Abstract
Three populations of the solar-powered sea slug Elysia crispata on reefs from the southern Gulf of Mexico and the Caribbean were analyzed. The aim was to describe and compare the changes in abundance and size of this species in different localities, as a function of depth and time-of-day. We hypothesized that differences in abundance would be related to locality, time of the day and depth, and differences in size would be related to locality and time of the day. Using snorkeling and SCUBA diving, all individuals within quadrats were counted and measured. A total of 680 organisms were recorded at Verde, Arcas and Puerto Morelos (PM) reefs at five times of the day (sunrise, morning, zenith, evening, and night) and depths of 0–13 m. Zero inflated negative binomial (ZINB) regressions adjusted to abundance data showed that E. crispata in Arcas and Verde reefs is expected to be more abundant (> 50) in shallow depths (< 2 m) at any time of the day except sunrise, whereas a low abundance (≤ 1 organism) is predicted in PM regardless of depth and time-of-day. According to linear models, size was not related to depth, but was related to locality and time-of-day, with sea slugs from Arcas and Verde having similar size, and both larger than those in PM. This information suggests that this sea slug is capable of moving within the reefs and helps to understand the unique biological phenomena of kleptoplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light conditions in the ocean depend on the amount of radiation reaching different areas, due to variations in the sun’s angle throughout the day, as well as depth, turbidity and other parameters (Kirk 2011). Light is a resource for autotroph organisms because it represents the source of energy to maintain metabolic functions, but it also represents a limiting condition. In mobile animals with photosynthetic capacity, it can determine physiological and behavioral aspects, such as activity peaks (circadian rhythms) or be the cue to initiate certain displays, such as food searching or predator avoidance (Begon et al. 2006). Light changes, thus, define the occurrence and distribution of all photosynthetic organisms (Lalli and Parsons 1997), and animals with photosymbioses are no exception (Melo Clavijo et al. 2018).
Sacoglossan sea slugs (Mollusca: Gastropoda) are the only metazoan group that can retain and keep functional for months the chloroplasts from the algae they feed on (Händeler et al. 2009). As these mollusks can benefit from the maintenance of the foreign organelles through the obtention of energetic products from their photosynthesis (Laetz et al. 2017; Cruz et al. 2020; Cartaxana et al. 2021), they have been named “solar-powered” sea slugs (Rumpho et al. 2000). The retention time of this process (also known as kleptoplasty) depends on the slug species, the algal source of the chloroplasts (kleptoplasts) and the light conditions (Cruz et al. 2013; de Vries et al. 2014). Light intensities mediate the photosynthesis within the animal cells, thereby affecting its fitness as well as kleptoplast survival (Vieira et al. 2009; Cruz et al. 2015). For instance, moderate irradiance in the habitat of Plakobranchus ianthobaptus can increase its fitness through kleptoplast survival (Donohoo et al. 2020), while high light intensities can decrease photosynthetic performance in Elysia timida and Elysia viridis (Vieira et al. 2009; Cruz et al. 2015; Cartaxana et al. 2018, 2019).
Elysia crispata is the most conspicuous solar-powered sea slug in the Gulf of Mexico and the Caribbean. Because of its large size (can reach 150 mm in length), it can be easier to find than other slugs. It is common on a broad variety of substrata all year long (e.g., dead coral, sea grass, algae and under rocks), inhabiting borrow pits and mangrove lagoons, as well as coral reefs throughout the Caribbean at up to 25 m depth (Sanvicente-Añorve et al. 2012; Camacho-García et al. 2014; Krug et al. 2016). Clark (1994) suggested the existence of two subspecies of E. crispata, one inhabiting mangrove areas and another living in coral reefs, and Pierce et al. (2006) later proposed a new species named Elysia clarki for the mangrove slugs. Krug et al. (2016) recently analyzed the populations and confirmed that E. crispata was a single species. Nonetheless, the latter study recognized two ecotypes coinciding with the proposed separation by Clark (1994): clarki animals of a more consistently green coloration, that inhabit shallow, low energy waters, with less light incidence; and crispata animals of more variable body coloration that are present in deeper, high energy waters with more light (Pierce et al. 2006; Krug et al. 2016). This species can keep functional kleptoplasts for up to 4 months, hence it is considered a long-term retention species (Curtis et al. 2010). These characteristics make E. crispata an ideal model to study the ecology of photosynthetic animals.
Field studies regarding sacoglossan sea slugs are difficult to perform, as they are small sized animals, cryptic in the substrate (host algae) they are usually associated to and a high effort is required to obtain data, challenging the studies of their populations (Clark 1994; Jensen 1994). Despite these adversities, some species have been studied in situ. For instance, Baumgartner and Toth (2014) found that size and abundance of E. viridis vary among seasons and depend on the host algae: larger but fewer individuals were found on Cladophora rupestris in autumn, whereas smaller ones were more abundant on Cladophora sericea in summer. These authors hypothesized that predation might explain the differences in abundance and size of E. viridis between algal hosts and time of year.
Research on E. crispata has focused on describing biochemical and biomolecular components (Gavagnin et al. 1996, 1997; Middlebrooks et al. 2012; Vital et al. 2021), photosynthetic activity (Curtis et al. 2006; Christa et al. 2015), physiology related to kleptoplasty (Curtis et al. 2006, 2007, 2010; Middlebrooks et al. 2019) and even microbiota (Mahadevan and Middlebrooks 2020). In general, this species has been found to have a long-term retention of chloroplasts from different algae, with a good physiological condition after months of starvation. Population studies of E. crispata have only been conducted in Florida in mangrove swamps and pits, and most of them focused on the clarki ecotype (Clark 1994; Middlebrooks et al. 2014, 2020). Equivalent studies of this species in coral reefs in their western distribution are still lacking and would provide relevant information of this interesting biological model.
Light conditions influence the transfer of carbon and nitrogen from kleptoplasts to sea slugs (Cruz et al. 2020; Cartaxana et al. 2021). While ecological research efforts have mainly focused on the relation between light and Elysia’s physiology and behavior (Schmitt and Wägele 2011; Miyamoto et al. 2015; Cartaxana et al. 2018), studies on its natural distribution are scarce. There is evidence that kleptoplast photosynthesis minimizes weight loss and size reduction, and increases survival in E. viridis, E. timida and E. chlorotica under starving conditions (Giménez-Casalduero and Muniain 2008; Pelletreau et al. 2014; Cartaxana et al. 2017). In addition, the growth rate of E. viridis relative to the rate in which it consumes algae (i.e., efficient growth) has been correlated with exposure to regular light and increased photosynthesis (Baumgartner et al. 2015). Thus, it is reasonable to expect that light conditions influence the abundance and size of E. crispata by limiting its access to photosynthetic resources.
