Abstract
The oil amounts of raw and roasted mustard seeds were reported between 12.05 (control) and 16.30% (oven). Total phenolic and flavonoid amounts of untreated (control) and roasted mustard seeds were recorded to be between 473.90 (control) and 569.96 mgGAE/100 g (oven) to 345.48 (control) and 479.76 mg/100 g (microwave), respectively. Antioxidant capacity results of untreated and roasted mustard seeds were determined to be between 6.71 (oven) and 6.97 mmol/kg (control). Total phenolic and flavonoid amounts of oven-roasted seeds were higher than those of control and microwave-roasted seeds. L* values of mustard seeds varied between 35.04 and 65.52. Heat treatment caused a decrease in L* values. The lowest L* value was observed in the sample dried in conventional oven. The quantitative values of gallic and 3,4-dihydroxybenzoic acids of mustard seeds were recorded to be between 8.47 (control) and 117.31 mg/100 g (microwave) to 2.16 (control) and 11.79 mg/100 g (microwave), respectively. Erucic acid values of the oils extracted from raw and roasted mustard seeds were reported between 41.38 (control) and 42.81% (microwave). Oleic and linoleic acid amounts of mustard oils differed between 26.06 (microwave) and 26.90% (control) and between 13.08 (oven) and 13.98% (control), respectively. The oven roasting system had the most effect on the element and protein contents of the seeds, followed by the microwave in decreasing order. As with the bioactive components of the seeds, the amounts of phenolic constituents of roasted mustard seeds increased significantly compared to the control.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White mustard (Sinapis alba L.), also known as yellow or light mustard, is an annual oilseed plant with great economic value due to its drought tolerance and resistance to plant diseases. Mustard is a herbaceous plant with yellow flowers that belongs to the cruciferous family and whose seeds are used as spices. Among the mustard varieties, three of them are known as spices and are widely used. The known and widely used mustards are white, black, and red mustard [1,2,3]. Its seed is also used directly as a condiment, spice, and salad dressing oil [2]. There are major and alternative oil plants used for different purposes today, among the oil plants that have high nutritional value, high oil quality and have been modified to adapt to environmental conditions [3]. Since additional studies have shown that mustard seed oil may contain high levels of erucic acid, mustard seed oil should not be consumed as food [4,5,6]. On the other hand, many researchers have begun to work on the breeding of varieties with low or zero erucic acid content. White mustard (Sinapsis alba L.) seeds, known as one of the oldest condiments, have a high oil content of up to 47%, rich in erucic acid, and the fatty acid profile of each mustard variety is different [7, 8]. The oil of mustard seed is widely used in cooking, mostly in Asian countries, due to its spicy and bitter taste, and its seeds contain various phenolic compounds [9]. The cake of mustard seed remaining after oil extraction is rich in phenolic compounds, and it may be possible to recover these compounds considered as nutraceuticals and food ingredients with potential applications [9, 10]. Roasting is the most important process causing substantial physical, chemical, structural and sensory changes in seed processes. Also, roasting has a controversial impact on the stability of the seeds that were defaced faster than fresh seeds. Roasting treatment disrupts the integrity of the cellular components and breaks the covalent bonds between the phenolic compounds and cell wall thereby promote the release of bound phenolic and flavonoid compounds [11]. Roasting treatment is the most common thermal treatment applied to oil seeds prior to oil extraction and approved to enhance the yield and nutritional quality oil [12]. Roasting can alter the texture, color, flavor, and appearance of the grains, whereas the resulting product develops unique features of crispness and taste as compared to the raw kernels [13]. In Europe, white mustard is generally grown as seed or green manure and mustard seeds are often used as a spice plant. Also, its leaves and seeds can also be used in the preparation of various dishes [7, 14]. Since the oil extracted from mustard seeds contains a high amount of erucic acid (approximately 40–45%) which has a potentially harmful effect on the human body, mustard varieties with reduced erucic acid content (such as Bamberka and Warta) began to be grown in Poland in 2006. These varieties have begun to be consumed safely due to their lower erucic acid and glucosinolate contents than other traditional varieties [15, 16]. Phenolic compounds, which are thought to be associated with antioxidant activity by contributing to various biological properties, have accumulated as secondary metabolites in different parts of plants [17]. Roasting, which causes significant physico-chemical and sensory changes in the processing of food products, causes many thermal and chemical reactions that increase the overall quality of the food product [8]. The aim of the present study was to investigate the effects of conventional oven and microwave roasting systems on color values, total phenols, flavonoids, antioxidant capacity, fatty acids, phenolic compounds, and biogenic element results in white mustard seeds and oils.
