Abstract
Purpose
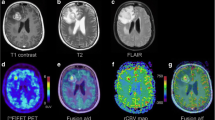
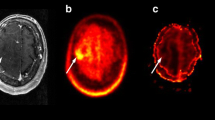
Perfusion-weighted (PWI) magnetic resonance imaging (MRI) and O‑(2-[18F]fluoroethyl-)-l-tyrosine ([18F]FET) positron emission tomography (PET) are both useful for discrimination of progressive disease (PD) from radiation necrosis (RN) in patients with gliomas. Previous literature showed that the combined use of FET-PET and MRI-PWI is advantageous; hhowever the increased diagnostic performances were only modest compared to the use of a single modality. Hence, the goal of this study was to further explore the benefit of combining MRI-PWI and [18F]FET-PET for differentiation of PD from RN. Secondarily, we evaluated the usefulness of cerebral blood flow (CBF), mean transit time (MTT) and time to peak (TTP) as previous studies mainly examined cerebral blood volume (CBV).
Methods
In this single center study, we retrospectively identified patients with WHO grades II–IV gliomas with suspected tumor recurrence, presenting with ambiguous findings on structural MRI. For differentiation of PD from RN we used both MRI-PWI and [18F]FET-PET. Dynamic susceptibility contrast MRI-PWI provided normalized parameters derived from perfusion maps (r(relative)CBV, rCBF, rMTT, rTTP). Static [18F]FET-PET parameters including mean and maximum tumor to brain ratios (TBRmean, TBRmax) were calculated. Based on histopathology and radioclinical follow-up we diagnosed PD in 27 and RN in 10 cases. Using the receiver operating characteristic (ROC) analysis, area under the curve (AUC) values were calculated for single and multiparametric models. The performances of single and multiparametric approaches were assessed with analysis of variance and cross-validation.
Results
After application of inclusion and exclusion criteria, we included 37 patients in this study. Regarding the in-sample based approach, in single parameter analysis rTBRmean (AUC = 0.91, p < 0.001), rTBRmax (AUC = 0.89, p < 0.001), rTTP (AUC = 0.87, p < 0.001) and rCBVmean (AUC = 0.84, p < 0.001) were efficacious for discrimination of PD from RN. The rCBFmean and rMTT did not reach statistical significance. A classification model consisting of TBRmean, rCBVmean and rTTP achieved an AUC of 0.98 (p < 0.001), outperforming the use of rTBRmean alone, which was the single parametric approach with the highest AUC. Analysis of variance confirmed the superiority of the multiparametric approach over the single parameter one (p = 0.002). While cross-validation attributed the highest AUC value to the model consisting of TBRmean and rCBVmean, it also suggested that the addition of rTTP resulted in the highest accuracy. Overall, multiparametric models performed better than single parameter ones.
Conclusion
A multiparametric MRI-PWI and [18F]FET-PET model consisting of TBRmean, rCBVmean and PWI rTTP significantly outperformed the use of rTBRmean alone, which was the best single parameter approach. Secondarily, we firstly report the potential usefulness of PWI rTTP for discrimination of PD from RN in patients with glioma; however, for validation of our findings the prospective studies with larger patient samples are necessary.



Similar content being viewed by others
Data availability
The datasets are available from the corresponding author on reasonable request.
Change history
05 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00062-024-01398-z
References
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: State of the art and future directions. CA: A Cancer Journal for Clinicians. 2020;70(4):299–312.
Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma—a review of the literature and current understanding. Acta Neurochir. 1. Februar 2012;154(2):191–201.
Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI. Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol Imaging. 2. Dezember. Radiation. 2018;2018:6828396.
Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M, u. a. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro-Oncology. 1. September 2015;17(9):1188–98.
Shah R, Vattoth S, Jacob R, Manzil FFP, O’Malley JP, Borghei P, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32(5):1343–59.
Nael K, Bauer AH, Hormigo A, Lemole M, Germano IM, Puig J, u. a. Multiparametric MRI for Differentiation of Radiation Necrosis From Recurrent Tumor in Patients With Treated Glioblastoma. American Journal of Roentgenology. 1. Januar 2018;210(1):18–23.
Boxerman JL, Ellingson BM, Jeyapalan S, Elinzano H, Harris RJ, Rogg JM, et al. Longitudinal DSC-MRI for Distinguishing Tumor Recurrence From Pseudoprogression in Patients With a High-grade Glioma. Am J Clin Oncol. 2017;40(3):228–34.
Young RJ, Gupta A, Shah AD, Graber JJ, Chan TA, Zhang Z, et al. MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clin Imaging. 2013;37(1):41–9.
Tsien C, Galbán CJ, Chenevert TL, Johnson TD, Hamstra DA, Sundgren PC, u. a. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol. 1. Mai 2010;28(13):2293–9.
Kong DS, Kim ST, Kim EH, Lim DH, Kim WS, Suh YL, et al. Diagnostic Dilemma of Pseudoprogression in the Treatment of Newly Diagnosed Glioblastomas: The Role of Assessing Relative Cerebral Blood Flow Volume and Oxygen-6-Methylguanine-DNA Methyltransferase Promoter Methylation Status. AJNR Am J Neuroradiol. 2011;32(2):382–7.