Solar-powered sea slugs will thrive in habitats where optimum light conditions are met. Light conditions vary both between localities and with depth due to the vertical attenuation of light caused by absorption, scattering and diffraction (Kirk 2011), and other factors related to it (e.g., turbidity). In addition, individuals at any site will experience variations in the quality and quantity of light throughout a 24-h period. Circadian rhythms in sea slugs have been documented to be present in swimming and crawling behaviors (Melibe leonina, Newcomb et al. 2014) and the opening and closing of the parapodia (E. timida, Monselise and Rahat 1980). However, the need of light by photosynthetic sacoglossans makes them vulnerable to photodamage and predation, especially at higher light intensities, where the probability of location by visual predators is increased (Weaver and Clark 1981). If sea slugs can move within a small spatial scale between places with varying light quality (e. g. from the top of a dead coral to a crevice nearby), then individuals of different size would occur in different numbers throughout the day. Information on changes in size and abundance of E. crispata throughout a 24-h period will help understand the patterns and time scales of sea slug activity and mobility within the reef. Therefore, the aim of the present study was to describe and compare changes in size and abundance of three populations of E. crispata in Southern Gulf of Mexico and the Mexican Caribbean, as a function of time of the day and depth. We hypothesized that differences in abundance would be related to locality, time of the day and depth, and differences in size would be related to locality and time of the day.
Materials and methods
Study area
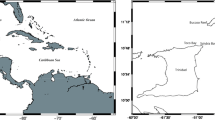
Field studies in three coral reefs of the Gulf of Mexico and Caribbean were conducted: Verde (19°12′09′′ N, 96°03′58′′ W) in August 2018 and June 2019, Cayo Arcas (20°13′12′′ N, 91°58′22′′ W) in April 2018 and Puerto Morelos (20°50′59′′ N, 86°52′23′′ W; Fig. 1) in March and July–August 2019. Verde, located 5 km off the coast, is part of the reef system “Sistema Arrecifal Veracruzano-SAV” in Southern Gulf of Mexico, and has a marked seasonal variation in salinity, temperature, and turbidity. These variation are due to the influence of three major rivers flowing into the Gulf of Mexico and winter winds locally known as “nortes” (Salas-Perez and Granados-Barba 2008; Mateos-Jasso et al. 2012). Arcas is a remote reef located 128 km off the coast of Campeche in the Yucatan and Campeche Bank-YCB (Tunnell 2010). Puerto Morelos (PM) is part of the Mesoamerican reef in the Mexican Caribbean-CAR and is located at an approximate distance ranging from 500 m to less than 3 km parallel to the coast. The continental platform in the Yucatan Peninsula is karstic with little or no transport of suspended sediments to the sea; thus, the coastal influence on the reef is minimal and waters are usually clear (SEMARNAP 2000). These coral reefs were selected because variations in the abundance and distribution of E. crispata between them are probably related to the diverse biological, geological, physical and chemical processes occurring within each region (SAV, YCB, CAR) (Carrillo et al. 2007).
Sampling design and fieldwork
Quadrats (1 and 25 m2) were haphazardly sampled in different sites of the three localities at five different times of day: sunrise (57), morning (48), zenith (78), evening (86) and night (55). Sampled quadrats were located at either side of different transects (20–40 m) which were used as guides to facilitate the identification of the area that had been searched and assure it was not sampled repeatedly. Searching times ranged between 0620 and 0745 h (sunrise), 0930 and 1110 h (morning), 1200 and 1340 h (zenith), 1700 and 1930 h (evening) and 2040 and 2300 h (night). By means of snorkeling and SCUBA diving, all individuals of E. crispata in each quadrat were counted and measured (head–tail length) with a Vernier caliper (± 0.1 mm). Sea slugs on all types of substrata occurring within each quadrat were counted and measured but were never collected. As sacoglossan sea slugs are cryptic, we reduced searching bias by only using experienced divers in sampling trips and dive lights were used during night searches. Depth and light intensity were measured with a dive computer (Hollis DG03) and a light logger (HOBO® Pendant MX2202, Onset Computer Pocasset, MA, USA, with a precision of ± 1–40 lx), respectively. Measurements were recorded every time that slugs were found and averaged per sampling unit. Light units were converted to µmol photons m–2 s–1 following Thimijan and Heins (1983) to facilitate comparison with other studies.
Statistical analyses
To model the number of sea slugs as a function of reef (Verde, Arcas and PM), time-of-day (sunrise, morning, zenith, evening, night) and depth (from 0 to 13 m), a zero-inflated negative binomial (ZINB) generalized linear model with a log link function was used. The log link function ensures positive fitted values, while the negative binomial distribution is typically used for count data with many zero observations and strong overdispersion (Zuur et al. 2009). The ZINB model has two components to estimate the expected values of abundance as a function of a set of explanatory variables: a logit (zero inflated) component to assess the probability of finding false zeros and a negative binomial (count) component to predict the number of individuals.
Absence of organisms (zeros in counts) could be the result of (1) sampling in habitats that are unsuitable for the species, (2) poor experimental design or sampling practices, (3) the lack of experience or otherwise ability to identify specimens by the observer, (4) sampling in habitats that are suitable, but contain unexploited sites or (5) sampling outside the distribution range of the species, among others (Zuur et al. 2009). Thus, true zeros refer to the real absence of organisms in that site, while false zeros refer to the inability to record organisms where they are most likely to be found. When the probability of finding a false zero is low, then, a recorded zero will truly reflect the absence of an organism (i.e., it is likely to be a true zero). By contrast, when the probability of finding a false zero is high, then, the study might not have been able to record true zeros (for instance, individuals could have been at the sampling site, but passed unnoticed by the observer). In ZINB models, if you find organisms in any of the conditions studied, their abundance will be predicted by the NB component of the model, hence the expected abundance would be consistent with having recorded them.
The terms in the ZINB model were reef (categorical factor with three levels), time-of-day (categorical factor with five levels), and depth (continuous). The interaction term was depth × time-of-day in the logit component:
ECi represents the abundance (counts) of E. crispata in observation i, which follow a negative binomial distribution with mean \({\mu }_{i}\), k as the dispersion parameter and \({\pi }_{i}\) as the probability that observation ECi is a false zero. We simplified the notation for the predictor function by omitting the regression parameters that are usually provided before the variable names (Zuur et al. 2009).
The procedure to select the optimal ZINB model consisted of dropping terms one by one in a systematic order and deciding whether they were likely to pertain to the model. To assist each decision the Akaike Information Criterion (AIC) and likelihood ratio tests were used (Zuur et al. 2009). Since all three reefs had suitable habitats for this species, we considered that origin of false zeros were most likely explained by differences in time-of-day (TOD) or depth, but not by differences between localities. Thus, the initial full model in the count component included reef, TOD, depth, and the interactions of depth with reef and time, whereas TOD, depth and their interaction were included only in the logit component.
In addition, a linear model was adjusted to the size data using depth, reef, TOD and the interaction of reef and TOD as explanatory variables. A model selection sequence based on F tests similar to the one described previously, was used to find the best combination of variables explaining changes in sea slug size. This procedure showed that the model including reef and TOD and their interaction was the optimal model. Tukey’s tests for unequal N samples were then performed to assess significant differences in size between reef and TOD once these terms resulted statistically significant (Zar 2009). As a low number of organisms were found in PM reef (N = 7), size data from this reef were only described and not considered in the hypothesis testing procedures.
