml of each diluted sample was transferred into capped polyethylene vials and stained according to the instructions attached by the manufacturer. All samples were incubated for 15 min in the dark at room temperature. After incubation, the samples were filtered with a Millipore apparatus through 0.2 μm pore size, 13 mm diameter black, polycarbonate filters. After filtration of the sample, the filter was washed with 1 ml−1 of distilled filtered water, dried, and then mounted on microscopic slides. Filters were mounted with low-fluorescent mounting oil (provided with the viability kit) and examined by an epifluorescence microscope (Olympus BX-41) at magnification of × 10 eyepiece and × 100 objective lens, equipped with a filter block B-2A for blue light (EX450-490 excitation filter, DM510 dichroic mirror and BA520 barrier filter; excitation λ = 480/490 nm, emission λ = 500/635 nm) [29] and camera Color Viev. Bacteria were counted in 20 randomly selected fields of view from each layer and sampling site. Green stained cells were classified as “live,” whereas cells stained red were classified as “dead” (Fig. 3). The number of cells in each sample was calculated according to Boulos et al. [41] using the formula T = N × A/a ÷ V where T is the number of bacteria/ml, N is the average number of bacteria/field, A is the surface filtration (mm2), a is the area of the microscopic field and V is the volume of a filtered sample (ml). According to Quéric et al. [38], Pearce et al. [28], and Säwström et al. [42], counts of live and dead bacterial cells were summed up to estimate the total bacterial abundance. The number of psammon bacteria was normalized to sand dry weight (d.w.) after drying at 105°Cfor 24 h.
Statistical Analyses
Statistical parameters (standard deviation — SD, coefficient of variation — CV, coefficient of dispersion — CD) used in the statistical analysis were based on Velji and Albright [43]. The statistical analysis of the obtained studies results was calculated using Statistica 9.0 software. The normal distribution of the data was checked by using the Shapiro–Wilk test before statistical analysis. According to Vignesh et al. [44], if the distribution of the variable met the condition of normality, ANOVA was used to compare the means. When mean values revealed a distribution other than normal, a non–parametric test was used — the Kruskal–Wallis ANOVA rank test and the median test as an equivalent of ANOVA. In order to examine possible inter-relationships between measured bacteriological parameters, discrete, single pair, simple regression analysis was made using Statistica 9.0 software.
Results
All the results of total psammon bacterial number (TBN) and abundance of live and dead bacterial cells are presented in Table 1. TBN absorbed on the grains of sand from the beaches in Darłowo, Rowy, and Ustka was similar and varied from 5.12 ± 2.25 to 5.65 ± 1.98 × 106 cells × g−1 d.w., whereas the lowest (4.10 ± 1.37 × 106 cells × g−1 d.w.) TBN was determined in the sand of the beach in Czołpino. Live cells of psammon bacteria constituted 50–53% of bacteria inhabiting the sand of the beaches in Darłowo and Rowy. In Czołpino and Ustka beach sand, a fraction of live bacteria was three times (31–39%) lower than the number of dead bacteria.
The data on the number of live and dead bacterial cells isolated from the transect marked along the profile perpendicular to the shoreline of the studied beaches are given in Fig. 4. The number of live and dead psammon bacteria isolated from the surface of sand grains was different in different parts of the studied beaches. Generally, the highest number of the two studied groups of bacteria inhabiting dry sand of Czołpino, Darłowo, and Rowy beaches was recorded in the middle part of the beach (site 3) and the dune (site 4). In the sand of Ustka beach, the number of active (live) and inactive (dead) psammon bacteria was similar in all horizontal profiles.
The data on the number of live and dead psammon bacteria in vertical profiles of the studied beaches are presented in Fig. 5. In the sand of Czołpino, Darłowo, and Rowy beaches, the highest number of two bacterial groups was found in the uppermost (0–10 cm) sand layer and the lowest at the deepest (11–15 cm) sand layer. In the sand of Ustka beach, we noted homogeneous distribution of live and dead bacterial cells in all examined sand cores (0–15 cm).
