Abstract
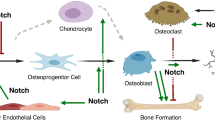
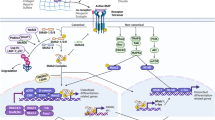
The skeleton originates from stem cells residing in the sclerotome and neural crest that undergo proliferation, migration, and commitment. The development of the skeletal stem cells is influenced by many signaling pathways that govern cell fate determination, proliferation, differentiation, and apoptosis. This review will focus on Notch signaling functions in regulating the different cell types that form the skeletal system as well as the interplay between them to maintain homeostasis. Osteochondroprogenitors require Notch signaling to maintain multipotency and to prevent premature differentiation into osteoblasts. Subsequently, overactivation of Notch signaling suppresses osteoblast maturation. Moreover, Notch signaling in osteochondroprogenitors is required for chondrocyte proliferation and hypertrophy and suppresses terminal differentiation. Translational studies demonstrated a crucial role of Notch signaling in osteosarcoma and osteoarthritis, where concepts derived from developmental pathways are often recapitulated. This brings hope of taking advantage of the molecular mechanisms learned from development to approach the pathological processes underlying abnormal bone/cartilage metabolism or tumorigenesis. Pharmacological agents that target Notch receptors or ligands in a tissue-specific fashion would offer new opportunities for treating bone/cartilage diseases caused by dysregulation of Notch signaling.


Similar content being viewed by others
References
Artavanis-Tsakonas S, Muskavitch MA (2010) Notch: the past, the present, and the future. Curr Top Dev Biol 92:1–29
Mumm JS, Kopan R (2000) Notch signaling: from the outside in. Dev Biol 228:151–165
Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Schroeter EH, Kisslinger JA, Kopan R (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393(6683):382–386
De Strooper B, Woodgett J (2003) Alzheimer’s disease: mental plaque removal. Nature 423:392–393
Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C (2003) Reconstitution of gamma-secretase activity. Nat Cell Biol 5:486–488
Honjo T (1996) The shortest path from the surface to the nucleus: RBP-J kappa/Su(H) transcription factor. Genes Cells 1:1–9
Zanotti S, Canalis E (2012) Notch regulation of bone development and remodeling and related skeletal disorders. Calcif Tissue Int 90:69–75
Engin F, Lee B (2010) NOTCHing the bone: insights into multi-functionality. Bone 46:274–280
Mukherjee T, Kim WS, Mandal L, Banerjee U (2011) Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science 332:1210–1213
Demehri S, Liu Z, Lee J, Lin MH, Crosby SD, Roberts CJ, Grigsby PW, Miner JH, Farr AG, Kopan R (2008) Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol 6:e123
Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V, Srivastava D (2011) Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat Cell Biol 13:1244–1251
Conlon RA, Reaume AG, Rossant J (1995) Notch1 is required for the coordinate segmentation of somites. Development 121:1533–1545
Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T (1995) Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development 121:3291–3301
Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S (1997) Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89:629–639
Kusumi K, Sun ES, Kerrebrock AW, Bronson RT, Chi DC, Bulotsky MS, Spencer JB, Birren BW, Frankel WN, Lander ES (1998) The mouse pudgy mutation disrupts Delta homologue Dll3 and initiation of early somite boundaries. Nat Genet 19:274–278
Hassed SJ, Wiley GB, Wang S, Lee JY, Li S, Xu W, Zhao ZJ, Mulvihill JJ, Robertson J, Warner J, Gaffney PM (2012) RBPJ mutations identified in two families affected by Adams–Oliver syndrome. Am J Hum Genet 91:391–395
Penton AL, Leonard LD, Spinner NB (2012) Notch signaling in human development and disease. Semin Cell Dev Biol 23:450–457
Narumi Y, Min BJ, Shimizu K, Kazukawa I, Sameshima K, Nakamura K, Kosho T, Rhee Y, Chung YS, Kim OH, Fukushima Y, Park WY, Nishimura G (2013) Clinical consequences in truncating mutations in exon 34 of NOTCH2: report of six patients with Hajdu–Cheney syndrome and a patient with serpentine fibula polycystic kidney syndrome. Am J Med Genet A 161:518–526