Models were validated by visual analysis of the residuals using plots of Pearson residuals versus fitted values and each explanatory variable (Zuur et al. 2010). The best ZINB model derived from these procedures included reef and depth for the count data, while the best model for the binomial data included TOD, depth, and the interaction between them. All statistical analyses and graphs were performed in R v. 3.5.3 (R Core Team 2019), using stats v3.5.3 (R Core Team 2019), pscl v.1.5.1 (Jackman et al. 2017), lmtest v. 0.9–37 (Hothorn et al. 2019) and ggplot2 v3.3.5 (Wickham et al. 2021) packages. Differences were considered significant at p < 0.05 (Zar 2009).
Results
A total of 680 organisms of E. crispata was recorded in Verde, Arcas and PM reefs at five moments of the day (sunrise, morning, zenith, evening, night) and depths ranging from 0 to 13 m. Sampling covered a similar area of ~ 1.5 km in PM and Verde, but was reduced to 0.44 km2 in Arcas. Despite differences in sampling effort, Arcas and Verde had a more similar sea slug density compared to PM, where only seven individuals of E. crispata were recorded (Table 1).
Arcas and Verde slugs resembled the description of the crispata ecotype in possessing a completely white foot, whereas sea slugs from PM had a darker green coloration and presented green diverticula in the foot, similar to the clarki ecotype (Fig. 2). Sea slugs were usually found exposed on top of hard substrates, such as coral fragments and coral pavement, yet some individuals were spotted on algae of the genus Halimeda and Padina. In general, few macroalgae species consistent with their potential food sources were observed near the sea slugs. An unidentified filamentous green alga was frequently found on the rocky substrates where sea slugs were common.
Measured at zenith (1200–1340 h), the overall mean light intensity was the highest at Arcas (309.78 ± 282.12 µmol photons m–2 s–1), followed by PM (289.68 ± 195.22 µmol photons m–2 s–1), and Verde with the lowest mean light intensity (148.52 ± 187.30 µmol photons m–2 s–1). Light intensity also varied with depth (Fig. 3). In Arcas and Verde, light intensity decreased as depth increased, with mean values of 258.76 ± 188.4 µmol photons m–2 s–1 at < 5 m and 131.73 ± 224 µmol photons m–2 s–1 at > 5 m deep. In PM, however, abnormally high values of light intensity were recorded at 10.5 m deep (Fig. 3).
Abundance
The highest numbers of slugs were found in Verde (521), followed by Arcas (152), and the lowest number of individuals was found in PM (7). When the times of day were considered, the highest number of organisms was found during night (203), followed by zenith (184) and evening (157), while the lowest during morning (58) and sunrise (78). Despite having a lower sampling effort in Arcas (i.e., less area sampled), the data was analyzed because it provides information of a very isolated population of sea slugs.
The zero-inflated component of the ZINB model describing the absence of sea slugs showed that the interaction between TOD and depth was statistically significant (Chi-square test, χ2 = 32.898, P < 0.001), indicating that the probability of finding E. crispata changes with depth and these changes vary depending on the time-of-day (Table 2). During sunrise, the likelihood of false zeros decreased with depth, while in the morning, zenith, evening, and night, the likelihood of finding false zeros increased with depth (Fig. 4a). Therefore, the model suggests that the true absence of sea slugs at any time-of-day, except sunrise, is likely to decrease with depth.
Zero inflated negative binomial (ZINB) regressions adjusted to abundance data of Elysia crispata in the three localities studied (reefs: Arcas, Verde and Puerto Morelos) at five times of the day (sunrise, morning, zenith, evening, and night). a) Probability of finding false zeros of E. crispata in different times of day and depths; b) predicted counts of E. crispata in the three localities and depths studied; c) expected values of E. crispata considering the explanatory variables included in both components of the ZINB model; “others” represent all times of day, except sunrise
Once the condition of finding slugs was met, the negative binomial component of the model predicted the number of individuals that would be found. This logistic component predicts a decrease in the number of slugs with increasing depth in all three reefs. Nevertheless, the overall predicted counts in Arcas and Verde are much higher at lower depths compared to PM (Fig. 4b). In summary, the ZINB model showed that E. crispata in Arcas and Verde is expected to be more abundant (> 50 individuals) in shallow depths (< 2 m) at any time of the day, except sunrise, when true zeros are most likely. By contrast, low abundance (≤ 1 organism) is estimated in PM at all depths considered (Fig. 4c). The model, for example, implies that if there was an unfruitful search for this species in waters deeper than 6 m during the night in Verde and Arcas, sea slugs were probably there, but we failed to find them. In the event of finding them, the abundance of slugs would nonetheless have very low numbers (< 2 individuals).
Size
The smallest recorded individual (9 mm) was found in Arcas during zenith, whereas the three largest (70 mm) were found in Verde in the evening. Only small organisms were observed in shallower waters, and no sea slugs smaller than 20 mm were found deeper than 7 m. Despite these contrasting numbers, the observed changes in size with depth did not show statistical significance (F (1,663) = 0.365, P = 0.545; Online Resource 1 and 2).
The size of E. crispata, however, was statistically related to reef and TOD, as showed by the significant interaction term (F (4,666) = 8.66, P < 0.001; Online Resource 2). Pair-wise comparisons indicated that sea slugs sampled during the morning, zenith and evening in Verde were larger than those sampled at the same TOD in Arcas (P < 0.05). Nonetheless, sea slugs sampled at sunrise and night were similar in size (P > 0.05; Fig. 5). Sea slugs from PM ranged from 10 to 25 mm in length and their mean size was 18.42 ± 4.64 mm (± SD).
Size of Elysia crispata at different times of day, sunrise, morning, zenith, evening and night in two localities, Arcas and Verde reefs. The line represents the median, top and bottom of the box are the 25th and 75th percentiles, the whiskers represent the maximum and minimum values, and dots are outliers
Discussion
Decades of research in the laboratory have addressed the role of kleptoplasts in the fitness and survival of solar-powered sea slugs (see review by Wägele and Martin 2014). Still, the aspects of their abundance and distribution in their natural environments are unknown for most of the species. Even though E. crispata is a widely distributed and common species in the Caribbean, there are few records in the three localities studied herein (Gavagnin et al. 1997; Zamora-Silva and Ortigosa 2012; Ortigosa and Simões 2019). In preliminary field studies in the remote coral reef islands of Alacranes and the submerged shallow reef (10 m) at Bajos del Norte, both located in the Southern Gulf of Mexico, we found 95 and 26 organisms of E. crispata, respectively. However, in coastal reefs 23 km off the coast of Sisal, Yucatan (Madagascar and Bajo de 10), no organisms of this species were found, confirming previous reports (Ortigosa et al. 2013) as well as potential distribution predictions (Jiménez et al. 2021). It appears that not only the abundance of E. crispata is markedly variable, but its distribution is patchy with high numbers in some localities (e.g., Verde and Arcas) compared to very low occurrence in others (e.g., PM). Such heterogeneity poses a challenge in terms of describing and predicting the occurrence of E. crispata in the natural environment. Together with the sea slug’s cryptic nature, these irregular patterns allow for debate on the causes that explain its absence in places where they could be plausibly expected. To attend this difficulty, the model used in our study (ZINB) enables to assess the probability of the true absence of sea slugs at different combinations of time-of-day and depth (in contrast with a false absence associated to a reduced ability of detection). Our results suggest that an effective detection of E. crispata depends on the light conditions found at certain depths and times of day.
