Abstract
During endochondral ossification bones are formed as cartilage templates in which chondrocytes proliferate, differentiate into hypertrophic chondrocytes and are gradually replaced by bone. Postnatally, remnants of embryonic chondrocytes remain in a restricted domain between the ossified regions of the bones forming the growth plate. The coordinated proliferation and differentiation of chondrocytes ensures the continuous elongation of the epiphyseal growth plates. The sequential changes between proliferation and differentiation are tightly regulated by secreted growth factors, which activate chondrocyte-specific transcription factors. Transcription factors that play critical roles in regulating cell-type-specific gene expression include Sox9, Gli2/3, and Runx2. The interaction of these transcription factors with general transcriptional regulators like histone-modifying enzymes provides an additional level of regulation to fine-tune the expression of target genes in different chondrocyte populations. This review will outline recent advances in the analysis of the complex transcriptional network that regulates the distinct steps of chondrocyte differentiation.



Similar content being viewed by others
References
Long F, Zhang XM, Karp S, Yang Y, McMahon AP (2001) Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 128:5099–5108
Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM (2002) PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 129:2977–2986
MacLean HE, Kronenberg HM (2005) Localization of Indian hedgehog and PTH/PTHrP receptor expression in relation to chondrocyte proliferation during mouse bone development. Dev Growth Differ 47:59–63
Hall BK, Miyake T (1995) Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol 39:881–893
Hall BK, Miyake T (2000) All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22:138–147
Goldring MB, Tsuchimochi K, Ijiri K (2006) The control of chondrogenesis. J Cell Biochem 97:33–44
Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ (1996) Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273:613–622
St-Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13:2072–2086
Pan Y, Wang C, Wang B (2009) Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol 326:177–189
Pan Y, Wang B (2007) A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome. J Biol Chem 282:10846–10852
Minina E, Schneider S, Rosowski M, Lauster R, Vortkamp A (2005) Expression of Fgf and Tgfbeta signaling related genes during embryonic endochondral ossification. Gene Expr Patterns 6:102–109
Goumans MJ, Mummery C (2000) Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44:253–265
Yoon BS, Lyons KM (2004) Multiple functions of BMPs in chondrogenesis. J Cell Biochem 93:93–103
Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM (2005) Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci U S A 102:5062–5067
Yoon BS, Pogue R, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM (2006) BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development 133:4667–4678
Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A (2002) Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell 3:439–449
Sahni M, Raz R, Coffin JD, Levy D, Basilico C (2001) STAT1 mediates the increased apoptosis and reduced chondrocyte proliferation in mice overexpressing FGF2. Development 128:2119–2129
Murakami S, Balmes G, McKinney S, Zhang Z, Givol D, de Crombrugghe B (2004) Constitutive activation of MEK1 in chondrocytes causes Stat1-independent achondroplasia-like dwarfism and rescues the Fgfr3-deficient mouse phenotype. Genes Dev 18:290–305
Yamaguchi TP, Rossant J (1995) Fibroblast growth factors in mammalian development. Curr Opin Genet Dev 5:485–491
Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C (2005) Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8:727–738
Reinhold MI, Kapadia RM, Liao Z, Naski MC (2006) The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J Biol Chem 281:1381–1388
Guo X, Mak KK, Taketo MM, Yang Y (2009) The Wnt/beta-catenin pathway interacts differentially with PTHrP signaling to control chondrocyte hypertrophy and final maturation. PLoS One 4:e6067
Hartmann C (2007) Skeletal development—Wnts are in control. Mol Cells 24:177–184
Yang Y, Topol L, Lee H, Wu J (2003) Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 130:1003–1015
Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B (1999) Sox9 is required for cartilage formation. Nat Genet 22:85–89
Koziel L, Wuelling M, Schneider S, Vortkamp A (2005) Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development 132:5249–5260
Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, Kronenberg HM (2005) Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J Clin Invest 115:1734–1742
Wuelling M, Kaiser FJ, Buelens LA, Braunholz D, Shivdasani RA, Depping R, Vortkamp A (2009) Trps1, a regulator of chondrocyte proliferation and differentiation, interacts with the activator form of Gli3. Dev Biol 328:40–53
Suemoto H, Muragaki Y, Nishioka K, Sato M, Ooshima A, Itoh S, Hatamura I, Ozaki M, Braun A, Gustafsson E, Fassler R (2007) Trps1 regulates proliferation and apoptosis of chondrocytes through Stat3 signaling. Dev Biol 312:572–581
Napierala D, Sam K, Morello R, Zheng Q, Munivez E, Shivdasani RA, Lee B (2008) Uncoupling of chondrocyte differentiation and perichondrial mineralization underlies the skeletal dysplasia in tricho-rhino-phalangeal syndrome. Hum Mol Genet 17:2244–2254
LuValle P, Beier F (2000) Cell cycle control in growth plate chondrocytes. Front Biosci 5:D493–D503