Based on the data collected in this study (Fig. 6), a distinct seasonal variation in the abundance of live and dead psammon bacteria was shown. Generally, the maximum number of two bacterial groups at four studied beaches was noted in spring. The lower number of active and inactive psammon bacteria was observed in the summer season.
The two-way ANOVA analysis on the beach of Rowy (live — p ≤ 0.01 dead — p ≤ 0.001) and Kruskal–Wallis test for the beach in Ustka (live — p ≤ 0.001 dead — p ≤ 0.001) indicated that both number of studied live and dead bacteria displayed significant differences with seasons and on the beach in Darłowo (Kruskal–Wallis test) (live — p ≤ 0.01 dead — p ≤ 0.001) with sites (Table 2). In the sand of Czołpino, beach number of live bacteria varied significantly with seasons (Kruskal–Wallis test live — p ≤ 0.01).
Statistical analysis of the examined bacteriological parameters, which was based on linear regression (Fig. 7) showed that there was a highly (p ≤ 0.001) significant positive correlations between number of live and dead bacteria inhabited sand grains in the Darłowo (R2 = 0.39), Rowy (R2 = 0.35) and Ustka (R2 = 0.49) beaches. In the sand of Czołpino beach, no statistical correlation was found between the number of both the studied bacterial groups.
Discussion
Bacteria are now recognized as a major biological force decisive for functioning of marine ecosystems, for example marine beaches [5, 45]. Knowledge of the number of bacteria and their metabolic activity is very important in the studies of microbial secondary production, bacterial growth rates, and division [13, 29, 46]. The total bacterial number in the studied sandy beaches varied from 4.1 to 5.6 × 106 cells × g−1 d.w. This range was consistent with those reported from other beaches, such as sandy beaches located in Puck (Poland) (2.9–16.1 × 106 cells × g−1 d.w.) [3], Collelungo beach (Italy) (2.0–15.0 × 106 cells × g−1 d.w.) [47], Nova Scotia (Canada) (19.0–73.0 × 106 cells × g−1 d.w.) [48] and Sopot (Poland) (34.0–91.0 × 106 cells × g–1 d.w.) [49] but lower (0.9–1.8 × 108 cells × g−1 d.w.) than the values recorded on Baia Blu beach (Italy) [50]. The authors of this study are fully aware that the determined number of microorganisms may be underestimated during sonification due to insufficient desorption from the sand grains; however, studies by Lee et al. [51] on soil samples showed that sonification lasting up to 3 min gives the highest results in desorbing bacterial cells from sediment particles.
Despite the ecological role of benthic bacteria in biogeochemical cycles at a global scale, information on the fraction of live versus dead bacterial cells in marine sediments is very important in ecological studies [24, 52, 53]. Natural bacterial assemblages display different metabolic levels and vital states and only a part of aquatic bacteria are live and actively growing, while a large fraction is dormant and dead [24, 26, 53]. The results of our study also showed that among all bacteria recorded on the surface of sand grains on the four studied beaches, only 31 to 53% were live cells. Sherr et al. [54] and Pearce et al. [28] suggest that only a small fraction of bacterial community in the studied marine beaches was metabolically active and contributed to bacterial secondary production, growth and division, respiration and enzymatic activity in that marine environment. These results are in accordance with previous studies conducted in lakes [42], rivers [55], ponds [26], estuaries [29], coastal [56] and marine ecosystems [24, 53, 57]. According to Davidson et al. [26] and Papageorgiou et al. [58], a low abundance of live bacterial cells may result from grazing these metabolically active organisms by protozoa especially bacterivorous nanoflagellates and also meio- and macrofauna. Numerous authors [59, 60] reported size-specific grazing of bacterivores leading to preferential grazing of live, active and/or dividing bacteria. Grazing selectivity may result in active bacteria being grazed at rates around 4 times that of inactive cells [26, 61]. This process has been identified as a dominant factor modifying and controlling bacterial number and mortality in aquatic ecosystems [60]. Another factor that influences the abundance of live bacterial cells are viruses [46]. According to Maranger et al. [62], viral infections of active bacteria may be responsible for a large fraction of live bacteria mortality in water basins. Viral infection is known to impair the integrity of bacterial cell walls [42, 63]. It has been suggested that viruses may cause up to 30% mortality of bacteria in aquatic ecosystems [42, 64].