Jiménez et al. (2021) found that distance to the coast was the best predictor with the highest contribution in the niche modeling of Elysia (including E. crispata) from the Caribbean. The authors associated this result to the fact that areas closer to the coast usually have more light availability because they are shallower, warmer and more productive. The characteristics of shallow waters can be found in reefs far from the coast, where large numbers of these organisms can be expected. Despite Arcas is noticeably more distant from the coast than Verde and PM, it has three islands that fulfill the description of the niche modeled for Elysia (Jiménez et al. 2021): high light availability due to shallow, warm, and productive waters. Accordingly, less favorable conditions for sea slugs should include turbidity, and excess of sediment. These conditions are present in Verde (at least in certain seasons; Mateos-Jasso et al. 2012; Avendaño et al. 2019), yet this reef had a high abundance of sea slugs. In addition, mangrove lagoons and borrow pits are characteristic of another recognized habitat of the clarki ecotype in Florida (Middlebrooks et al. 2014). Taken as a whole, these results suggest that this species can inhabit a wide range of environmental conditions.
Studies in laboratory have shown that sacoglossan sea slugs regulate the potentially harmful excess of light through behavioral and physiological mechanisms (Jesus et al. 2010; Cartaxana et al. 2018). The differences in abundance of E. crispata found between Arcas, Verde and PM might be related to different light conditions (Fig. 3), particularly if turbidity is acting as a light regulator through the absorption by suspended particles (Carruthers et al. 2001). Verde is reported to have a high turbidity as the result of substantial discharge of Jamapa, La Antigua and Papaloapan rivers (Avendaño et al. 2019; Liaño-Carrera et al. 2019), while Arcas and Puerto Morelos usually present clear waters (SEMARNAP 2000; Chávez et al. 2007). Despite the contrasting conditions of Arcas and Verde, E. crispata were found in similar abundances, signaling that other factors such as food accessibility, larval availability or conditions at a microhabitat scale might be determinant in the distribution of this species.
While sampling in Arcas, Verde and PM took place from March to August, it is unlikely that seasonal variability in environmental factors are responsible for the differences in sea slug abundance found between the reefs. Differences in temperature and photoperiod, which have been signaled as important factors regulating sea slug temperate populations (Clark 1975; Mondy and Pierce 2005), are less extreme in tropical than in temperate waters, and E. crispata occurs almost all year around in coral reefs of the Southern Gulf of Mexico and Mexican Caribbean (Gavagnin et al. 1997; Sanvicente-Añorve et al. 2012; Zamora-Silva and Ortigosa 2012; Ortigosa and Simões 2019). Moreover, the most contrasting abundances found in this study were between Puerto Morelos and both, Arcas and Verde, yet PM and Verde were both sampled in the same months. During this study, massive sargassum arrivals affected the area near PM. Sargassum so vastly accumulated can decrease light illuminance almost 75% (van Tussenbroek et al. 2017; Hendy et al. 2021) and such events can also increase temperature and decrease pH and oxygen concentrations (van Tussenbroek et al. 2017; Hendy et al. 2021). These factors are known to affect both the normal development and kleptoplasty of E. crispata, through bleaching and causing body deformities (Dionísio et al. 2017, 2018). As there is no previous data on the occurrence of E. crispata in PM, we can only suggest that sargassum arrivals could have been a cause for the low density of individuals found in this study.
Microhabitat availability is distinct between localities (Withers and Tunnell 2007), and could be providing different types of refuge for the slugs to take cover at different times of the day. As previously reported (Weaver and Clark 1981; Middlebrooks et al. 2014; Krug et al. 2016), most individuals in the present study were found on top of hard substrates without algae. Weaver and Clark (1981) suggest that this could be a tactic to increase photosynthesis; however, in Bahamas it is common to find E. crispata on the underside of rocks in < 2 m depth (Redfern 2013). It is possible that the occurrence of sea slugs on substrate depends on microhabitat conditions at a smaller spatial scale, which in turn, vary with depth (Chávez et al. 2007). In Verde, for example, crevices are common at most of the depths, but they are less frequent both in shallow waters and close to the reef lagoon. By contrast, the reef lagoon has larger rocks and sea grass patches that provide shade in these otherwise luminous shallow waters. The great variety of microhabitats found in these coral reefs can be expected to serve as shelter for slugs to avoid highly irradiated waters, a condition that could be physiologically limiting (Vieira et al. 2009).
The low abundance of E. crispata with increasing depth could be related to the reduction of resources at deeper waters. Macroalgae abundance and diversity can change with increasing depth in the reef system (SAV) where Verde is located (Horta-Puga et al. 2020). Adults from this species apparently consume a wide variety of macroalgae (Vital et al. 2021) but we rarely observed sea slugs associated to any macroalgae in the present study. Middlebrooks et al. (2014) also found low occurrence of E. crispata associated to their algal food in a completely different habitat in Florida. Adults might be temporarily staying at lower depths to lay eggs in algae and, moving back to deeper waters, as it has been suggested in other sea slugs (Willan 1979). While juvenile E. crispata have a narrower food range compared to adults (Curtis et al. 2007), the induction of metamorphosis in the larvae does not depend on the presence of a particular food source (Krug 2009). It is possible that juveniles were consuming filamentous green algae other than Bryopsis plumosa or Derbesia tenuissima (Curtis et al. 2007), such as the one we observed near the rocky substrates where organisms were frequently found. Overall, these results suggest that the presence of food sources is unlikely to be the main factor determining the occurrence of this species within its geographical distribution, but further research should consider the feeding ecology of larval and juvenile stages.
A weak association of E. crispata to its food sources would allow sea slugs to explore deeper waters under conditions of low abundance or even absence of food. Within aquatic ecosystems irradiance decreases and the spectral composition of light changes with depth, thereby influencing photosynthetic activity (Hill 1996). Long (red) wavelengths are absorbed at the first few meters and short (blue) wavelengths are the last to be absorbed as the depth increases (Kirk 2011). Moving deeper could be an advantageous strategy for sea slugs exploring lower light intensities, although limits to such advantages are surely imposed by the pigments in their chloroplasts and how these respond to the varying wavelengths. Elysia crispata has a wide variety of pigments in its chloroplasts and its composition and concentration do not seem to be related to depth (Vital et al. 2021), which might represent an advantage to them. The relationship between pigment concentration and light preferences by sea slugs, hence, constitutes an interesting area of research yet to be investigated.
