Abstract
Purpose
The use of dynamic susceptibility contrast (DSC) perfusion and 11C-methionine positron emission tomography (MET-PET) for glioma grading is currently not standardized. The purpose of this study was to identify regions of interest (ROIs) that enable the best performance and clinical applicability in both methods, as well as to evaluate the complementarity of DSC perfusion and MET-PET in spatial hotspot definition.
Methods
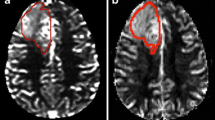
In 41 patient PET/MRI datasets, different ROIs were drawn: in T2-hyperintense tumour, in T2-hyperintense tumour and adjacent oedema and in tumour areas with contrast enhancement, altered perfusion or pathological radiotracer uptake. The performance of DSC perfusion and MET-PET using the different ROIs to distinguish high- and low-grade gliomas was assessed. The spatial overlap of hotspots identified by DSC perfusion and MET-PET was assessed visually.
Results
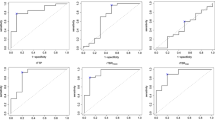
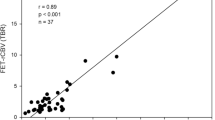
ROIs in T2 fluid attenuated inversion recovery (FLAIR) sequence-hyperintense tumour revealed the most significant differences between high- and low-grade gliomas and reached the highest diagnostic performance in both DSC perfusion (p = 0.046; area under the curve = 0.74) and MET-PET (p = 0.007; area under the curve = 0.80). The combination of methods yielded an area under the curve of 0.80. Hotspots were completely overlapped in one half of the patients, partially overlapped in one third of the patients and present in only one method in approximately 20% of the patients.
Conclusions
For multi-parametric examinations with DSC perfusion and MET-PET, we recommend an ROI definition based on T2-hyperintense tumour. DSC perfusion and MET-PET contain complementary information concerning the spatial hotspot definition.




Similar content being viewed by others
References
Faehndrich J, Weidauer S, Pilatus U, Oszvald A, Zanella FE, Hattingen E (2011) Neuroradiological viewpoint on the diagnostics of space-occupying brain lesions. Clin Neuroradiol 21(3):123–139. https://doi.org/10.1007/s00062-011-0073-6
Fink JR, Muzi M, Peck M, Krohn KA (2015) Multimodality brain tumor imaging: MR imaging, PET, and PET/MR imaging. J Nucl Med: Off Publ, Soc Nucl Med 56(10):1554–1561. https://doi.org/10.2967/jnumed.113.131516
Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191(1):41–51. https://doi.org/10.1148/radiology.191.1.8134596
Sahin N, Melhem ER, Wang S, Krejza J, Poptani H, Chawla S, Verma G (2013) Advanced MR imaging techniques in the evaluation of nonenhancing gliomas: perfusion-weighted imaging compared with proton magnetic resonance spectroscopy and tumor grade. Neuroradiol J 26(5):531–541
Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, Shigematsu Y, Liang L, Ge Y, Ushio Y, Takahashi M (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 171(6):1479–1486. https://doi.org/10.2214/ajr.171.6.9843274
Barajas RF, Jr., Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, Parsa AT, Aghi MK, McDermott MW, Berger MS, Cha S, Chang SM, Nelson SJ (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neuro-Oncology 14 (7):942–954. doi:https://doi.org/10.1093/neuonc/nos128
Server A, Graff BA, Orheim TE, Schellhorn T, Josefsen R, Gadmar OB, Nakstad PH (2011) Measurements of diagnostic examination performance and correlation analysis using microvascular leakage, cerebral blood volume, and blood flow derived from 3T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in glial tumor grading. Neuroradiology 53(6):435–447. https://doi.org/10.1007/s00234-010-0770-x
Lin Y, Xing Z, She D, Yang X, Zheng Y, Xiao Z, Wang X, Cao D (2017) IDH mutant and 1p/19q co-deleted oligodendrogliomas: tumor grade stratification using diffusion-, susceptibility-, and perfusion-weighted MRI. Neuroradiology 59(6):555–562. https://doi.org/10.1007/s00234-017-1839-6
Usinskiene J, Ulyte A, Bjornerud A, Venius J, Katsaros VK, Rynkeviciene R, Letautiene S, Norkus D, Suziedelis K, Rocka S, Usinskas A, Aleknavicius E (2016) Optimal differentiation of high- and low-grade glioma and metastasis: a meta-analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology 58(4):339–350. https://doi.org/10.1007/s00234-016-1642-9
Bergstrom M, Lundqvist H, Ericson K, Lilja A, Johnstrom P, Langstrom B, von Holst H, Eriksson L, Blomqvist G (1987) Comparison of the accumulation kinetics of L-(methyl-11C)-methionine and D-(methyl-11C)-methionine in brain tumors studied with positron emission tomography. Acta Radiol 28(3):225–229
Kubota K (2001) From tumor biology to clinical Pet: a review of positron emission tomography (PET) in oncology. Ann Nucl Med 15(6):471–486