Materials and methods
Materials
The seeds of white mustard (Sinapis alba L.) were purchased from a market in Ankara district in Turkey in 2023. The foreign substances in the seeds were removed before analytical procedures.
Methods
Heat treatment
Mustard seeds were spread homogeneously on both oven and microwave Teflon trays with a thickness of 3 mm, and the seeds were roasted in an oven and in a microwave oven at 220 °C for 10 min and at 900 W for 15 min, respectively. After the roasted seeds were cooled in the desiccator, the seeds were ground using a grinder before analysis.
Moisture content
The sample amount used for moisture determination of each sample was 0.450 g. The moisture amounts of white mustard seeds were recorded by the infrared moisture analyzer (KERN & SOHN GmbH; 60 g/200 °C) [19].
Determination of protein contents of mustard samples
Protein results of the seeds were determined according to the report pointed out in AOAC method [19].
Color values
Minolta Chroma meter CR 400 (Konica Minolta, Inc. Osaka, Japan) was used to determine the color values of mustard seeds. After the device was calibrated according to the white surface calibration plate before the device measurement, L*, a* and b* values were determined according to the CIELab color scale [20].
Extraction process
Extraction of mustard seed samples was carried out with partial modifications according to the report published by Lee et al. [21]. After adding 10 ml methanol:water (80:20, v/v) to 1 g of ground sample, the resulting mixture was kept in an ultrasonic bath for 30 min. At the end of this period, the mixture was centrifuged at 6000 rpm for 10 min, and then the collected supernatant was filtered through a 0.45 µm membrane filter and made ready for analysis.
Total phenolic amount of mustard seeds
The Folin–Ciocalteu (FC) chemical was used to report total phenolic amounts of mustard seed extracts according to the study revealed by Yoo et al. [22]. FC (1 ml) and Na2CO3 (10 ml) were added to extract and mixed with vortex. The deionised water was added until the final volume was 25 ml and kept at dark for 1 h. The absorbance of each sample was read at 750 nm. The results obtained were recorded as mg gallic acid equivalent (GAE)/100 g.
Total flavonoid amount of mustard seeds
In line with the study conducted by Hogan et al.[23], the total flavonoid content of mustard seed extract was investigated. After 1 mL of mustard seed extract was added with 0.3 ml NaNO2, 0.3 mL AlCl3 and 2 mL NaOH, respectively, the solution was mixed thoroughly with the help of a vortex (2 min). Then, the solution was stored in the dark for 15 min and the absorbance value of each sample was determined at 510 nm. Results are given as mg quercetin equivalent (QE)/100 g.
Antioxidant activity
The antioxidant capacity results of mustard seed sample were measured using DPPH (1,1-diphenyl-2-picrylhydrazyl) according to the study explained by Lee et al. [24]. After preprocessing, the absorbance of each sample was read at 517 nm. The results are stated as mmol trolox equivalent (TE)/kg. % DPPH radical scavenging effect values were calculated according to the equation below:
DPPH radical scavenging effect (%) = [ (A control–A sample)/A control] × 100 A Control = Absorbance of the control.
A sample = Absorbance of sample or standards.
Determination of phenolic constituents
HPLC (Shimadzu) mounted with a PDA detector and an Inertsil ODS-3 (5 µm; 4.6 × 250 mm) column was used for chromatographic separation of phenolic compounds of mustard seed extracts. The mobile phase was a mixture of 0.05% acetic acid in water (A) and acetonitrile (B) with the flow rate of 1 ml/min at 30 °C. The injection volume was 20 µl. The peaks were taken at 280 using a PDA detector. The elution program was employed: 0–0.10 min 8% B; 0.10–2 min 10% B; 2–27 min 30% B; 27–37 min 56% B; 37–37.10 min 8% B; 37.10–45 min 8% B. The total running time per sample was 60 min [25].
