Abstract
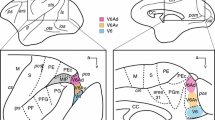
In four squirrel monkeys (Saimiri sciureus), the tracer biotin dextranamine (BDA) was injected into the ventrolateral pons at a site at which injection of the glutamate antagonist kynurenic acid blocked vocalization electrically elicited from the periaqueductal gray (PAG). Anterograde projections could be traced into all cranial motor and sensory nuclei involved in phonation, that is, the nucleus ambiguus, facial, hypoglossal and trigeminal motor nuclei, the motorneuron column in the ventral gray substance innervating the extrinsic laryngeal muscles, the nucleus retroambiguus, solitary tract and spinal trigeminal nuclei. Projections were also found into a number of auditory nuclei, namely the nucleus cochlearis-complex, superior olive, ventral and dorsal nuclei of the lateral lemniscus and inferior colliculus. Furthermore, there were projections into the reticular formation of the lateral and dorsocaudal medulla and lateral pons, into nucleus gracilis, inferior and medial vestibular nuclei, lateral reticular nucleus, ventral raphe, pontine gray, superior colliculus, PAG and mediodorsal thalamic nucleus. Injection of the tracer wheat germ agglutinin-conjugated horseradish peroxidase into the ventrolateral pontine vocalization-blocking area in one animal yielded retrograde labeling throughout the PAG. Injection of BDA into a vocalization-eliciting site of the PAG in another animal yielded projections into the ventrolateral pontine vocalization-blocking area. It is concluded that the ventral paralemniscal area in the ventrolateral pons represents a relay station of the descending periaqueductal vocalization-controlling pathway.







Similar content being viewed by others
Abbreviations
- Ab:
-
Nucl. ambiguus
- APH:
-
Area posterior hypothalami
- Apr:
-
Area praerubralis
- Apt:
-
Area praetectalis
- AS:
-
Aquaeductus Sylvii
- BC:
-
Brachium conjunctivum
- BM:
-
Nucl. basalis (Meynert)
- BP:
-
Brachium pontis
- Cb:
-
Cerebellum
- CC:
-
Corpus callosum
- Cc:
-
Canalis centralis
- Cd:
-
Nucl. caudatus
- CeI:
-
Nucl. centralis inferior th.
- CeL:
-
Nucl. centralis lateralis th.
- CeM:
-
Nucl. centralis medialis th.
- CI:
-
Capsula interna
- CM:
-
Centrum medianum
- Cn:
-
Nucl. cuneatus
- CnL:
-
Nucl. cuneatus lateralis
- Co:
-
Nucl. cochlearis
- CoI:
-
Colliculus inferior
- CoS:
-
Colliculus superior
- CP:
-
Commissura posterior
- CPf:
-
Cortex piriformis
- CRf:
-
Corpus restiforme
- CS:
-
Nucl. centralis superior th.
- CSi:
-
Colliculus superior, stratum intermediale
- CSL:
-
Nucl. centralis superior lateralis
- CSp:
-
Colliculus superior, stratum profundum
- CSs:
-
Colliculus superior, stratum superficiale
- CT:
-
Corpus trapezoideum
- DBC:
-
Decussation of brachium conjunctivum
- DG:
-
Nucl. dorsalis tegmenti
- DLM:
-
Decussation of lemniscus medialis
- DPy:
-
Decussation of pyramidal tract
- DR:
-
Dorsal raphe
- Dv:
-
Nucl. dorsalis n. vagi
- Ep:
-
Epiphysis
- FC:
-
Funiculus cuneatus
- FL:
-
Funiculus lateralis
- FLM:
-
Fasciculus longitudinalis medialis
- FRM:
-
Formatio reticularis myelencephali
- FRPc:
-
Formatio reticularis pontis caudalis
- FRPo:
-
Formatio reticularis pontis oralis
- FRTM:
-
Formatio reticularis tegmenti mesencephali
- FV:
-
Funiculus ventralis
- G:
-
Nucl. gracilis
- GL:
-
Geniculatum laterale
- GM:
-
Geniculatum mediale
- GP:
-
Globus pallidus
- GPH:
-
Griseum periventriculare hypothalami
- GPM:
-
Griseum periventriculare mesencephali
- Gpo:
-
Griseum pontis
- Hipp:
-
Hippocampus
- HL:
-
Habenula lateralis
- II:
-
Nervus opticus
- III:
-
Nervus oculomotorius
- IP:
-
Nucl. interpeduncularis
- IV:
-
Nervus trochlearis
- LC:
-
Locus coeruleus
- LD:
-
Nucl. lateralis dorsalis th.
- Lim:
-
Nucl. limitans
- LLd:
-
Nucl. dorsalis lemnisci lateralis
- LLv:
-
Nucl. ventralis lemnisci lateralis
- LM:
-
Lemniscus medialis
- LP:
-
Nucl. lateralis posterior th.
- MD:
-
Nucl. medialis dorsalis th.