The interactions between sand and sea on marine beach transfer bacteria between different parts of this ecotone [7, 12]. For this reason, horizontal zonation of the distribution of psammon bacterial communities is a well-known global phenomenon on marine sandy beaches [19, 65]. The results of the present study also support these findings. In our study especially on the beach in Ustka, some difference in the abundance of live and dead psammon bacteria along the horizontal profile was observed. However, in the case of the beach in Darłowo, a clear trend of increase in the abundance of both studied groups of bacteria with distance from the shoreline was noted. On the studied beaches, the maximum density of bacteria was noted in the dune and the middle part of the beach (dry zones), whereas bacteria were much less abundant in wet zones, i.e., in the sand samples collected under seawater surface and at the swash zone. According to Schumann et al. [46] and Podgórska and Mudryk [19], a high number of bacteria recorded in the dune and middle part of the beach may result from the accumulation of considerable amounts of particular and dissolved organic matter of anthropogenic, plant and animal origin. These organic compounds can generate growth, production, and high number of bacteria in these zones of marine beach. Moreover, dune which acts as a biocatalytic filter is enriched with humic substances originating from decaying roots of grasses growing on it [66, 67]. Humic substances may be utilized by bacteria very intensively in metabolic processes as a good source of carbon or energy [68]. The low number of bacteria in the sand at waterline as well as under seawater surface may be due to grazing pressure of bacterivorous organisms, which, as has been shown by Haque et al. [69], are very numerous in these parts of the beach. Nanoflagellates, ciliates, macrofauna, and meiofauna for which bacteria represent a readily available source of food and main energy input, are important factors controlling the numbers of bacteria in wet sand on marine beaches [2, 47, 58]. The low number of bacteria in wet sand may be also due to a high-energy character of this marine beach region which is an inhospitable place for bacteria not able to cope with sheer stress associated with a high energy environment [48, 58].
In the sand of Czołpino, Darłowo, and Rowy beaches, the highest number of two studied bacterial groups was found in the surface sand layer and it decreased with increasing sediment depth. Previous studies carried out on marine sediments [19, 65, 70] demonstrated the characteristic vertical distribution of bacteria. These organisms were the most abundant in the top layer of sand, while their number significantly decreased with increasing bottom depth. The concurrent decline in bacterial number with increasing sediment depth might be determined by vertically changing different environmental conditions [38, 71]. This characteristic vertical distribution of psammon bacteria in sand of marine beaches most probably results from the fact that temperature [20], better mixing of sand (also caused by tourist movement) [3], concentration of organic matter [34] as well as better oxygenation [72], which are main stimulators of growth for psammon bacteria, decrease with depth. In the sand of Ustka beach, the fraction of live and dead bacteria was not significantly different between all examined depths. This homogenous distribution of psammon bacteria in the vertical profile on this beach could have been the result of annual dredging, which supplies this beach with huge amounts of sediments collected from the seabed of the Baltic Sea. This process may disturb typical heterogeneous vertical structure of this beach.
The results of our study showed seasonal dynamics in the number of live and dead bacteria inhabiting sand of all studied beaches. Generally the maximum number of two bacterial groups was recorded in spring and their minimum was noted in summer. This finding supports earlier studies carried out in seawater and coastal water in the Netherlands [57], in the Warnow River in the northeast Germany [55], and the Słupia River estuary in Poland [29]. According to Davidson et al. [26], Alonso-Saez et al. [73], and Freese et al. [55], lower number of live and dead psammon bacteria in summer in beaches may result from bacteriostatic or bactericidal inhibitory effect on these organisms due to intensified sunlight insolation, especially with the shortest wavelength fraction of ultraviolet radiation. Solar ultraviolet radiation (290 to 400 nm) causes cellular damage on different cell targets, including nucleic acids, proteins and lipids, which may end up in mutations, cell inactivation, and death [74, 75]. Besides the negative influence of solar radiation, Perliński and Mudryk [29] indicated that excretion of bacteriostatic and bactericidal substances, particularly those synthesized by cyanobacteria and chlorophyta during summer algal bloom in seawater flooding, the sandy beach swash zone may be another factor causing increased psammon bacteria mortality at this time of the year.