Oil content
Soxhlet system was used for oil extraction of mustard seeds. After 10 g of ground mustard powder were weighed into the Soxhlet cartridge, the mouth of the cartridge was tightly closed with cotton and placed in the Soxhlet extractor. After adding 250 mL of petroleum ether to the Soxhlet flask, extraction was carried out at 50 °C for 5 h. At the end of this period, the solvent in the micelle in the flask was evaporated with a rotary evaporator and the solvent was collected in a separate flask. remaining crude oil was calculated (%) [19].
Fatty acid composition
Mustard seed oil was esterified according to the method of Ahmed et al.[25]. Fatty acid methyl esters of mustard seed oils were analyzed by gas chromatography (Shimadzu GC-2010) equipped with flame-ionization detector and capillary column. Nitrogen gas with a flow rate of 1.51 ml/min was used as the mobile phase, and the total flow rate and division rate were 80 ml/min and were applied as 1/40.
Mineral results of mustard seeds
After powdered mustard seeds (0.5 g) was incinerated using 5 ml of 65% HNO3 and 2 ml of 35% H2O2 in a microwave, the volume of samples was completed to 20 ml with distilled water. ICP-OES was used for biogenic element amounts of white mustard seeds [26].
Statistical analyses
The sample results were subjected to analysis of variance by averaging the triple analysis data. Duncan's Multiple Range Test was applied for significant differences in results between control, oven and microwave roasting types (p < 0.05). To examine the correlation between phenolic constituents, and between biogenic element amounts of mustard seeds roasted in different ways, a multivariate cluster analysis was performed using the PAST statistical program to perform principal component analysis (PCA) [27].
Results and discussion
Physicochemical properties of raw and roasted mustard seeds
The moisture and oil results, color values, bioactive compounds and antioxidant capacity results of raw (control) and roasted white mustard seeds are displayed in Table 1. The results obtained regarding physicochemical and bioactive properties exhibited some differences based on roasting types compared to the control (unroasted seeds). The moisture and oil amounts of raw and roasted mustard seeds were reported to be between 2.78 (oven) and 6.75% (control) to 12.05 (control) and 16.30% (oven), respectively. It can be seen that while the moisture amounts of the seeds decreased with the applied heat treatment, the oil content of the seeds increased. There were significant fluctuations between the analysis values of seeds roasted in oven and microwave based on the heat treatment applied (p < 0.05). These differences may be due to the roasting process time as well as the heat intensity and the intermolecular transfer of heat within the seed. Total phenolic and flavonoid amounts of raw and roasted white mustard seeds were recorded to be between 473.90 (control) and 569.96 mg GAE/100 g (oven) to 345.48 (control) and 479.76 mg/100 g (microwave), respectively. Antioxidant capacity results of raw and roasted wild mustard seeds were measured to be between 6.71 (oven) and 6.97 mmol/kg (control). Total phenolic and flavonoid contents of oven-roasted seeds were higher than control and microwave-roasted seeds. This may be due to the fact that the non-enzymatic browning and Maillard reaction occurring in the seeds during roasting in the oven occurs more frequently than in the microwave. Because the roasting time is short due to the rapid distribution of heat between molecules in the microwave depending on the time, it may be due to the lack of non-enzymatic browning and Maillard reaction products. Mustard seeds contained 8.02% moisture [28]. In previous studies, the fat amounts of the mustard seeds varied between 2.5 and 43.85% [29,30,31]. The total phenolic compound content in mustards has been reported to vary between 3.26 ± 0.25 and 404.33 ± 2.52 mg GAE/g [32]. In previous studies, the flavonoid amounts of Korean mustard seeds were found to be between 4.02 and 395.33 mg QE/g [32, 33]. Total phenol, flavonoid and antioxidant activity (DPPH assay) values of raw mustard seeds were 10.03 mgGAE/g, 0.36 mgCE/g and 1.72 mgAE/g, respectively [13]. Total phenol, flavonoid and antioxidant capacity values (DPPH assay) of white mustard seeds (NS Bela cv) were determined as 11.09 mgGAE/g (dw), 5.59 mgQE/g (dw) and 2.39 mg AAE/g (dw) [3], respectively. Antioxidant capacity, total phenolic, and total flavonoid results of mustard seeds were reported as 87.07%, 13.21 mg GAE g/1 dw and t 14.50 mg QE g/1 (dw) [34]. When our results were compared with the results of the last study, some differences were detected. These differences are likely due to factors such as mustard variety, growing conditions, and harvest time.