In the present study we confirmed the significant effect of TOD as a relevant factor predicting both sacoglossan occurrence and size, and suggest that they have circadian rhythms as do other sea slug groups (Newcomb et al. 2014). A daily pattern with a lower number of organisms of E. crispata at sunrise was statistically identified in Arcas and Verde, suggesting that sea slugs are capable of moving within the reef. Light wavelengths vary with the angle of the sun as it changes throughout the day. If sea slugs respond to daily variations in light by moving, its quality and quantity could be determining sea slug presence in these reefs. Research on circadian rhythms in sea slugs is very scarce, but studies on E. timida in the laboratory and their habitat suggested the presence of a biological clock that partially controls its parapodial behavior (Rahat and Monselise 1979; Monselise and Rahat 1980). During daylight, Plakobranchus ocellatus seems to take cover on the underside of rocks or by burrowing in the sand (Tanamura and Hirose 2016); likewise, E. crispata might move to crevices in the reef or under rocks. While only experienced observers participated in the present study, sampling bias cannot be completely overruled. The use of lights during night dives might have increased the detection of organisms by focusing the observer’s attention to a better illuminated, yet reduced field. It is unsure to what extent does E. crispata present nocturnal activity but results herein suggest mobility during that period of the day.
One of the main features of day light is that it synchronizes physiological mechanisms of living organisms to a period that allows the recognition of 24-h cycles. Changes in ambient light constitute an external signal to initiate certain activities (Takahashi 1991). The fact that E. crispata of varying size were found in certain times of day and that these differed between Arcas and Verde leads to suggest that sea slugs are capable of a relatively wide range of mobility within 0 and 13 m deep, and that this is influenced, at least partially by day light. Such light-dependent movement could be the result of one or more of the following explanations: (1) light stress avoidance, (2) circadian rhythms, and/or (3) predation avoidance.
Avoiding light stress to maintain functional chloroplasts has been supported by laboratory experiments in other species, such as E. timida (Jesus et al. 2010). Some of the algae used as food by slugs have photoprotection mechanisms through changes in pigments or by acclimating to depth and time of the day (Raniello et al. 2006). However, not all E. crispata present these pigments (Vital et al. 2021), and the macroalgae consumed most frequently by this species (e.g., Bryopsis, Penicillus and Halimeda) might not have them (Middlebrooks et al. 2019; Giossi et al. 2021). Other potential mechanisms for the protection of excessive light in E. crispata are mucus excretion, which has been speculated to be used as a sunscreen (Ireland and Scheuer 1979; Gavagnin et al. 1996; Havurinne et al. 2021), and behavioral strategies, such as closing the parapodia (Cartaxana et al. 2018, 2019) or moving towards areas in the reef that provide light protection.
Dial changes of light could also trigger movement of sea slugs within the reef. While the solar elevation changes throughout the day, there is no simple relation between this and the spectral distribution (i.e., the proportion of different wavelengths) or total irradiance (i.e., light intensity/photon flux). When the solar elevation is reduced at sunrise, the ratio of short to long wavelength light in the direct solar beam decreases. This is due to a removal of short wavelength (blue) light in the atmospheric path caused by scattering (Kirk 2011). It might be that E. crispata detects changes of the spectral distribution at certain moments of the day and modifies its position in the reef by moving towards more sheltered areas. For this behavior to be displayed, photoreceptors detecting different wavelengths must exist. The eyes of E. timida perceive light at 540 nm and the presence of extraocular receptors has also been considered (Rahat and Monselise 1979). While E. crispata has eyes, the extent to which they can detect quality of light and display avoidance, or preference accordingly is still unknown. Either confirming light selective behaviors or finding other type of photoreceptors in E. crispata would constitute an additional element supporting the idea of behavioral mechanisms of photoprotection in this species and may help to explain some of the patterns in which they naturally occur.
Crypsis and secondary metabolites have been mentioned as mechanisms used by sacoglossans to avoid predation (Gavagnin et al. 1997, 2000). Predators of this sea slug group include crustaceans, fish, other sea slugs and even corals (Trowbridge 1994; Mehrotra et al. 2019). While we witnessed sea slug attacks by different fish species during our field work, no consumption of E. crispata was observed. Predation avoidance could be considered the most unlikely explanation of the patterns described herein, but it should not be fully discarded until further studies assess the consumption of Elysia by visual predators.
Our research provided novel population information, showing that the abundance and size of E. crispata in coral reefs of Southern Gulf of Mexico and Mexican Caribbean Sea depend on locality, depth, and time of the day. Such information suggests that this sea slug is capable of moving a few meters within the reefs, and its mobility may be triggered by light quality and intensity. Further research is needed to better understand the unique biological phenomena of solar-powered sea slugs as they interact with their natural habitat and use it in management and conservation initiatives.
Data availability
The datasets analyzed during the current study are available in the Zenodo repository and can be found at https://doi.org/10.5281/zenodo.7686727.
References
Avendaño O, Salas-Monreal D, Anis A, Salas-de-Leon DA, Monreal-Gomez MA (2019) Monthly surface hydrographical variability in a coral reef system under the influence of river discharges. Estuar Coast Shelf Sci 222:53–65. https://doi.org/10.1016/j.ecss.2019.04.012
Baumgartner FA, Toth GB (2014) Abundance and size distribution of the sacoglossan Elysia viridis on co-occurring algal hosts on the Swedish west coast. PLoS ONE. https://doi.org/10.1371/journal.pone.0092472
Baumgartner FA, Pavia H, Toth GB (2015) Acquired phototrophy through retention of functional chloroplasts increases growth efficiency of the sea slug Elysia viridis. PLoS ONE 10:1–14. https://doi.org/10.1371/journal.pone.0120874
Begon M, Townsend C, Harper JL (2006) Ecology: From individuals to ecosystems, 4th edn. Blackwell Publishing
Camacho-García YE, Pola M, Carmona L, Padula V, Villani G, Cervera JL (2014) Diversity and distribution of the heterobranch sea slug fauna on the Caribbean of Costa Rica. Cah Biol Mar 55: 109-127