Conclusion
The psammon bacteria play a central role in regulating accumulation, export, and transformation of the largest part of organic matter in the marine ecosystems, for example marine beaches. Therefore, the number of bacteria and their metabolic activity influence microbial secondary production, bacterial growth rates and division. Among all bacteria recorded on the surface of sand grains on four studied beaches, only 31 to 53% were live cells, metabolically active, and they are important in microbial loop, biological balance, and for functioning of these marine beach ecosystems. In the sand of Darłowo beach, a clear difference in the abundance of live and dead psammon bacteria was observed along the horizontal profile. This is the example of well-known bacteria transfer between sand and sea in marine beach ecotone. Figure 5 shows the results of the present study in the sand of Czołpino, Darłowo, and Rowy beaches demonstrated the characteristic (but not statistically significant) vertical distribution of bacteria. The psammon bacteria were the most abundant in the top layer of sand, while their number significantly decreased with increasing bottom depth. Seasonal dynamics in the number of live and dead bacteria inhabiting sand of all studied beaches was observed too. Generally, the maximum number of two bacterial groups was recorded in spring and their minimum was noted in summer. Many physical, chemical, and biological factors may be causing changes in the number of psammon bacteria at different time of year.
Data availability
Data will be made available on reasonable request.
References
Gupta AA (2012) Longitudinal assessment of shifting microbial community composition in sandy beaches. Following the 2010 deepwater horizon oil spill and remediation effort. Master thesis, 4269 Louisiana State University pp.111
Velonakis E, Dimitriadi D, Papadogiannakis E (2014) Present status of effect of microorganisms from sand beach on public health. Journal of Coastal Life Medicine 2:746–756. https://doi.org/10.12980/JCLM.2.2014JCLM-2014-0067
Astel AM, Bigus K, Stec M (2018) Microbial enzymatic activity and its relation to organic matter abundance on sheltered and exposed beaches on the Polish coast. Oceanologia 60:312–330. https://doi.org/10.1016/j.oceano.2018.01.001
Amyot J, Grant J (2014) Environmental function analysis: a decision support tool for integrated sandy beach planning. Ocean Coast Manage 102:317–327. https://doi.org/10.1016/j.ocecoaman.2014.10.009
Graham KE, Prussin AJ II, Marr LC, Sassoubre LM, Boehm AB (2018) Microbial community structure of sea spray aerosols at three California beaches. FEMS Microbiol Ecol 94:fiy005. https://doi.org/10.1093/femsec/fiy005
Vieira RHSF, Rodrigues DP, Menezes EA (2001) Microbial contamination of sand from major beaches in Fortaleza, Ceará State, Brazil. Braz J Microbiol 32:77–80. https://doi.org/10.1590/S1517-83822001000200001
Graham KE, Prussin AJ II, Marr LC, Sassoubre LM, Boehm AB (2018) Microbial community structure of sea spray aerosol at three California beaches. FEMS Microbiol Ecol 94:3. https://doi.org/10.1093/femsec/fiy005
Januário AP, Afonso CN, Mendes S, Rodrigues MJ (2019) Faecal indicator bacteria and Pseudomonas aeruginosa in marine coastal waters: is there a relationship Pathogens 9:13. https://doi.org/10.3390/2Fpathogens9010013
Urban-Malinga B, Zalewski M, Jakubowska A, Wodzinowski T, Malinga M, Pałys B, Dąbrowska A (2020) Microplastics on sandy beaches of the southern Baltic Sea. Mar Pollut Bull 155:111170. https://doi.org/10.1016/j.marpolbul.2020.111170
Brown AC, McLachlan A (2002) Sandy shore ecosystems and the treats facing them: some prediction for the year 2025. Environ Conserv 29:62–77. https://doi.org/10.1017/S037689290200005X
Brown AC, McLachlan A (2006) The ecology of sandy shores. Academic Press pp 392.