Color results of mustard seeds
L* values of mustard seeds were measured to be between 35.04 and 65.52. Heat treatment caused a decrease in L* values, and the lowest L* value was observed in the sample roasted in conventional oven (Table 1). The a* and b* values were detected as 5.33–8.63 and 10.74–32.75, respectively. Heat application caused an increase in a* values, while a reduction in b* values was found after heating. The mustard seeds heated in conventional or microwave ovens showed higher a* value than the control sample. The lowest b* result was observed in sample heated in conventional oven. Chahbani et al. [35] pointed out that L* and b* results of peas reduced from 72 to 45; from 41 to 20 after microwave drying, while a* values increased from -21 to -2. The long drying time caused differences in color values because of the enzymic and nonenzymic browning reactions [36]. The color values of raw and roasted mustard seeds differed depending on the roasting type and control. L* and b* results of heat-treated seeds were determined to be lower than the control. When the color of the seeds turned brown during roasting, the brightness and b* results of the seeds decreased significantly. This decrease could possibly be due to non-enzymatic browning or the Maillard reaction during roasting.
The phenolic compound profiles of mustard seeds
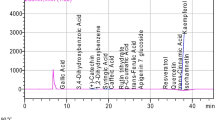
The phenolic compound profiles and quantitative results of raw and heated mustard seeds are given in Table 2. Catechin, gallic acid, 3,4-dihydroxybenzoic acid and p-coumaric acid recorded in raw and roasted mustard seeds were the major phenolic constituents (Fig. 1). As with the bioactive components of the seeds, the phenolic component amounts of heated mustard seeds increased significantly compared to the control (p < 0.05). This increase may be due to the relative increase in the concentrations of phenolic constituents in the seeds due to water loss (about 55–58%) in the seeds, the resistance of the components to heat or the products of the Maillard reaction. While gallic acid amounts of raw and roasted mustard seeds vary between 8.47 (control) and 117.31 mg/100 g (microwave), 3,4-dihydroxybenzoic acid amounts of mustard seeds were reported to be between 2.16 (control) and 11.79 mg/100 g (microwave). While catechin contents of raw and roasted mustard seeds ranged from 11.58 (control) to 41.35 mg/100 g (microwave), p-coumaric acid amounts of raw and roasted seeds were found to be between 0.83 (control) and 86.96 mg/100 g (oven). Quercetin and kaempferol amounts of raw and roasted mustard seeds were determined to be between 0.56 (control) and 11.02 mg/100 g (oven) to 0.29 (control) and 3.09 mg/100 g (oven), respectively. In general, the phenolic component amounts of mustard seeds roasted in the microwave were higher than those roasted in the oven. The p-coumaric acid and quercetin amounts of the seeds were significantly higher than both the control and microwave roasted seeds (p < 0.05). White mustard seeds contained 80.53 p-hydroxybenzoic acid, 0.093 protocatechuic acid, 0.037 vanillic acid, 0.044 syringic acid, 0.018 gallic acid, 0.009 chlorogenic acid, 0.324 p-coumaric acid, 0.636 ferulic acid, 0.072 caffeic acid, 6.12 sinapic acid, 13.78 4-vinylsyringol, 2.76 methyl sinapate, 0.181 epicatechin, 0.012 naringenin, 0.652 quercetin, 0.077 kaempferol and 0.076 mg/kg rutin [9]. It has been stated that factors such as species, variety, processing method and detection method can have a significant impact on the phenolic constituents of mustard [32]. The final differences may have a critical role in this regard, as can differences in oilseed crops due to the different genetic potential, as well as different growing conditions and cultivation practices [3].
Pearson correlation (r) between phenolic compounds
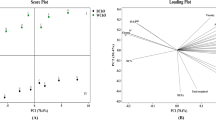
Pearson correlation (r) between phenolic compound contents in different drying states of mustard (control, conventional dried, and microwave dried) is given in Fig. 2. As can be seen from the examination of Fig. 2, although there are positive relationships between the phenolic compound contents of mustard seeds in different drying conditions, it was determined that the relationship between the gallic acid phenolic compound and the syringic acid phenolic compound was a significant and highly positive relationship (p < 0.05, r > 0.70). It was stated that the relationships between 3,4-dihydroxybenzoic acid, one of the phenolic compound contents of mustard, and catechin contents were significant and highly positive relationships. This study revealed that there are significant and highly positive relationships between resveratrol and kaempferol.