Carrillo L, Horta-Puga G, Carricart-Ganivet JP (2007) Climate and oceanography. In: Tunnell JW, Chávez EA, Withers K (eds) Coral reefs of the southern Gulf of Mexico. Texas A&M University Press, Corpus Christi, pp 34–40
Carruthers TJB, Longstaff BJ, Dennison WC, Abal EG, Aioi K (2001) Measurement of light penetration in relation to seagrass. Glob Seagrass Res Methods. https://doi.org/10.1016/b978-044450891-1/50020-7
Cartaxana P, Trampe E, Kühl M, Cruz S (2017) Kleptoplast photosynthesis is nutritionally relevant in the sea slug Elysia viridis. Sci Rep 7:7714. https://doi.org/10.1038/s41598-017-08002-0
Cartaxana P, Morelli L, Quintaneiro C, Calado G, Calado R, Cruz S (2018) Kleptoplasts photoacclimation state modulates the photobehaviour of the solar-powered sea slug Elysia viridis. J Exp Biol 221:1–23. https://doi.org/10.1242/jeb.180463
Cartaxana P, Morelli L, Jesus B, Calado G, Calado R, Cruz S (2019) The photon menace: Kleptoplast protection in the photosynthetic sea slug Elysia timida. J Exp Biol 222:3–6. https://doi.org/10.1242/jeb.202580
Cartaxana P, Rey F, LeKieffre C, Lopes D, Hubas C, Spangenberg JE, Escrig S, Jesus B, Calado G, Domingues R, Kühl M, Calado R, Meibom A, Cruz S (2021) Photosynthesis from stolen chloroplasts can support sea slug reproductive fitness. Proc R Soc B 288:20211779. https://doi.org/10.1098/rspb.2021.1779
Chávez EA, Tunnell JWJ, Withers K (2007) Reef zonation and ecology: Veracruz shelf and Campeche Bank. Coral reefs of the southern Gulf of Mexico. Texas A&M University Press. Corpus Christi, College Station, pp 41–67
Christa G, Händeler K, Kück P, Vleugels M, Franken J, Karmeinski D, Wägele H (2015) Phylogenetic evidence for multiple independent origins of functional kleptoplasty in Sacoglossa (Heterobranchia, Gastropoda). Org Divers Evol 15:23–36. https://doi.org/10.1007/s13127-014-0189-z
Clark KB (1975) Nudibranch life cycles in the Northwest Atlantic and their relationship to the ecology of fouling communities. Helgoländer Meeresun 27:28–69. https://doi.org/10.1007/BF01611686
Clark KB (1994) Ascoglossan (=Sacoglossa) molluscs in the Florida Keys: rare marine invertebrates at special risk. Bull Mar Sci 54:900–916
Cruz S, Calado R, Serôdio J, Cartaxana P (2013) Crawling leaves: photosynthesis in sacoglossan sea slugs. J Exp Bot 64:3999–4009. https://doi.org/10.1093/jxb/ert197
Cruz S, Cartaxana P, Newcomer R, Dionísio G, Calado R, Serôdio J, Pelletreau KN, Rumpho ME (2015) Photoprotection in sequestered plastids of sea slugs and respective algal sources. Sci Rep 5:1–8. https://doi.org/10.1038/srep07904
Cruz S, LeKieffre C, Cartaxana P, Hubas C, Thiney N, Jakobsen S, Escrig S, Jesus B, Kühl M, Calado R, Meibom A (2020) Functional kleptoplasts intermediate incorporation of carbon and nitrogen in cells of the Sacoglossa sea slug Elysia viridis. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-66909-7
Curtis NE, Massey SE, Pierce SK (2006) The symbiotic chloroplasts in the sacoglossan Elysia clarki are from several algal species. Invertebr Biol 125:336–345. https://doi.org/10.1111/j.1744-7410.2006.00065.x
Curtis NE, Pierce SK, Massey SE, Schwartz JA, Maugel TK (2007) Newly metamorphosed Elysia clarki juveniles feed on and sequester chloroplasts from algal species different from those utilized by adult slugs. Mar Biol 150:797–806. https://doi.org/10.1007/s00227-006-0398-x
Curtis NE, Schwartz JA, Pierce SK (2010) Ultrastructure of sequestered chloroplasts in sacoglossan gastropods with differing abilities for plastid uptake and maintenance. Invertebr Biol 129:297–308. https://doi.org/10.1111/j.1744-7410.2010.00206.x
de Vries J, Rauch C, Christa G, Gould SB (2014) A sea slug’s guide to plastid symbiosis. Acta Soc Bot Pol 83:415–421. https://doi.org/10.5586/asbp.2014.042
Dionísio G, Faleiro F, Bilan M, Rosa IC, Pimentel M, Serôdio J, Calado R, Rosa R (2017) Impact of climate change on the ontogenetic development of “solar-powered” sea slugs. Mar Ecol Prog Ser 578:87–97. https://doi.org/10.3354/meps12227
Dionísio G, Faleiro F, Bispo R, Lopes AR, Cruz S, Paula JR, Repolho T, Calado R, Rosa R (2018) Distinct bleaching resilience of photosynthetic plastid-bearing mollusks under thermal stress and high CO2 conditions. Front Physiol 9:1–11. https://doi.org/10.3389/fphys.2018.01675
Donohoo SA, Wade RM, Sherwood AR (2020) Finding the sweet spot: Sub-ambient light increases fitness and kleptoplast survival in the sea slug Plakobranchus cf. ianthobaptus Gould, 1852. Biol Bull 238:154–166. https://doi.org/10.1086/709371
Gavagnin M, Mollo E, Cimino G, Ortea J (1996) A New γ-Dihydropyrone-Propionate from the Caribbean Sacoglossan Tridachia crispata. Tetrahedron Lett 37:4259–4262
Gavagnin M, Mollo E, Castelluccio F, Montanaro D, Ortea J, Cimino G (1997) A novel dietary sesquiterpene from the marine sacoglossan Tridachia crispata. Nat Prod Lett 10:151–156. https://doi.org/10.1080/10575639708043731
Gavagnin M, Mollo E, Montanaro D, Ortea J, Cimino G (2000) Chemical studies of Caribbean sacoglossans: Dietary relationships with green algae and ecological implications. J Chem Ecol 26:1563–1578. https://doi.org/10.1023/A:1005526526884
Giménez-Casalduero F, Muniain C (2008) The role of kleptoplasts in the survival rates of Elysia timida (Risso, 1818): (Sacoglossa: Opisthobranchia) during periods of food shortage. J Exp Mar Bio Ecol 357:181–187. https://doi.org/10.1016/j.jembe.2008.01.020
Giossi CE, Cruz S, Rey F, Marques R, Melo T, Domingues do MR, Cartaxana RP (2021) Light induced changes in pigment and lipid profiles of Bryopsidales algae. Front Mar Sci 8:745083. https://doi.org/10.3389/fmars.2021.745083
Händeler K, Grzymbowski YP, Krug PJ, Wägele H (2009) Functional chloroplasts in metazoan cells—a unique evolutionary strategy in animal life. Front Zool 6:1–18. https://doi.org/10.1186/1742-9994-6-28
Havurinne V, Aitokari R, Mattila H, Käpylä V, Tyystjärvi E (2021) Ultraviolet screening by slug tissue and tight packing of plastids protect photosynthetic sea slugs from photoinhibition. Photosynth Res 152:373–387. https://doi.org/10.1007/s11120-021-00883-7
Hendy IW, Woolford K, Vincent-Piper A, Burt O, Schaefer M, Cragg SM, Sanchez-Navarro P, Ragazzola F (2021) Climate-driven golden tides are reshaping coastal communities in Quintana Roo, Mexico. Clim Chang Ecol 2:100033. https://doi.org/10.1016/j.ecochg.2021.100033
Hill W (1996) Effects of light. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology. Academic Press Inc, San Diego, pp 121–148
Horta-Puga G, Tello-Musi JL, Córdova A, Gutiérrez-Carrillo A, Gutiérrez-Martínez J, Morales-Aranda AA (2020) Spatio-temporal variability of benthic macroalgae in a coral reef system highly influenced by fluvial discharge: Veracruz, Gulf of Mexico. Mar Ecol 41:1–18. https://doi.org/10.1111/maec.12596
Hothorn T, Zeileis A, Farebrother RW, Cummins C, Millo G, Mitchell D (2019) lmtest. 1–47.