Boehem AB, Yamahara KM, Sassobure LM (2014) Diversity and transport of microorganism in intertidal sands of the California coast. Appl Environ Microb 80:3943–3951. https://doi.org/10.1128/AEM.00513-14
Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M (2011) Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the deepwater horizon oil spill. Appl Environ Microb 77:7962–7974. https://doi.org/10.1128/aem.05402-11
Huettel M, Berg PL, Kostka JE (2014) Benthic exchange and biogeochemical cycling permeable sediments. Annu Rev Mar Sci 6:23–51. https://doi.org/10.1146/annurev-marine-051413-012706
Staley C, Sadowsky MJ (2016) Regional similarities and consistent patterns of local variation in beach sand bacterial communities throughout the Northern Hemisphere. Appl Environ Microb 82:2751–2762. https://doi.org/10.1128/2FAEM.00247-16
Whitman R, Harwood VJ, Edge TA, Nevers M, Byappanahalli M, Vijayavel K, Brandão J, Sadowsky MJ, Alm EW, Crowe A, Ferguson D, Ge Z, Halliday E, Kinzelman J, Kleinheinz G, Przybyla-Kelly K, Staley C, Staley Z, Solo-Gabriele HM (2014) Microbes in beach sands: integrating environment, ecology and public health. Rev Environ Sci Bio/Technol 13:329–368. https://doi.org/10.1007/s11157-014-9340-8
Probandt D, Eickhorst T, Elliott A, Amann R, Knittel K (2018) Microbial life on a sand grain: from bulk sediment to single grains. ISME J 12:623–633. https://doi.org/10.1038/ismej.2017.197
Oyelade A, Fagad O, Sanuth H, Anjolaiya L, Nwadike B (2018) Microbiological quality of some recreational beaches along the shoreline of Lagos State, Nigeria. J Environ Earth Sci 8:2224–3216
Podgórska B, Mudryk Z (2007) Physiological properties of bacteria inhabiting polluted and unpolluted marine sandy beaches (Southern Baltic Sea). Pol J Ecol 55:15–26
Bigus K, Astel A, Niedzielski P (2016) Seasonal distribution of metals in vertical and horizontal profiles of sheltered and exposed beaches on Polish coast. Mar Pollut Bull 106:347–359. https://doi.org/10.1016/j.marpolbul.2016.03.009
Mudryk ZJ, Podgórska B (2007) Culturable microorganisms in sandy beaches in south Baltic Sea. Pol J Ecol 55:221–231
Mudryk Z, Podgorska B, Ameryk A (2001) Bacteriological characterisation of a Baltic sandy beach in summer. Int J Ecohydrol Hydrobiol 1:503–509
Mudryk ZJ, Podgórska B (2005) Generic composition and respiratory activity of heterotrophic bacteria of marine sandy beach (southern Baltic Sea). Pol J Ecol 53:97–103
Manini E, Danovaro R (2006) Synoptic determination of living/dead and active/dormant bacterial fractions in marine sediments. FEMS Microbiol Ecol 55:416–423. https://doi.org/10.1111/j.1574-6941.2005.00042.x
Morono Y, Terada T, Masui N, Inagaki F (2009) Discriminative detection and enumeration of microbial life in marine subsurface sediments. ISME J 3:503–511. https://doi.org/10.1038/ismej.2009.1
Davidson AT, Thomson PG, Westwood K, van den Enden R (2004) Estimation of bacterioplankton activity in Tasmanian coastal waters and between Tasmania and Antarctica using stains. Aquat Microb Ecol 37:33–45. https://doi.org/10.3354/ame037033
Perliński P, Skórczewski P, Zdanowicz M, Mudryk Z (2015) Participation of MEM+ bacteria in the bacterioplankton community in Ustka harbor, the River Słupia estuary, Southern Baltic Sea. European J Biological Res 5:17–24
Pearce I, Davidson AT, Bell E, Wright S (2007) Seasonal changes in the concentration and metabolic activity of bacteria and viruses at an Antarctic coastal site. Aquat Microb Ecol 47:11–23. https://doi.org/10.3354/AME047011