Pearson correlation (r) between phenolic compound contents (Gallic acid, 3,4-dihydroxybenzoic acid, catechin, caffeic acid, syringic acid, rutin, p-coumaric acid, ferulic acid, resveratrol, quercetin, cinnamic acid and kaempferol)) in different drying states of mustard (control, conventional dried and microwave dried)
The fatty acid compositions of the mustard seed oils
The fatty acid compositions of the oils extracted from raw and roasted mustard seeds are illustrated in Table 3. The fatty acid found in the highest amounts in mustard seed oil was erucic acid (Fig. 3). It is understood from here that heat treatment did not have a positive effect on the reduction of erucic acid. However, statistically significant changes were monitored between the fatty acid compositions of oils extracted from mustard seeds (p < 0.05). While the myristic, oleic, and linoleic acid contents of the oils partially decreased by roasting the seeds, the arachidic, linolenic, behenic and erucic acid amounts of the oils increased (Fig. 3). Palmitic and stearic acid contents were higher in the oil obtained from seeds roasted in the oven than in the control and microwave. Erucic acid amounts of raw and roasted mustard oils were found between 41.38 (control) and 42.81% (microwave). While oleic acid amounts of mustard oils changed between 26.06 (microwave) and 26.90% (control), linoleic acid results of the oils were determined to be between 13.08 (oven) and 13.98% (control). Also, linolenic acid results of the oils obtained from mustard seeds were detected between 11.14 (control) and 11.46% (oven). The highest palmitic (3.87%) and stearic (1.24%) acids were recorded in the oil sample extracted from mustard seeds roasted in oven system. Results exhibited some changes based on roasting types compared to the control. Mustard oil contained 4.1% palmitic, 1.7% stearic, 17.4% oleic, 15.8% linoleic, 18.2% linolenic, 42.8% erucic acids [37]. Wendlinger et al. [29] determined 1.5–4.3% palmitic, 1.0–2.0% stearic, 9.2–61.0% oleic, 9.8–15.1% linoleic, 7.8–15.2% linolenic and 0.3–50.8% erucic acids in nine different mustard oils. Stojanovi´c et al. [3] determined 2.24% palmitic, 0.72% stearic, 19.28% oleic, 8.93% linoleic, 6.45% linolenic, 9.79% 20:1 and 46.13% erucic acids in the oil of white mustard seeds (NS Bela cv). Mustard oil contained 4.51% palmitic, 2.78% stearic, 38.21% oleic, 25.31% linoleic, 11.30% linolenic, 10:86% arachidonic, 11.35% erucic acids [38]. Mustard seeds contained approximately 24–40% oil and this oil was defined by the presence of higher levels of erucic acid [37]. It has been shown that mustard oil contains low amounts of saturated fatty acids and high levels of erucic acid (42.8%) and linolenic acid (18.2%) [37]. The mean daily intake of erucic acid based on the mustard sample ranged from 13 to 86 mg [29]. In another study, white and black mustard oils from the Brassicaceae family were distinguished by their high erucic acid content of 46.13% and 41.82%, respectively. Other studies have also confirmed that the level of erucic acid in mustard oil varied between 35.7% and 51.4%, respectively [39, 40]. This description agrees with the amount of erucic acid we obtained [41] while mustard oil, which is a rich source of erucic acid, is still frequently used to prepare meals in Asian cuisine [4]. The findings obtained with the fatty acids of mustard seed oil were similar to the fatty acids obtained in high amounts as a result of the studies of Stojanovic et al. [3], Abdul Alim et al. [37], and Wendlinger et al. [29]. However, there are minor differences in the amounts of fatty acids in each study. These changes may probably be due to the climate where mustard grows, soil structure, variety, harvest time and extraction processes.