Ireland C, Scheuer PJ (1979) Photosynthetic marine mollusks: In vivo 14 C incorporation into metabolites of the sacoglossan Placobranchus ocellatus. Science 80(205):922–923. https://doi.org/10.1126/science.205.4409.922
Jackman S, Tahk A, Zeileis A, Maimone C, Fearon J, Meers Z (2017) pscl. 1–100.
Jensen KR (1994) Behavioural adaptations and diet specificity of sacoglossan opisthobranchs. Ethol Ecol Evol 6:87–101. https://doi.org/10.1080/08927014.1994.9523011
Jesus B, Ventura P, Calado G (2010) Behaviour and a functional xanthophyll cycle enhance photo-regulation mechanisms in the solar-powered sea slug Elysia timida (Risso, 1818). J Exp Mar Bio Ecol 395:98–105. https://doi.org/10.1016/j.jembe.2010.08.021
Jiménez LM, Simões N, Yáñez-Arenas C (2021) Where is the genus Elysia in the western Atlantic? Potential distribution, species richness and representation in marine protected areas. J Molluscan Stud. https://doi.org/10.1093/mollus/eyab003
Kirk JTO (2011) Light and photosynthesis in aquatic ecosystems, 3rd edn. Cambridge University Press, New York
Krug PJ (2009) Not my “type”: larval dispersal dimorphisms and bet-hedging in Opistobranch life histories. Biol Bull 216:355–372. https://doi.org/10.2307/25548166
Krug PJ, Vendetti JE, Valdés Á (2016) Molecular and morphological systematics of Elysia Risso, 1818 (Heterobranchia: Sacoglossa) from the Caribbean region. Zootaxa 4148:1–137. https://doi.org/10.11646/zootaxa.4148.1.1
Laetz EMJ, Moris VC, Moritz L, Haubrich AN, Wägele H (2017) Photosynthate accumulation in solar-powered sea slugs - starving slugs survive due to accumulated starch reserves. Front Zool 14:1–9. https://doi.org/10.1186/s12983-016-0186-5
Lalli CM, Parsons TR (1997) Biological oceanography, an introduction, 2nd edn. The Open University, Burlington, USA
Liaño-Carrera F, Camarena-Luhrs T, Gómez-Barrero A, Martos-Fernández FJ, Ramírez-Macias JI, Salas-Monreal D (2019) New coral reef structures in a tropical coral reef system. Lat Am J Aquat Res 47:270–281. https://doi.org/10.3856/vol47-issue2-fulltext-7
Mahadevan P, Middlebrooks ML (2020) Bacterial diversity in the clarki ecotype of the photosynthetic sacoglossan, Elysia crispata. Microbiologyopen 9:1–9. https://doi.org/10.1002/mbo3.1098
Mateos-Jasso A, Zavala-Hidalgo J, Romero-Centeno R, Allende-Arandía ME (2012) Variability of the thermohaline structure in the northern Veracruz Coral Reef System, Mexico. Cont Shelf Res 50–51:30–40. https://doi.org/10.1016/j.csr.2012.10.001
Mehrotra R, Monchanin C, Scott CM, Phongsuwan N, Gutierrez MC, Chavanich S, Hoeksema BW (2019) Selective consumption of Sacoglossan sea slugs (Mollusca: Gastropoda) by scleractinian corals (Cnidaria: Anthozoa). PLoS ONE 14:1–22. https://doi.org/10.1371/journal.pone.0215063
Melo Clavijo J, Donath A, Serôdio J, Christa G (2018) Polymorphic adaptations in metazoans to establish and maintain photosymbioses. Biol Rev 93:2006–2020. https://doi.org/10.1111/brv.12430
Middlebrooks ML, Bell SS, Pierce SK (2012) The kleptoplastic sea slug Elysia clarki prolongs photosynthesis by synthesizing chlorophyll a and b. Symbiosis 57:127–132. https://doi.org/10.1007/s13199-012-0187-x
Middlebrooks ML, Bell SS, Curtis NE, Pierce SK (2014) Atypical plant-herbivore association of algal food and a kleptoplastic sea slug (Elysia clarki) revealed by DNA barcoding and field surveys. Mar Biol 161:1429–1440. https://doi.org/10.1007/s00227-014-2431-9
Middlebrooks ML, Curtis NE, Pierce SK (2019) Algal sources of sequestered chloroplasts in the sacoglossan sea slug Elysia crispata vary by location and ecotype. Biol Bull 236:88–96. https://doi.org/10.1086/701732
Middlebrooks ML, Curtis NE, Pierce SK (2020) The complete disappearance of a long standing sacoglossan sea slug population following Hurricane Irma, despite recovery of the local algal community. Symbiosis 80:231–237. https://doi.org/10.1007/s13199-020-00670-3
Miyamoto A, Sakai A, Nakano R, Yusa Y (2015) Phototaxis of sacoglossan sea slugs with different photosynthetic abilities: a test of the ‘crawling leaves’ hypothesis. Mar Biol 162:1343–1349. https://doi.org/10.1007/s00227-015-2673-1
Mondy WL, Pierce SK (2005) Apoptotic-like morphology is associated with annual synchronized death in kleptoplastic sea slugs (Elysia chlorotica). Invertebr Biol 122:126–137. https://doi.org/10.1111/j.1744-7410.2003.tb00078.x
Monselise EBI, Rahat M (1980) Photobiology of Elysia timida (Mollusca: Opisthobranchia): Observations in the sea. Isr J Zool 29:125–128. https://doi.org/10.1080/00212210.1980.10688489
Newcomb JM, Kirouac LE, Naimie AA, Bixby KA, Lee C, Malanga S, Raubach M, Watson WHI (2014) Circadian rhythms of crawling and swimming in the nudibranch mollusc Melibe leonina. Biol Bull 227:263–273. https://doi.org/10.1002/adma.201403943
Ortigosa D, Simões N (2019) Sea slugs (Gastropoda: Heterobranchia) from two remote reefs of the southern Gulf of Mexico: Cayo Arenas and Cayo Arcas. Rev Mex Biodivers. https://doi.org/10.22201/ib.20078706e.2019.90.2596
Ortigosa D, Simoes N, Calado G (2013) Sea slugs (Mollusca: Opisthobranchia) from Campeche Bank, Yucatan Peninsula, Mexico. Thalassas 29:59–75
Pelletreau KN, Weber APM, Weber KL, Rumpho ME (2014) Lipid accumulation during the establishment of kleptoplasty in Elysia chlorotica. PLoS ONE 9:1–16. https://doi.org/10.1371/journal.pone.0097477
Pierce SK, Curtis NE, Massey SE, Bass AL, Karl SA, Finney CM (2006) A morphological and molecular comparison between Elysia crispata and a new species of kleptoplastic sacoglossan sea slug (Gastropoda: Opisthobranchia) from the Florida Keys, USA. Molluscan Res 26:23–38
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rahat PM, Monselise EB (1979) Photobiology of the chloroplast hosting mollusc Elysia timida- (Opisthobranchia). J Exp Biol 79:225–233
Raniello R, Lorenti M, Brunet C, Buia MC (2006) Photoacclimation of the invasive alga Caulerpa racemosa var. cylindracea to depth and daylight patterns and a putative new role for siphonaxanthin. Mar Ecol 27:20–30. https://doi.org/10.1111/j.1439-0485.2006.00080.x
Redfern C (2013) Bahamian seashells: 1161 species from Abaco Bahamas. Bahamianseashellscom Incorporated, Boca Ratón