Perliński P, Mudryk ZJ (2017) Abundance of live and dead cells bacterioneuston and bacterioplankton from the Słupia river estuary. Baltic Coastal Zone 21:61–72
Deng Y, Wang L, Chen Y, Long Y (2020) Optimization of staining with SYTO 9/propidium iodide: interplay, kinetics and impact on Brevibacillus brevis. Biotechniques 69:88–98. https://doi.org/10.2144/btn-2020-0036
Stiefel P, Schmidt-Emrich S, Maniura-Weber K, Ren Q (2015) Critical aspects of using bacterial cell viability assays with the fluorophores SYTO 9 and propidium iodide. BMC Microbiol 15:36. https://doi.org/10.1186/s12866-015-0376-x
Ou F, McGovering C, Swift S, Vanholsbeeck F (2019) Rapid and cost-effective evaluation of bacterial viability using fluorescence spectroscopy. Anal Bioanal Chem 411:3653–3663. https://doi.org/10.1007/s00216-019-01848-5
Ou F, McGovering C, Switf S, Vanholsbeeck F (2019) Near-real-time enumeration of live and dead bacteria using a fibre-base spectroscopic device. Sci Rep 9:4807. https://doi.org/10.1038/s41598-019-41221-1
Trojanowski J, Bigus K, Trojanowska C (2011) Differences of chemical components in beaches sediments with dissimilar anthropopressure. Baltic Coastal Zone 15:109–126
Zawadzka-Kahlau E (1999) Trends in South Baltic coast development during the last hundred years. Peribalticum 7:115–136
Bigus K, Astel A, Stec M, Piskuła P (2017) Spatiotemporal variation of biochemical composition of organic matter and number of bacteria in core sediments of selected beaches of the southern Baltic Sea. Baltic Coastal Zone 21:155–176
Ramírez M, Muñoz A, López-Piñeiro A, Albarrán Á, Peña D, Nunes JMR, Gama J, Loures L (2017) Evaluation of the microbial viability of soil samples from maize crops in freeze-storage under different management conditions in a semi-arid climate. Sustainability 9:690. https://doi.org/10.3390/su9050690
Quéric NV, Soltwedel T, Arntz WE (2004) Application of a rapid direct viable count method to deep-sea sediment bacteria. J Microbiol Meth 57:351–367. https://doi.org/10.1016/j.mimet.2004.02.005
Ríos A, Wierzchos J, Sancho LG, Ascaso C (2004) Exploring the physiological state of continental Antarctic endolithic microorganisms by microscopy. FEMS Microbiol Ecol 50:143–152. https://doi.org/10.1016/j.femsec.2004.06.010
Joyce E, Phull SS, Lorimer JP, Mason TJ (2017) The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason Sonochem 10:315–318. https://doi.org/10.1016/S1350-4177(03)00101-9
Boulos L, Prevost M, Barbeau J, Coallar J, Desjardins R (1999) LIVE/DEAD BactLight: application of a new rapid standing method for direct enumeration of viable and total bacteria in drinking water. J Microbiol Meth 37:77–86. https://doi.org/10.1016/S0167-7012(99)00048-2
Säwström C, Pearce I, Davidson AT (2008) Influence of environmental conditions, bacterial activity and viability on the viral component in 10 Antarctic lakes. FEMS Microbiol Ecol 63:12–22. https://doi.org/10.1111/j.1574-6941.2007.00407.x
Velji MJ, Albright J (1986) Microscopic enumeration of attached marine bacteria of seawater, marine sediment, fecal matter and kelp blade samples following pyrophosphate and ultrasound treatments. Can J Microbiol 32:121–126
Vignesh S, Dahms HU, Emmanuel KV (2014) Physicochemical parameters aid microbial community? A case study from marine recreational beaches, Southern India. Environ Monit Assess 186:1875–1887. https://doi.org/10.1007/s10661-013-3501-z
Martin A, Hall JA, O’Toole R, Davy SK, Ryan KG (2008) High single-cell metabolic activity in Antarctic sea ice bacteria. Aquat Microb Ecol 52:25–31. https://doi.org/10.3354/AME01205