- Mv :
-
Nucl. motorius n. trigemini
- NCI:
-
Colliculus inferior, subnucleus centralis
- NCS:
-
Nucl. centralis superior (Bechterew)
- NCT:
-
Nucl. trapezoidalis
- Niv :
-
Nucl. trochlearis
- NiiiC:
-
Nucl. oculomotorius, pars centralis
- NiiiD:
-
Nucl. oculomotorius, pars dorsalis
- Nucl. iiiV:
-
Nucl. oculomotorius, pars ventralis
- NiiiW:
-
Nucl. Edinger–Westphal
- NR:
-
Nucl. ruber
- NSv :
-
Nucl. spinalis n. trigemini
- NTS:
-
Nucl. solitarius
- OI:
-
Oliva inferior
- OS:
-
Oliva superior
- P:
-
Nucl. posterior th.
- PAG:
-
Periaqueductal gray
- PbL:
-
Nucl. parabrachialis lateralis
- PbM:
-
Nucl. parabrachialis medialis
- PC:
-
Pedunculus cerebri
- Pd:
-
Nucl. peripeduncularis
- Pf:
-
Nucl. parafascicularis
- Pg:
-
Nucl. parabigeminalis
- Pp:
-
Nucl. praepositus
- PuI:
-
Nucl. pulvinaris inferior
- PuM:
-
Nucl. pulvinaris medialis
- Put:
-
Putamen
- Pv:
-
Nucl. principalis n. trigemini
- Py:
-
Tractus pyramidalis
- RAb:
-
Nucl. retroambiguus
- RL:
-
Nucl. reticularis lateralis
- RTP:
-
Nucl. reticularis tegmenti pontis
- Sf:
-
Nucl. subfascicularis th.
- SGD:
-
Substantia grisea dorsalis
- SGV:
-
Substantia grisea ventralis
- SN:
-
Substantia nigra
- ST:
-
Stria terminalis
- St:
-
Subthalamus
- TRM:
-
Tractus retroflexus (Meynert)
- TSc:
-
Tractus spinocerebellaris
- Ves:
-
Vestibular complex
- VesI:
-
Nucl. vestibularis inferior
- VesM:
-
Nucl. vestibularis medialis
- VI:
-
Nucl. and Nervus abducens
- VII:
-
Nucl. and Nervus facialis
- Vii :
-
Lateral ventricle
- Viii :
-
Third ventricle
- Viv :
-
Fourth ventricle
- VPI:
-
Nucl. ventralis posterior inferior th.
- VPL:
-
Nucl. ventralis posterior lateralis th.
- VPM:
-
Nucl. ventralis posterior medialis th.
- VPMp:
-
Nucl. ventralis posterior medialis th., pars parvocellularis
- VR:
-
Ventral raphe; XII, Nucl. hypoglossus
- ZI:
-
Zona incerta
References
Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR (1989) Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283:248–268
Behrend O, Schuller G (2000) The central acoustic tract and audiovocal coupling in the horseshoe bat, Rhinolophus rouxi. Eur J Neurosci 12:4268–4280
Brandt HM, Apkarian AV (1992) Biotin-dextran. A sensitive anterograde tracer for neuroanatomic studies in rat and monkey. J Neurosci Meth 45:35–40
Chandler SH, Goldberg LJ (1988) Effects of pontomedullary reticular formation stimulation on the neuronal networks responsible for rhythmical jaw movements in the guinea pig. J Neurophysiol 59:819–832
Dressnandt J, Jürgens U (1992) Brain stimulation-induced changes of phonation in the squirrel monkey. Exp Brain Res 89:549–559
Emmers R, Akert K (1963) A stereotaxic atlas of the brain of the squirrel monkey (Saimiri sciureus). University of Wisconsin Press, Madison
Gergen JA, MacLean PD (1962) A stereotaxic atlas of the squirrel monkey’s brain (Saimiri sciureus), Public Health Service Publication, No. 933, Bethesda/Maryland
Hayakawa T, Takanaga A, Maeda S, Seki M, Yajima Y (2001) Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: a study using transganglionic transport of cholera toxin. Neurosci Res 39:221–232
Holstege G (1989) Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol 284:242–252
Jürgens U (1979) Vocalization as an emotional indicator. A neuroethological study in the squirrel monkey. Behaviour 69:88–117
Jürgens U (2000) Localization of a pontine vocalization-controlling area. J Acoust Soc Am 108:1393–1396
Jürgens U (2002) Neural pathways underlying vocal control. Neurosci Biobehav Rev 26:235–258
Jürgens U, Ploog D (1970) Cerebral representation of vocalization in the squirrel monkey. Exp Brain Res 10:532–554
Jürgens U, Pratt R (1979) Role of the periaqueductal grey in vocal expression of emotion. Brain Res 167:367–378
Jürgens U, Richter K (1986) Glutamate-induced vocalization in the squirrel monkey. Brain Res 373:349–358
Jürgens U, Schriever S (1991) Respiratory muscle activity during vocalization in the squirrel monkey. Folia Primatol 56:121–132
Kalia M, Mesulam M-M (1980) Brain stem projections of sensory and motor components of the vagus complex in the cat. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol 193:467–508
Kanai T, Wang SC (1962) Localization of the central vocalization mechanism in the brainstem of the cat. Exp Neurol 6:426–434
Kirzinger A, Jürgens U (1985) The effects of brain stem lesions on vocalization in the squirrel monkey. Brain Res 358:150–162
Kirzinger A, Jürgens U (1991) Vocalization-correlated single-unit activity in the brain stem of the squirrel monkey. Exp Brain Res 84:545–560