Sherr CJ, Roberts JM (2004) Living with or without cyclins and cyclin-dependent kinases. Genes Dev 18:2699–2711
Rossi F, MacLean HE, Yuan W, Francis RO, Semenova E, Lin CS, Kronenberg HM, Cobrinik D (2002) p107 and p130 coordinately regulate proliferation, Cbfa1 expression, and hypertrophic differentiation during endochondral bone development. Dev Biol 247:271–285
Scheijen B, Bronk M, van der Meer T, Bernards R (2003) Constitutive E2F1 overexpression delays endochondral bone formation by inhibiting chondrocyte differentiation. Mol Cell Biol 23:3656–3668
Beier F, Ali Z, Mok D, Taylor AC, Leask T, Albanese C, Pestell RG, LuValle P (2001) TGFbeta and PTHrP control chondrocyte proliferation by activating cyclin D1 expression. Mol Biol Cell 12:3852–3863
Nakajima M, Negishi Y, Tanaka H, Kawashima K (2004) p21(Cip-1/SDI-1/WAF-1) expression via the mitogen-activated protein kinase signaling pathway in insulin-induced chondrogenic differentiation of ATDC5 cells. Biochem Biophys Res Commun 320:1069–1075
Mau E, Whetstone H, Yu C, Hopyan S, Wunder JS, Alman BA (2007) PTHrP regulates growth plate chondrocyte differentiation and proliferation in a Gli3 dependent manner utilizing hedgehog ligand dependent and independent mechanisms. Dev Biol 305:28–39
Hilton MJ, Tu X, Cook J, Hu H, Long F (2005) Ihh controls cartilage development by antagonizing Gli3, but requires additional effectors to regulate osteoblast and vascular development. Development 132:4339–4351
Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, Rosenblum ND (2006) GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development 133:569–578
Provot S, Nachtrab G, Paruch J, Chen AP, Silva A, Kronenberg HM (2008) A-raf and B-raf are dispensable for normal endochondral bone development, and parathyroid hormone-related peptide suppresses extracellular signal-regulated kinase activation in hypertrophic chondrocytes. Mol Cell Biol 28:344–357
Long F, Schipani E, Asahara H, Kronenberg H, Montminy M (2001) The CREB family of activators is required for endochondral bone development. Development 128:541–550
Provot S, Schipani E (2005) Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun 328:658–665
Reimold AM, Grusby MJ, Kosaras B, Fries JW, Mori R, Maniwa S, Clauss IM, Collins T, Sidman RL, Glimcher MJ, Glimcher LH (1996) Chondrodysplasia and neurological abnormalities in ATF-2-deficient mice. Nature 379:262–265
Beier F, LuValle P (2002) The cyclin D1 and cyclin A genes are targets of activated PTH/PTHrP receptors in Jansen's metaphyseal chondrodysplasia. Mol Endocrinol 16:2163–2173
Retting KN, Song B, Yoon BS, Lyons KM (2009) BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136:1093–1104
Dailey L, Laplantine E, Priore R, Basilico C (2003) A network of transcriptional and signaling events is activated by FGF to induce chondrocyte growth arrest and differentiation. J Cell Biol 161:1053–1066
Dailey L, Ambrosetti D, Mansukhani A, Basilico C (2005) Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 16:233–247
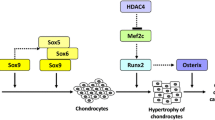
Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN (2007) MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell 12:377–389
James CG, Appleton CT, Ulici V, Underhill TM, Beier F (2005) Microarray analyses of gene expression during chondrocyte differentiation identifies novel regulators of hypertrophy. Mol Biol Cell 16:5316–5333
Zhang S, Xiao Z, Luo J, He N, Mahlios J, Quarles LD (2009) Dose-dependent effects of Runx2 on bone development. J Bone Miner Res 24:1889–1904
Yamashita S, Andoh M, Ueno-Kudoh H, Sato T, Miyaki S, Asahara H (2009) Sox9 directly promotes Bapx1 gene expression to repress Runx2 in chondrocytes. Exp Cell Res 315:2231–2240
Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B (2002) The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16:2813–2828
Huh YH, Ryu JH, Chun JS (2007) Regulation of type II collagen expression by histone deacetylase in articular chondrocytes. J Biol Chem 282:17123–17131
Di Renzo F, Cappelletti G, Broccia ML, Giavini E, Menegola E (2008) The inhibition of embryonic histone deacetylases as the possible mechanism accounting for axial skeletal malformations induced by sodium salicylate. Toxicol Sci 104:397–404
Westendorf JJ (2007) Histone deacetylases in control of skeletogenesis. J Cell Biochem 102:332–340
Furumatsu T, Tsuda M, Yoshida K, Taniguchi N, Ito T, Hashimoto M, Ito T, Asahara H (2005) Sox9 and p300 cooperatively regulate chromatin-mediated transcription. J Biol Chem 280:35203–35208
Furumatsu T, Ozaki T, Asahara H (2009) Smad3 activates the Sox9-dependent transcription on chromatin. Int J Biochem Cell Biol 41:1198–1204
Hong S, Derfoul A, Pereira-Mouries L, Hall DJ (2009) A novel domain in histone deacetylase 1 and 2 mediates repression of cartilage-specific genes in human chondrocytes. FASEB J 23:3539–3552
Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN (2004) Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119:555–566
Jensen ED, Nair AK, Westendorf JJ (2007) Histone deacetylase co-repressor complex control of Runx2 and bone formation. Crit Rev Eukaryot Gene Expr 17:187–196
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wuelling, M., Vortkamp, A. Transcriptional networks controlling chondrocyte proliferation and differentiation during endochondral ossification. Pediatr Nephrol 25, 625–631 (2010). https://doi.org/10.1007/s00467-009-1368-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-009-1368-6