The protein and mineral contents of mustard seeds
The protein and mineral contents of mustard seeds roasted by microwave and oven systems are displayed in Table 4. Protein and element contents of mustard seeds were affected by the roasting process. The roasting in the oven had the most effect on the mineral and protein contents of the mustard seeds, followed by the microwave and control (untreated seed) in decreasing order. Because, as a result of roasting in the oven, the element and protein contents of the seeds were found to be lower than in the microwave. However, the biogenic element and protein contents of mustard seeds roasted in both systems were found to be higher compared to the control. This may be due to the relative increase in element and protein concentrations in the dry matter due to water loss in the grain as a result of the roasting process. P and K contents of raw and roasted mustard seeds were established to be between 7072.85 (control) and 8424.89 mg/kg (microwave oven) to 6967.01 (control) and 9024.81 mg/kg (microwave), respectively. While Ca amounts of the seeds varied between 377,789.05 (microwave oven) and 398,394.95 mg/kg (control), Mg results of mustard seeds were established to be between 3617.64 (control) and 4224.11 mg/kg (microwave oven). Also, S contents of mustard seeds changed between 11,482.90 (control) and 12,999.10 mg/kg (microwave oven). The highest Fe (214.33), Cu (7.19), Mn (33.56), Zn (49.50), B (11.99 mg/kg) and protein (32.56%) were determined in mustard seeds roasted in microwave system. As seen in Table 4, the highest amount of biogenic element in mustard seeds was Ca, followed by S, K, P, Mg, Fe, Zn, Mn, B and Cu in decreasing order. Therefore, it can be said that mustard seed can be a good source of Ca, P, K, Mg, Fe and protein. After the oil contained in mustard seed was completely removed due to erucic acid, the remaining pulp can be a source of additives in both human food and animal rations due to its high concentrations of elements and protein content. Mustard seeds contain protein (17–32.4) [28, 30, 31]. Mustard seeds contained 55 Mn, 7 Cu, 713 Fe, 1277 Ca, 1804 Mg, 3612 P, 1161 Na, 3511.8 mg/kg K [30]. White mustard seed (NS Bela cv) contained 26.72 Na, 1195.04 K, 508.93 Ca, 229.22 Mg, 3.62 Zn, 3.42 Fe, 2.16 Mn, 0.28 Cu 907.07 mg/100 g P [3]. The findings revealed that mustard seed powder was relatively high in potassium, phosphorus, calcium, magnesium and sulfur, and relatively low in copper, manganese and iron. The data are in good agreement with the results of Youssef et al. [30] and Stojanovi´c et al. [3]. Genetic characteristics may cause plants to absorb and accumulate some elements from the soil more than others, and the mineral amount of the soil where the oilseed plant grows may also affect the mineral profile of plant parts [42].
Pearson correlation between macro and micro element contents
Pearson correlation between macro- and micro-nutritional element contents and protein contents of mustard seed samples roasted in different ways (control, oven and microwave) (r) is illustrated in Fig. 4. Although there are positive relationships between the protein content and nutritional elements of the mustard samples, it was determined that the relationship between the N contents was significant and highly positive (p < 0.05, r > 0.70). Although there was a positive relationship between the P contents and other nutritional elements of mustard samples dried in different ways, it was found that there were significant and highly strong positive relationships between the Mg contents and the Zn contents. While a significant and highly positive relationship was found between the K amounts of the mustard samples and their Mg, Zn and Cu contents, a significant and highly positive relationship was also determined between their Mg content and Zn and Cu contents. In addition, the study determined that there were significant positive relationships between Fe and Mn contents of dried mustard samples and similarly between Fe contents and Mn and B contents, respectively, and Mn contents with B contents.
Conclusion
-
-The highest total phenol and flavonoid amounts were detected in oven-roasted seeds.
-
Roasting in conventional and microwave ovens increased the "a" color values of the seeds.
-
The phenolic component amounts of heated mustard seeds increased significantly compared to the control.
-
Heat treatment did not have a positive effect on the reduction of erucic acid.
-
The roasting in the oven had the most effect on the mineral and protein contents of the mustard seeds, followed by the microwave and control (untreated seed) in decreasing order.