Isidor B, Lindenbaum P, Pichon O, Bezieau S, Dina C, Jacquemont S, Martin-Coignard D, Thauvin-Robinet C, Le Merrer M, Mandel JL, David A, Faivre L, Cormier-Daire V, Redon R, Le Caignec C (2011) Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat Genet 43:306–308
Majewski J, Schwartzentruber JA, Caqueret A, Patry L, Marcadier J, Fryns JP, Boycott KM, Ste-Marie LG, McKiernan FE, Marik I, Van Esch H, Michaud JL, Samuels ME (2011) Mutations in NOTCH2 in families with Hajdu–Cheney syndrome. Hum Mutat 32:1114–1117
Hsu YH, Kiel DP (2012) Genome-wide association studies of skeletal phenotypes: what we have learned and where we are headed. J Clin Endocrinol Metab 97:E1958–E1977. doi:10.1210/jc.2012-1890
Kung AW, Xiao SM, Cherny S, Li GH, Gao Y, Tso G, Lau KS, Luk KD, Liu JM, Cui B, Zhang MJ, Zhang ZL, He JW, Yue H, Xia WB, Luo LM, He SL, Kiel DP, Karasik D, Hsu YH, Cupples LA, Demissie S, Styrkarsdottir U, Halldorsson BV, Sigurdsson G, Thorsteinsdottir U, Stefansson K, Richards JB, Zhai G, Soranzo N, Valdes A, Spector TD, Sham PC (2010) Association of JAG1 with bone mineral density and osteoporotic fractures: a genome-wide association study and follow-up replication studies. Am J Hum Genet 86:229–239
Xiao SM, Kung AW, Gao Y, Lau KS, Ma A, Zhang ZL, Liu JM, Xia W, He JW, Zhao L, Nie M, Fu WZ, Zhang MJ, Sun J, Kwan JS, Tso GH, Dai ZJ, Cheung CL, Bow CH, Leung AY, Tan KC, Sham PC (2012) Post-genome wide association studies and functional analyses identify association of MPP7 gene variants with site-specific bone mineral density. Hum Mol Genet 21:1648–1657
Cole AG (2011) A review of diversity in the evolution and development of cartilage: the search for the origin of the chondrocyte. Eur Cell Mater 21:122–129
van der Kraan PM, van den Berg WB (2012) Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage 20:223–232
Wuelling M, Vortkamp A (2010) Transcriptional networks controlling chondrocyte proliferation and differentiation during endochondral ossification. Pediatr Nephrol 25:625–631
Miraoui H, Marie PJ (2010) Fibroblast growth factor receptor signaling crosstalk in skeletogenesis. Sci Signal 3(146):re9. doi:10.1126/scisignal.3146re9
Plaas A, Velasco J, Gorski DJ, Li J, Cole A, Christopherson K, Sandy JD (2011) The relationship between fibrogenic TGFbeta1 signaling in the joint and cartilage degradation in post-injury osteoarthritis. Osteoarthritis Cartilage 19:1081–1090
Baldridge D, Shchelochkov O, Kelley B, Lee B (2010) Signaling pathways in human skeletal dysplasias. Annu Rev Genomics Hum Genet 11:189–217
Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long F (2008) Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med 14:306–314
Kohn A, Dong Y, Mirando AJ, Jesse AM, Honjo T, Zuscik MJ, O’Keefe RJ, Hilton MJ (2012) Cartilage-specific RBPjkappa-dependent and -independent Notch signals regulate cartilage and bone development. Development 139:1198–1212
Shapiro IM, Adams CS, Freeman T, Srinivas V (2005) Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res C Embryo Today 75:330–339
Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, de Crombrugghe B (2005) Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA 102:14665–14670
Mead TJ, Yutzey KE (2009) Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc Natl Acad Sci USA 106:14420–14425
Dong Y, Jesse AM, Kohn A, Gunnell LM, Honjo T, Zuscik MJ, O’Keefe RJ, Hilton MJ (2010) RBPjkappa-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development 137:1461–1471
Zanotti S, Canalis E (2013) Notch suppresses nuclear factor of activated T cells (NFAT) transactivation and Nfatc1 expression in chondrocytes. Endocrinology 154:762–772
Song I, Kim JH, Kim K, Jin HM, Youn BU, Kim N (2009) Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett 583:2435–2440
Tu X, Chen J, Lim J, Karner CM, Lee SY, Heisig J, Wiese C, Surendran K, Kopan R, Gessler M, Long F (2012) Physiological notch signaling maintains bone homeostasis via RBPjk and Hey upstream of NFATc1. PLoS Genet 8:e1002577