Rumpho ME, Summer EJ, Manhart JR (2000) Solar-Powered sea slugs. Mollusc/algal Chloroplast Symbiosis. Plant Physiol 123:29–38. https://doi.org/10.1104/pp.123.1.29
Salas-Perez JJ, Granados-Barba A (2008) Oceanographic characterization of the Veracruz reefs system. Atmosfera 21:281–301
Sanvicente-Añorve L, Hermoso-Salazar M, Ortigosa J, Solís-Weiss V (2012) Opisthobranch assemblages from a coral reef system: the role of habitat type and food availability. Bull Mar Sci 88:1061–1074. https://doi.org/10.5343/bms.2011.1117
Schmitt V, Wägele H (2011) Behavioral adaptations in relation to long-term retention of endosymbiotic chloroplasts in the sea slug Elysia timida (Opisthobranchia, Sacoglossa). Thalassas 27:225–238
SEMARNAP (2000) Programa de Manejo del Parque Nacional Arrecife de Puerto Morelos. Distrito Federal, México
Takahashi JS (1991) Circadian rhythms: from gene expression to behavior. Curr Opin Neurobiol 1:556–561. https://doi.org/10.1016/S0959-4388(05)80028-5
Tanamura D, Hirose E (2016) Population dynamics of the sea slug Plakobranchus ocellatus (Opisthobranch: Sacoglossa: Elysioidea) on a subtropical coral reef off Okinawa-Jima Island, Ryukyu Archipelago, Japan. Zool Stud 55:1–8. https://doi.org/10.6620/ZS.2016.55-43
Thimijan RW, Heins RD (1983) Photometric, radiometric, and quantum light units of measure: a review of procedures for interconversion. HortScience 18:818–822. https://doi.org/10.1002/rhc3.12091
Trowbridge CD (1994) Defensive responses and palatability of specialist herbivores—predation on NE Pacific ascoglossan gastropods. Mar Ecol Prog Ser 105:61–70. https://doi.org/10.3354/meps105061
Tunnell JWJ (2010) Distribución de los arrecifes. In: Tunnell JWJ, Chávez EA, Withers K (eds) Arrecifes coralinos del sur del Golfo de México. Centro Interdisciplinario de Ciencias Marinas IPN México, pp 17–29
van Tussenbroek BI, Hernández Arana HA, Rodríguez-Martínez RE, Espinoza-Avalos J, Canizales-Flores HM, González-Godoy CE, Barba-Santos MG, Vega-Zepeda A, Collado-Vides L (2017) Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar Pollut Bull 122:272–281. https://doi.org/10.1016/j.marpolbul.2017.06.057
Vieira S, Calado R, Coelho H, Serôdio J (2009) Effects of light exposure on the retention of kleptoplastic photosynthetic activity in the sacoglossan mollusc Elysia viridis. Mar Biol 156:1007–1020. https://doi.org/10.1007/s00227-009-1144-y
Vital XG, Rey F, Cartaxana P, Cruz S, Domingues MR, Calado R, Simões N (2021) Pigment and fatty acid heterogeneity in the sea slug Elysia crispata is not shaped by habitat depth. Animals 11:1–17. https://doi.org/10.3390/ani11113157
Wägele H, Martin WF (2014) Endosymbioses in sacoglossan seaslugs: Plastid-bearing animals that keep photosynthetic organelles without borrowing genes. In: Löffelhardt W (ed) Endosymbiosis. Springer-Verlag Wien, pp 291–324
Weaver S, Clark KB (1981) Light intensity and color preferences of five Ascoglossan (=Sacoglossan) Molluscs (Gastropoda: Opisthobranchia): a comparison of chloroplast-symbiotic and aposymbiotic species. Mar Behav Physiol 7:297–306
Wickham H, Chang W, Henry L, Pedersen TL, Takahashi K, Wilke C, Woo K, Yutani H, Dunnington D (2021) ggplot2. 1–292.
Willan RC (1979) The ecology of two New Zealand opisthobranch molluscs. University of Auckland
Withers K, Tunnell JWJ (2007) Reef biodiversity. Coral reefs of the southern Gulf of Mexico. Texas A&M University Press. Corpus Christi, College Station, pp 68–87
Zamora-Silva A, Ortigosa D (2012) Nuevos registros de opistobranquios en el Parque Nacional Sistema Arrecifal Veracruzano, México. Rev Mex Biodivers 83:359–369
Zar JH (2009) Biostatistical Analysis, 5th edn. Prentice-Hall/Pearson, Upper Saddle River
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210x.2009.00001.x
Acknowledgements
First author (Xochitl G. Vital) was given a PhD scholarship from Consejo Nacional de Ciencia y Tecnología (CVU: 564148), this publication is one of the requirements of the Doctoral Program of Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México. We are very thankful to Diana Ugalde, Lilian Palomino-Álvarez and Deneb Ortigosa who helped during field trips and data collection, and to Alberto Hernández and Gemma L. Martínez-Moreno from UMDI-F. Ciencias, UNAM who provided technical support. We acknowledge the help and enriching comments from the entire team of Biodiversidad Marina de Yucatán (BDMY). We thank Dorado Buceo, especially Manuel Victoria and Manuel Andrade (¡Muchas gracias Capi!), for helping and providing diving services in Veracruz and Puerto Morelos. We acknowledge Secretaría de Marina Armada de México for their support in transportation to Cayo Arcas. Parque Nacional Sistema Arrecifal Veracruzano and Parque Nacional Arrecife de Puerto Morelos are acknowledged for allowing the entrance to the parks. Antar Pérez-Botello assisted in data visualization. We thank the reviewers for their comments to improve the manuscript. This study was partially funded by the Harte Research Institute, the Harte Charitable Foundation and Texas A&M University. Sónia Cruz acknowledges funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 949880), and from Fundação para a Ciência e a Tecnologia, grants no. UIDB/50017/2020+UIDP/50017/2020 (CESAM) and 2020.03278 (CEECIND). This is a BDMY publication.
Funding
This study was partially funded by the Harte Research Institute, the Harte Charitable Foundation and Texas A&M University.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by XGV and MM. Funding for fieldwork and travel was procured by NS. The first draft of the manuscript was written by XGV and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is an observational study. The study was conducted according to all applicable international, national and/or institutional guidelines for the care and use of animals.
Additional information
Responsible Editor: R. Rosa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vital, X.G., Simões, N., Cruz, S. et al. Photosynthetic animals and where to find them: abundance and size of a solar-powered sea slug in different light conditions. Mar Biol 170, 154 (2023). https://doi.org/10.1007/s00227-023-04301-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04301-5