References
Özcan M, Akgül A, Bayrak A (1998) Yabani hardal (Sinapis arvensis L.) tohum ve yağlarının bazı bileşim özellikleri (Some compositional (Sinapis arvensis L.) characteristics of wild mustard seed and oils. Gıda 23:285–289
Klóska L, Cegielska-Taras T, Pietka T (2012) Regeneration capacity of selected genotypes of white mustard (Sinapis alba L.). Vitr Cell Dev Biol- Plant 48:180–188
Stojanovi´c ZS, Uletilovi´c DD, Kravi´c SŽ, Kevrešan NL, Grahovac IS, Ðurovic Loncarevi´c AD, Marjanovi´c Jeromela AM (2023) Comparative study of the nutritional and chemical composition of new oil rape, safflower and mustard seed varieties developed and grown in Serbia. Plants 12:2160
Rastogi T, Reddy KS, Vaz M, Spiegelman D, Prabhakaran D, Willett WC, Stampfer MJ, Ascherio A (2004) Diet and risk of ischemic heart disease in India. Am J Clin Nutr 79:582–592
Mortuza MG, Dutta PC, Das ML (2006) Erucic acid content in some rapeseed/mustard cultivars developed in Bangladesh. J Sci Food Agric 86:135–139
Abul-Fadl MM, El-Badry N, Ammar MS (2011) Nutritional and chemical evaluation for two different varieties of mustard seeds. World Appl Sci J 15(9):1225–1233
Lietzow J (2021) Biologically active compounds in mustard seeds: aA toxicological perspective. Foods 10(9):2089
Grygier A (2022) Mustard seeds as a bioactive component of food. Food Rev Int 2022:1–14
Martinovic N, Polak T, Ulrih NP, Abramovic H (2020) mustard seed: phenolic composition and effects on lipid oxidation in oil, oil-in-water emulsion and oleogel. Ind Crops Prod 156:112851
Kim YT, Kim BK, Park KY (2007) Antimutagenic and anticancer effects of leaf mustard and leaf mustard kimchi. J Korean Soc Food Sci Nutr 12(2):84–88
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50(10):3010–3014
Hama JR (2017) Comparison of fatty acid profile changes between unroasted and roasted brown sesame (Sesamum indicum L.) seeds oil. Int J Food Prop 20:957–967
Nicoli M, Anese M, Parpinel M (1999) Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci Technol 10(3):94–100
Rahman M, Khatun A, Liu L, Barkla BJ (2018) Brassicaceae mustards: Traditional and agronomic uses in Australia and New Zealand. Molecules 23:231
Pietka T, Krzymanski J, Krótka K, Bartkowiak-Broda I (2014) Double low white mustard (Sinapis alba L. syn. Brassica hirta) is a source of protein and oil. Ro´sl Oleiste-Oilseed Crops 5:21–35
Sadowska U, Jewiarz K, Kopak M, Dziadek K, Francik R, Kope´c A (2023) Proximate analysis and antioxidant properties of young plants of Sinapis alba L. Depend on the Time of Harvest and Variety. Appl Sci 13:7980
Koeduka T, Sugimoto K, Watanabe B, Someya N, Kawanishi D, Gotoh T (2014) Bioactivity of natural O-prenylatedphenylpropenes from Illicium anisatum leaves and their derivatives against spider mites and fungal pathogens. Plant Biol (Stuttg) 16(2):451–456
Hojjati M, Noguera-Artiaga L, Wojdylo A, Carbonell-Barrachina AA (2015) Effects of microwave roasting on physicochemical properties of pistachio (Pistacia vera L.). Food Sci Biotechnol 24(6):1995–2001
AOAC (1990) Official Methods of Analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC
Pagliarini E (1994) Rastelli C, Sensory and instrumental assessment of olive oil appearance. Grasas Aceites 45:1–2
Lee YH, Choo C, Waisundara VY (2015) Determination of the total antioxidant capacity and quantification of phenolic compounds of different solvent extracts of black mustard seeds (Brassica nigra). Int J Food Propert 18:2500–2507
Yoo KM, Lee KW, Park JB, Lee HJ, Hwang IK (2004) Variation in major antioxidants and total antioxidant activity of Yuzu (Citrusjunos SiebexTanaka) during maturation and between cultivars. J Agric Food Chem 52:5907–5913
Hogan S, Zhang L, Li J, Zoecklein B, Zhou K (2009) Antioxidant properties and bioactive components of Norton (Vitis aestivalis) and Cabernet Franc (Vitis vinifera) wine grapes. LWT Food Sci Technol 42:1269–1274
Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Games EJC, Mehta RG (1998) Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen 1:35–46