Tietze A, Boldsen JK, Mouridsen K, Ribe L, Dyve S, Cortnum S, Ostergaard L, Borghammer P (2015) Spatial distribution of malignant tissue in gliomas: correlations of 11C-L-methionine positron emission tomography and perfusion- and diffusion-weighted magnetic resonance imaging. Acta Radiol 56(9):1135–1144. https://doi.org/10.1177/0284185114550020
Filss CP, Galldiks N, Stoffels G, Sabel M, Wittsack HJ, Turowski B, Antoch G, Zhang K, Fink GR, Coenen HH, Shah NJ, Herzog H, Langen KJ (2014) Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med: Off Publ Soc Nucl Med 55(4):540–545. https://doi.org/10.2967/jnumed.113.129007
Falk A, Fahlstrom M, Rostrup E, Berntsson S, Zetterling M, Morell A, Larsson HB, Smits A, Larsson EM (2014) Discrimination between glioma grades II and III in suspected low-grade gliomas using dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging: a histogram analysis approach. Neuroradiology 56(12):1031–1038. https://doi.org/10.1007/s00234-014-1426-z
Cicone F, Filss CP, Minniti G, Rossi-Espagnet C, Papa A, Scaringi C, Galldiks N, Bozzao A, Shah NJ, Scopinaro F, Langen KJ (2015) Volumetric assessment of recurrent or progressive gliomas: comparison between F-DOPA PET and perfusion-weighted MRI. Eur J Nucl Med Mol Imaging 42(6):905–915. https://doi.org/10.1007/s00259-015-3018-5
Thomsen H, Steffensen E, Larsson EM (2012) Perfusion MRI (dynamic susceptibility contrast imaging) with different measurement approaches for the evaluation of blood flow and blood volume in human gliomas. Acta Radiol 53(1):95–101. https://doi.org/10.1258/ar.2011.110242
Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, Slart RH (2013) Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging 40(4):615–635. https://doi.org/10.1007/s00259-012-2295-5
Wu R, Watanabe Y, Arisawa A, Takahashi H, Tanaka H, Fujimoto Y, Watabe T, Isohashi K, Hatazawa J, Tomiyama N (2017) Whole-tumor histogram analysis of the cerebral blood volume map: tumor volume defined by 11C-methionine positron emission tomography image improves the diagnostic accuracy of cerebral glioma grading. Jpn J Radiol 35(10):613–621. https://doi.org/10.1007/s11604-017-0675-2
Verger A, Filss CP, Lohmann P, Stoffels G, Sabel M, Wittsack HJ, Kops ER, Galldiks N, Fink GR, Shah NJ, Langen KJ (2017) Comparison of (18)F-FET PET and perfusion-weighted MRI for glioma grading: a hybrid PET/MR study. Eur J Nucl Med Mol Imaging 44(13):2257–2265. https://doi.org/10.1007/s00259-017-3812-3
Obuchowski NA (1997) Nonparametric analysis of clustered ROC curve data. Biometrics 53(2):567–578
Nihashi T, Dahabreh IJ, Terasawa T (2013) Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol 34(5):944–950, S941-911. https://doi.org/10.3174/ajnr.A3324
Takano K, Kinoshita M, Arita H, Okita Y, Chiba Y, Kagawa N, Fujimoto Y, Kishima H, Kanemura Y, Nonaka M, Nakajima S, Shimosegawa E, Hatazawa J, Hashimoto N, Yoshimine T (2016) Diagnostic and prognostic value of 11C-methionine PET for nonenhancing gliomas. AJNR Am J Neuroradiol 37(1):44–50. https://doi.org/10.3174/ajnr.A4460
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, Zilles K, Coenen HH, Langen KJ (2005) O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain: J Neurol 128(Pt 3):678–687. https://doi.org/10.1093/brain/awh399
Hatakeyama T, Kawai N, Nishiyama Y, Yamamoto Y, Sasakawa Y, Ichikawa T, Tamiya T (2008) 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur J Nucl Med Mol Imaging 35(11):2009–2017. https://doi.org/10.1007/s00259-008-0847-5
Berntsson SG, Falk A, Savitcheva I, Godau A, Zetterling M, Hesselager G, Alafuzoff I, Larsson EM, Smits A (2013) Perfusion and diffusion MRI combined with (1)(1)C-methionine PET in the preoperative evaluation of suspected adult low-grade gliomas. J Neuro-Oncol 114(2):241–249. https://doi.org/10.1007/s11060-013-1178-3
Albert NL, Winkelmann I, Suchorska B, Wenter V, Schmid-Tannwald C, Mille E, Todica A, Brendel M, Tonn JC, Bartenstein P, la Fougere C (2015) Early static F-FET-PET scans have a higher accuracy for glioma grading than the standard 20-40 min scans. Eur J Nucl Med Mol Imaging 43:1105–1114. https://doi.org/10.1007/s00259-015-3276-2
Acknowledgements
CB is supported by the TÜFF program of the Faculty of Medicine, Eberhard Karls University Tuebingen (Application Number 2395-0-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Department of Diagnostic and Interventional Radiology has a collaboration contract with Siemens Healthcare concerning the technical development of PET/MRI Biograph mMR.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Brendle, C., Hempel, JM., Schittenhelm, J. et al. Glioma grading by dynamic susceptibility contrast perfusion and 11C-methionine positron emission tomography using different regions of interest. Neuroradiology 60, 381–389 (2018). https://doi.org/10.1007/s00234-018-1993-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-1993-5