Kirzinger A, Jürgens U (1994) Motoneuronal location of external laryngeal and hyoid muscles involved in primate phonation. J Brain Res 35:559–565
Larson CR, Sutton D, Lindeman RC (1978) Cerebellar regulation of phonation in rhesus monkey (Macaca mulatta). Exp Brain Res 33:1–18
Lechtenberg R, Gilman S (1978) Speech disorders in cerebellar disease. Ann Neurol 3:285–290
MacLean PD (1967) A chronically fixed stereotaxic device for intracerebral exploration with macro- and micro-electrodes. Electroenceph Clin Neurophysiol 22:180–182
Magoun HW, Atlas D, Ingersoll EH, Ranson SW (1937) Associated facial, vocal and respiratory components of emotional expression: an experimental study. J Neurol Psychopath 17:241–255
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Metzner W (1993) An audio-vocal interface in echolocating horseshoe bats. J Neurosci 13:1899–1915
Metzner W(1996) Anatomical basis for audio-vocal integration in echolocating horseshoe bats. J Comp Neurol 368:252–269
Mori S, Nishimura H, Kurakami C, Yamamura T, Aoki M (1978) Controlled locomotion in the mesencephalic cat: distribution of facilitatory and inhibitory regions within pontine tegmentum. J Neurophysiol 41:1580–1591
Pieper F, Jürgens U (2003) Neuronal activity in the inferior colliculus and bordering structures during vocalization in the squirrel monkey. Brain Res 979:153–164
Ploog D (1995) Mutuality and dialogue in nonhuman primate communication. In: Markova I, Graumann CF, Foppa K (eds) Mutualities in dialogue, Cambridge University Press, Cambridge, pp 27–57
Richter K, Jürgens U (1986) A comparative study on the elicitability of vocalization by electrical brain stimulation, glutamate, aspartate and quisqualate in the squirrel monkey. Neurosci Lett 66:239–244
Sakamoto T, Wada K, Yamanaka Y, Shiba K, Nakajima Y (1993) Effects of microinjection of a GABA antagonist into the periaqueductal gray upon electrically-induced vocalization in decerebrate cats. Neurosci Res 18:235–240
Schuller G, Radtke-Schuller S (1990) Neural control of vocalization in bats: mapping of brainstem areas with electrical microstimulation eliciting species-specific echolocation calls in the rufous horseshoe bat. Exp Brain Res 79:192–206
Schuller G, Fischer S, Schweizer H (1997) Significance of the paralemniscal tegmental area for audio-motor control in the mustached bat, Pteronotus parnellii: The afferent and efferent connections of the paralemniscal area. Eur J Neurosci 9:342–355
Sessle BJ, Ball GJ, Lucier GE (1981) Suppressive influences from periaqueductal gray and nucleus raphe magnus on respiration and related reflex activities and on solitary tract neurons, and effect of naloxone. Brain Res 216:145–161
Shiba K, Umezaki T, Zheng Y, Miller AD (1997) The nucleus retroambigualis controls laryngeal muscle activity during vocalization in the cat. Exp Brain Res 115:513–519
Siebert S, Jürgens U (2003) Vocalization after periaqueductal grey inactivation with the GABA agonist muscimol in the squirrel monkey. Neuroci Lett 340:111–114
Thoms G, Jürgens U (1987) Common input of the cranial motor nuclei involved in phonation in squirrel monkey. Exp Neurol 95:85–99
Todd NPM, Merker B (2004) Siamang gibbons exceed the saccular threshold: intensity of the song of Hylobates syndactilus. J Acoust Soc Am 115:3077–3080
Ueyama T, Satoda T, Tashiro T, Sugimoto T, Matsushima R, Mizuno N (1990) Infrahyoid and accessory motoneurons in the Japanese monkey (Macaca fuscata). J Comp Neurol 291:373–382
Veenman CL, Reiner A, Honig MG (1992) Biotinylated dextranamine as an anterograde tracer for single-labeling and double-labeling studies. J Neurosci Meth 41:239–254
Winter P, Ploog D, Latta J (1966) Vocal repertoire of the squirrel monkey, its analysis and significance. Exp Brain Res 1:359–384
Zhang SP, Bandler R, Davis PJ (1995) Brain stem integration of vocalization: role of the nucleus retroambigualis. J Neurophysiol 74:2500–2512
Zhang SP, Davis PJ, Carrive P, Bandler R (1992) Vocalization and marked pressor effect evoked from the region of the nucleus retroambigualis in the caudal ventrolateral medulla of the cat. Neurosci Lett 140:103–107
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Schmidt
Rights and permissions
About this article
Cite this article
Hannig, S., Jürgens, U. Projections of the ventrolateral pontine vocalization area in the squirrel monkey. Exp Brain Res 169, 92–105 (2006). https://doi.org/10.1007/s00221-005-0128-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0128-5