Özcan MM, Uslu N (2023) The effect of fermentation with different additives on bioactive compounds, antioxidant activity, phenolic component, fatty acid composition and mineral substance contents of capers fruits. J Food Meas Charact 17:3896–3908
Skujins S (1998) Handbook for ICP-AES (Varian-Vista). A short guide to vista series ICP-AES operation. VarianInt, AG. Zug., Version 1.0
Pulliainen TK, Wallin HC (1996) Determination of total phosphorus in foods by colorimetry: Summary of NMKL. J AOAC Int 79(6):1408–1410
Gagandeep A, Dhiman M, Mendiz E, Rao AR, Rale RK (2005) Chemopreventive effects of mustard (Brassica compestris) on chemically induced tumorigenesis in murine forestomach and uterine cervix. Hum Experiment Toxicol 24(6):303–312
Wendlinger C, Hammann S, Vetter W (2014) Various concentrations of erucic acid in mustard oil and mustard. Food Chem 153:393–397
Youssef MKE, El Newihi AM, Omar SM, Ahmed ZS (2014) Assessment of proximate chemical composition, nutritional status, fatty acid composition and antioxidants of curcumin (Zingiberaceae) and mustard seeds powders (Brassicaceae). Food and Public Health 4(6):286–292
Sehwag SS, Das M (2015) A brief overview: Present status on utilization of mustard oil and cake. Indian J Tradit Knowl 14:244–250
Sun B, Tian YX, Jiang M (2018) Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var gemmifera). Royal Soc Chem 8(59):33845–33854
Oh SK, Tsukamoto C, Kim KW, Choi MR (2016) LC-PDA/MS/ MS analysis of glucosinolates in dolsan leaf mustard Kimchi and Dolsan leaf mustard pickles. Korean Soc Biotechnol Bioeng 31(1):1–7
Montaner C, Mallor C, Laguna S, Zufiaurre R (2023) Bioactive compounds, antioxidant activity, and mineral content of bróquil: A traditional crop of Brassica oleracea var. italica. Front Nut 9:1006012
Chahbani A, Fakhfakh N, Balti MA, Mabrouk M, El-Hatmi H, Zouari N, Kechaou N (2018) Microwave drying effects on drying kinetics, bioactive compounds and antioxidant activity of green peas (Pisum sativum L.). Food Biosci 25:32–38
Briki S, Zitouni B, Bechaa B, Amiali M (2019) Comparison of con-vective and infrared heating as means of drying pomegranate arils (Punica granatum L.). Heat Mass Transfer 55(11):3189–3199
AbdulAlimMd Z, Iqbal Z, Dutta PC (2023) Studies on the characterization and distribution of fatty acids and minör components of high-erucic acid mustard oil and low-erucic acid rapeseed oil. Emir J Food Agric 24(4):281–287
Chowdhury K, Banu LA, Khan S, Latif A (2007) Studies on the fatty acid composition of edible oil. Bangladesh J Sci Ind Res 42:311–316
Kozłowska M, Gruczynska E, Scibisz I, Rudzinska M (2016) Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem 213:450–456
Kok W, Mainal A, Chuah C, Cheng S (2018) Content of erucic acid in edible oils and mustard by quantitative 13 C NMR. Eur J Lipid Sci Technol 120:1700230
Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Dinovi M, Edler L, Grasl-Kraupp B, Hogstrand C et al (2016) Erucic acid in feed and food. EFSA J 14:4593
Singh B, Schulze DG (2015) Soil minerals and plant nutrition. Nat Educ Know 6:1
Acknowledgements
The authors of this study extend their appreciation to researchers.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Mehmet Musa Özcan: investigation, formal analysis, writing, and editing; Nurhan Uslu: formal analysis and data curation; Nazlı Yalım and Merve Kandil: statistical analysis and investigation; Zeynep Namalan and Zehra Beyza Atasoy: formal analysis and validation.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflicts of interest.
Compliance with ethics requirements
This study is not a study with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Özcan, M.M., Uslu, N., Yalım, N. et al. White mustard (Sinapis alba L.) seeds: the role of oven and microwave roasting on their bioactive components, antioxidant potential, fatty acids and mineral contents. Eur Food Res Technol 250, 1563–1572 (2024). https://doi.org/10.1007/s00217-024-04491-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04491-2