Chen S, Tao J, Bae Y, Jiang MM, Bertin T, Chen Y, Yang T, Lee B (2013) Notch gain of function inhibits chondrocyte differentiation via Rbpj-dependent suppression of Sox9. J Bone Miner Res 28:649–659
Hosaka Y, Saito T, Sugita S, Hikata T, Kobayashi H, Fukai A, Taniguchi Y, Hirata M, Akiyama H, Chung UI, Kawaguchi H (2013) Notch signaling in chondrocytes modulates endochondral ossification and osteoarthritis development. Proc Natl Acad Sci USA 110:1875–1880
Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK (2009) Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther 11:R85
Mahjoub M, Sassi N, Driss M, Laadhar L, Allouche M, Hamdoun M, Romdhane KB, Sellami S, Makni S (2012) Expression patterns of Notch receptors and their ligands in human osteoarthritic and healthy articular cartilage. Tissue Cell 44:182–194
Sassi N, Laadhar L, Driss M, Kallel-Sellami M, Sellami S, Makni S (2011) The role of the Notch pathway in healthy and osteoarthritic articular cartilage: from experimental models to ex vivo studies. Arthritis Res Ther 13:208
Karlsson C, Brantsing C, Egell S, Lindahl A (2008) Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs 188:287–298
Tezuka K, Yasuda M, Watanabe N, Morimura N, Kuroda K, Miyatani S, Hozumi N (2002) Stimulation of osteoblastic cell differentiation by Notch. J Bone Miner Res 17:231–239
McLarren KW, Lo R, Grbavec D, Thirunavukkarasu K, Karsenty G, Stifani S (2000) The mammalian basic helix loop helix protein HES-1 binds to and modulates the transactivating function of the runt-related factor Cbfa1. J Biol Chem 275:530–538
Sciaudone M, Gazzerro E, Priest L, Delany AM, Canalis E (2003) Notch 1 impairs osteoblastic cell differentiation. Endocrinology 144:5631–5639
Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E (2006) Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/beta-catenin but not bone morphogenetic protein signaling. J Biol Chem 281:6203–6210
Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, Chen Y, Wang L, Zheng H, Sutton RE, Boyce BF, Lee B (2008) Dimorphic effects of Notch signaling in bone homeostasis. Nat Med 14:299–305
Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E (2008) Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology 149:3890–3899
Salie R, Kneissel M, Vukevic M, Zamurovic N, Kramer I, Evans G, Gerwin N, Mueller M, Kinzel B, Susa M (2010) Ubiquitous overexpression of Hey1 transcription factor leads to osteopenia and chondrocyte hypertrophy in bone. Bone 46:680–694
Zanotti S, Smerdel-Ramoya A, Canalis E (2011) HES1 (hairy and enhancer of split 1) is a determinant of bone mass. J Biol Chem 286:2648–2657
Canalis E, Parker K, Feng JQ, Zanotti S (2012) Osteoblast lineage-specific effects of notch activation in the skeleton. Endocrinology 154:623–634
Aarden EM, Burger EH, Nijweide PJ (1994) Function of osteocytes in bone. J Cell Biochem 55:287–299
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Xiong J, O’Brien CA (2012) Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res 27:499–505
Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 5:464–475
Tao J, Chen S, Yang T, Dawson B, Munivez E, Bertin T, Lee B (2010) Osteosclerosis owing to Notch gain of function is solely Rbpj-dependent. J Bone Miner Res 25:2175–2183
Teitelbaum SL (2007) Osteoclasts: what do they do and how do they do it? Am J Pathol 170:427–435
Yamada T, Yamazaki H, Yamane T, Yoshino M, Okuyama H, Tsuneto M, Kurino T, Hayashi S, Sakano S (2003) Regulation of osteoclast development by Notch signaling directed to osteoclast precursors and through stromal cells. Blood 101:2227–2234
Bai S, Kopan R, Zou W, Hilton MJ, Ong CT, Long F, Ross FP, Teitelbaum SL (2008) NOTCH1 regulates osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblast lineage cells. J Biol Chem 283:6509–6518
Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K (2008) The association of Notch2 and NF-kappaB accelerates RANKL-induced osteoclastogenesis. Mol Cell Biol 28:6402–6412
Sethi N, Dai X, Winter CG, Kang Y (2011) Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19:192–205
Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D (1996) Organ-specific disease provoked by systemic autoimmunity. Cell 87:811–822
Sekine C, Koyanagi A, Koyama N, Hozumi K, Chiba S, Yagita H (2012) Differential regulation of osteoclastogenesis by Notch2/Delta-like 1 and Notch1/Jagged1 axes. Arthritis Res Ther 14:R45
Visnjic D, Kalajzic I, Gronowicz G, Aguila HL, Clark SH, Lichtler AC, Rowe DW (2001) Conditional ablation of the osteoblast lineage in Col2.3deltatk transgenic mice. J Bone Miner Res 16:2222–2231
Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL (2004) Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103:3258–3264
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846
Mancini SJ, Mantei N, Dumortier A, Suter U, MacDonald HR, Radtke F (2005) Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood 105:2340–2342
Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, Pear WS (2008) Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell 2:356–366
Varnum-Finney B, Halasz LM, Sun M, Gridley T, Radtke F, Bernstein ID (2011) Notch2 governs the rate of generation of mouse long- and short-term repopulating stem cells. J Clin Invest 121:1207–1216
Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar RN, Shapiro CL, Shields A, Smith MR, Srinivas S, Van Poznak CH (2009) NCCN Task Force Report: Bone health in cancer care. J Natl Compr Canc Netw 7 Suppl 3:S1–32 quiz S33–S35
Tanaka M, Setoguchi T, Hirotsu M, Gao H, Sasaki H, Matsunoshita Y, Komiya S (2009) Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br J Cancer 100:1957–1965
Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE, Donehower LA, Lee B (2009) Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum Mol Genet 18:1464–1470
Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T (2004) Mutant p53 gain of function in two mouse models of Li–Fraumeni syndrome. Cell 119:847–860
Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, El-Naggar AK, Lozano G (2004) Gain of function of a p53 hot spot mutation in a mouse model of Li–Fraumeni syndrome. Cell 119:861–872
Hughes DP (2009) How the NOTCH pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat Res 152:479–496
Zhang P, Yang Y, Zweidler-McKay PA, Hughes DP (2008) Critical role of notch signaling in osteosarcoma invasion and metastasis. Clin Cancer Res 14:2962–2969
Bae Y, Yang T, Zeng HC, Campeau PM, Chen Y, Bertin T, Dawson BC, Munivez E, Tao J, Lee BH (2012) miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet 21:2991–3000
He C, Xiong J, Xu X, Lu W, Liu L, Xiao D, Wang D (2009) Functional elucidation of MiR-34 in osteosarcoma cells and primary tumor samples. Biochem Biophys Res Commun 388:35–40
Zhang P, Yang Y, Nolo R, Zweidler-McKay PA, Hughes DP (2010) Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex 1 and its role in osteosarcoma invasiveness. Oncogene 29:2916–2926
Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW (2010) Therapeutic antibody targeting of individual Notch receptors. Nature 464:1052–1057
Aste-Amezaga M, Zhang N, Lineberger JE, Arnold BA, Toner TJ, Gu M, Huang L, Vitelli S, Vo KT, Haytko P, Zhao JZ, Baleydier F, L’Heureux S, Wang H, Gordon WR, Thoryk E, Andrawes MB, Tiyanont K, Stegmaier K, Roti G, Ross KN, Franlin LL, Wang F, Chastain M, Bett AJ, Audoly LP, Aster JC, Blacklow SC, Huber HE (2010) Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PLoS One 5:e9094
Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, Radojcic V, Friedman A, Gridley T, Shelton A, Reddy P, Samuelson LC, Yan M, Siebel CW, Maillard I (2013) Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest 123:1590–1604
Acknowledgements
The authors thank Terry Bertin for critical reading and editing of the manuscript. Some of the work was supported by National Institutes of Health grants DE016990 (to B. H. L.) and HD022657 (to B. H. L.) and Cancer Prevention Institute of Texas grant RP 101017 (to B. H. L.).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, S., Lee, B.H. & Bae, Y. Notch Signaling in Skeletal Stem Cells. Calcif Tissue Int 94, 68–77 (2014). https://doi.org/10.1007/s00223-013-9773-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9773-z