Schumann R, Schiewier U, Karsyen U (2003) Viability of bacteria from different aquatic habitats. II Cellular fluorescent markers for membrane integrity and metabolic activity. Aquat Microb Ecol 32:137–150. https://doi.org/10.3354/ame032137
Moreno M, Ferrero T, Granelli V (2006) Across shore variability and trophodynamic features of meiofauna in a microtidal beach of the NW Mediterranean. Estuar Coast Shelf S 66:357–367. https://doi.org/10.1016/j.ecss.2005.08.016
Novitsky JA, MacSween MC (1989) Microbiology of a high energy beach sediment: evidence for an active and growing community. Mar Ecol Progr Ser 52:71–75
Podgórska B, Mudryk Z, Skórczewski P (2008) Abundance and production of bacteria in a marine beach (southern Baltic Sea) in summer. Pol J Ecol 56:405–414
Misic C, Harriague AC (2007) Enzymatic activity and organic substrates on sandy beach of the Ligurian Sea (NW Mediterranean) influenced by anthropogenic pressure. Aquat Microb Ecol 47:239–251. https://doi.org/10.3354/ame047239
Lee J, Kim HS, Jo HY, Kwon MJ (2021) Revisiting soil bacterial counting methods: optimal soil storage and pretreatment methods and comparison of culture-dependent and independent methods. PLoS One 16:e0246142. https://doi.org/10.1371/journal.pone.0246142
Proctor LM, Souza AC (2001) Method for enumeration of 5 cyano 2,3diotyl tetrazolium chloride (CTC)-active cells and cells specific CTC activity of benthic bacteria in riverine estuarine and coastal sediments. J Microbiol Meth 43:213–222. https://doi.org/10.1016/s0167-7012(00)00218-9
Luna GM, Manini E, Danovaro R (2002) Large fraction of dead and inactive bacteria in coastal marine sediments: comparison of protocols for determination and ecological significance. Appl Environ Microb 68:3509–3513. https://doi.org/10.1128/AEM.68.7.3509-3513.2002
Sherr BF, del Giorgio P, Sherr EB (1999) Estimating abundance and single-cell characteristics respiring bacteria via the redox dye CTC. Aquat Microb Ecol 18:117–131. https://doi.org/10.3354/ame018117
Freese HM, Karsten U, Schumann R (2006) Bacterial abundance, activity, and viability in the eutrophic river Warnow, Northeast Germany. Microb Ecol 51:117–127. https://doi.org/10.1007/s00248-005-0091-5
Choi JW, Sherr BF, Sherr EB (1999) Dead or alive? A large fraction of ETS-inactive marine bacterioplankton cells, as assessed by reduction of CTC, can become ETS-active with incubation and substrate addition. Aquat Microb Ecol 18:105–115. https://doi.org/10.3354/ame018105
Nocker A, Richter-Heitmann T, Montijn R, Schuren F, Kort R (2010) Discrimination between live and dead cells in bacterial communities from environmental water samples analyzed by 454 pyrosequencing. Int Microbiol 13:59–65. https://doi.org/10.2436/20.1501.01.111
Papageorgiou N, Moreno M, Marin V, Baidaro S, Arvanitidis Ch, Fabiano M, Eleftheriou A (2007) Interrelationships of bacteria, meiofauna and macrofauna in a Mediterranean sedimentary beach (Maremma Park, NW Italy). Helgoland Mar Res 61:31–42. https://doi.org/10.1007/s10152-006-0051-6
Gasol JM, del Giorgio PA, Massana R, Durate CM (1995) Active versus inactive bacteria: size-dependence in a coastal marine plankton community. Mar Ecol Progr Ser 128:91–97. https://doi.org/10.3354/meps128091
Hahn MW, Höfle MG (2001) Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol Ecol 35:113–121. https://doi.org/10.1111/j.1574-6941.2001.tb00794.x
Gasol JM, del Giorgio PA (2000) Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci Mar 64:197–224. https://doi.org/10.3989/scimar.2000.64n2197