Hu LS, Eschbacher JM, Heiserman JE, Dueck AC, Shapiro WR, Liu S, u. a. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro-Oncology. 1. Juli 2012;14(7):919–30.
Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000;21(5):901–9.
Barajas RF, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, u. a. Differentiation of Recurrent Glioblastoma Multiforme from Radiation Necrosis after External Beam Radiation Therapy with Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging1. Radiology. November 2009;253(2):486–96.
Henriksen OM, Larsen VA, Muhic A, Hansen AE, Larsson HBW, Poulsen HS, u. a. Simultaneous evaluation of brain tumour metabolism, structure and blood volume using [18F]-fluoroethyltyrosine (FET) PET/MRI: feasibility, agreement and initial experience. Eur J Nucl Med Mol Imaging. 1. Januar 2016;43(1):103–12.
Heo YJ, Kim HS, Park JE, Choi CG, Kim SJ. Uninterpretable Dynamic Susceptibility Contrast-Enhanced Perfusion MR Images in Patients with Post-Treatment Glioblastomas: Cross-Validation of Alternative Imaging Options. PLOS ONE. 21. August 2015;10(8):e0136380.
Alsop DC, Detre JA. Reduced Transit-Time Sensitivity in Noninvasive Magnetic Resonance Imaging of Human Cerebral Blood Flow. J Cereb Blood Flow Metab. 1. November 1996;16(6):1236–49.
Jena A, Taneja S, Gambhir A, Mishra AK, D’souza MM, Verma SM, u. a. Glioma Recurrence Versus Radiation Necrosis: Single-Session Multiparametric Approach Using Simultaneous O‑(2-18F-Fluoroethyl)-L-Tyrosine PET/MRI. Clin Nucl Med. Mai 2016;41(5):e228–236.
Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–89.
Pyka T, Hiob D, Preibisch C, Gempt J, Wiestler B, Schlegel J, et al. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur J Radiol. 2018;103:32–7.
Sogani SK, Jena A, Taneja S, Gambhir A, Mishra AK, D’Souza MM, et al. Potential for differentiation of glioma recurrence from radionecrosis using integrated 18F-fluoroethyl-L-tyrosine (FET) positron emission tomography/magnetic resonance imaging: A prospective evaluation. Neurol India. 2017;65(2):293–301.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, u. a. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 10. April 2010;28(11):1963–72.
Boxerman JL, Schmainda KM, Weisskoff RM. Relative Cerebral Blood Volume Maps Corrected for Contrast Agent Extravasation Significantly Correlate with Glioma Tumor Grade, Whereas Uncorrected Maps Do Not. AJNR Am J Neuroradiol. 2006;27(4):859–67.
Hamacher K, Coenen HH. Efficient routine production of the 18F-labelled amino acid O‑2-18F fluoroethyl-L-tyrosine. Appl Radiat Isot. 2002;57(6):853–6.
Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–57.
Pichler R, Dunzinger A, Wurm G, Pichler J, Weis S, Nußbaumer K, u. a. Is there a place for FET PET in the initial evaluation of brain lesions with unknown significance? Eur J Nucl Med Mol Imaging. 1. August 2010;37(8):1521–8.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36.
Larsen VA, Simonsen HJ, Law I, Larsson HBW, Hansen AE. Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology. 1. März 2013;55(3):361–9.
Serkova NJ, Brown MS. Quantitative analysis in magnetic resonance spectroscopy: from metabolic profiling to in vivo biomarkers. Bioanalysis. 2012;4(3):321–41.
Chiang GC, Kovanlikaya I, Choi C, Ramakrishna R, Magge R, Shungu DC. Magnetic Resonance Spectroscopy, Positron Emission Tomography and Radiogenomics-Relevance to Glioma. Front Neurol. 2018;9:33.
Acknowledgements
The authors are grateful to Ms. Silke Kern, the technical team at the Department for Nuclear Medicine, Dr. Sibylle Wimmer and Dr. Raimund Kleiser from the Department of Neuroradiology for invaluable support for this study.
Author information
Authors and Affiliations
Contributions
RP, JP contributed to the study conception and design. Material preparation and data collection were performed by JP. Statistical analysis was performed by GW and BG. JP wrote the manuscript and was assisted by RP. OK and MS who contributed to the final manuscript by delivering important clinical expertise.
Corresponding author
Ethics declarations
Conflict of interest
J. Panholzer, G. Walli, B. Grün, O. Kalev, M. Sonnberger and R. Pichler declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was waived by the local Ethics Committee of the Medical Faculty of the Johannes Kepler University in view of the retrospective nature of the study and all the procedures performed were part of the routine care. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the author’s name Gertraud Malsiner-Walli was incorrectly written as Gertraud Walli. And the affiliation details were incorrectly given as ‘Institute for Applied Statistics, Johannes Kepler University, Linz, Austria’ but should have been ‘Institute for Statistics and Mathematics, WU University of Economics and Business, Vienna, Austria’.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panholzer, J., Malsiner-Walli, G., Grün, B. et al. Multiparametric Analysis Combining DSC-MR Perfusion and [18F]FET-PET is Superior to a Single Parameter Approach for Differentiation of Progressive Glioma from Radiation Necrosis. Clin Neuroradiol (2023). https://doi.org/10.1007/s00062-023-01372-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00062-023-01372-1