Maranger R, Bird DF, Juniper SK (1994) Viral and bacterial dynamics in Arctic sea and ice during the spring algal bloom near Resolute, N.W.T Canada. Mar Ecol Progr Ser 111:121–127
Riemann L, Meddelboe M (2002) Viral lysis of marine bacterioplankton: implication for organic matter cycling and bacterial clonal composition. Ophelia 56:57–68. https://doi.org/10.1080/00785236.2002.10409490
Bratbak G, Heldal M (2000) Viruses rule the waves-the smallest and the most abundant members in marine ecosystems. Microbiol Today 27:171–173
Olańczuk-Neyman K, Jankowska K (1998) Bacteriological investigations of the sandy beach ecosystem in Sopot. Oceanologia 40:137–151
Garcia-Lozano C, Pintó J (2018) Current status and future restoration of coastal dune systems on the Catalan shoreline (Spain, NW Mediterranean Sea). J Coast Conserv 22:519–532. https://doi.org/10.1007/s11852-017-0518-4
Nayak S, Behera S, Dash PK (2019) Potential of microbial diversity of coastal sand dunes. Need for exploration in Odisha of India. The Scientific World Journal. https://doi.org/10.1155/2019/2758501
Burkowska A, Donderski W (2007) Impact of humic substances on bacterioplankton in eutrophic lake. Pol J Ecol 55:155–160
Haque AM, Szymelfenig M, Weslawski JM (1996) The sandy littoral zoobenthos of the Polish Baltic coast. Oceanologia 38:361–378
Luna GM, Dell’Anno A, Giuliano L, Danovaro R (2004) Bacterial diversity in deep Mediterranean sediments: relationship with the active bacterial fraction and substrate availability. Environ Microbiol 6:745–753. https://doi.org/10.1111/j.1462-2920.2004.00611.x
Boetius A, Ferdelman T, Lochte K (2000) Bacterial activity in sediments of the deep Arabian Sea in relation to vertical flux. Deep Sea Res Part II 47:2835–2875. https://doi.org/10.1016/S0967-0645(00)00051-5
Uraban-Malinga B, Opaliński KW (2001) Interstitial community oxygen consumption in a Baltic sandy beaches: horizontal zonation. Oceanologia 43:455–468
Alonso-Sáez L, Gasol JM, Lefort T, Hofer J, Sommaruga R (2006) Effect of natural sunlight on bacterial activity and differential sensitivity of natural bacterioplankton groups in northwestern Mediterranean Coastal waters. Appl Environ Microb 72:5806–5813. https://doi.org/10.1128/aem.00597-06
Santos AL, Oliveira V, Baptista I (2013) Wavelength dependence of biological damage include vy UV radiation on bacteria. Arch Microbiol 195:63–74. https://doi.org/10.1007/s00203-012-0847-5
Schuch AP, Victor ML, Santos MB (2015) Molecular and sensory mechanism mitigate sunlight-induced DNA damage in tree frog tadpole. J Exp Biol 218:3059–3067. https://doi.org/10.1242/jeb.126672
Acknowledgements
The authors are grateful to Miss Aleksandra Woźniak and Paulina Bartosik for their contribution in performing bacteriological analyses in the MSc thesis.
Funding
The work was performed with internal fund of the Institute of Biology and Earth Science.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Piotr Perliński, Marta Zdanowicz, Zbigniew Jan Mudryk, Łukasz Kubera. Piotr Perliński was responsible for field work and collection of research samples. Zbigniew Jan Mudryk, Piotr Perliński, Łukasz Kubera drafted the manuscript which was critically reviewed by all of the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perliński, P., Mudryk, Z.J., Zdanowicz, M. et al. Abundance of Live and Dead Bacteriopsammon Inhabiting Sandy Ecosystems of Recreational Marine Beaches of the Southern Baltic Sea. Microb Ecol 86, 350–363 (2023). https://doi.org/10.1007/s00248-022-02079-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-02079-5