Abstract
In this study, the effects of roasting chia seeds at different temperatures (90 and 120 °C) on total phenol, flavonoid, and carotenoid contents and antioxidant activities were examined. Additionally, the effects of different extraction methods on the phenolic components of chia seeds and the chemical properties and fatty acids of chia seed oils were investigated. Chia seed oil was extracted via Soxhlet without roasting as control, yielded 29.62%. The oils from seeds roasted at 90 °C and 120 °C had yields of 32.65% and 33.85%. The control oil had an acidity value of 4.70%. In comparison, the acidity values were 4.20% and 3.61% for the oils from seeds roasted at 90 °C and 120 °C. The total phenol result of the control sample was found to be 2.55 mg GAE/g, while the total phenol contents in Chia samples roasted at 90 °C and 120 °C are determined as 2.34 mgGAE/g and 2.14 mg GAE/g, respectively. Also, the flavonoid contents of the roasted chia samples (90 °C and 120 °C) were reported as 13.71 mgCE/g and 12.91 mgCE/g, respectively. It was observed that the application of roasting caused a decrease in quercetin and resveratrol values (p < 0.01). The highest antioxidant value (80.13%) was established in chia sample roasted at 90 °C. Quercetin, catechin, resveratrol, kaempferol and izorhamnetin were the main phenolic components of chia seeds. Other phenolic components such as syringic acid, p-cuoumeric acid, caffeic acid, and gallic acid were found at quite low levels. It was observed that of roasting application caused a decrease in quercetin and resveratrol values. The highest fatty acids in chia seeds were linoleic and linolenic acids. Linolenic and linoleic acid results of chia oils were recorded to be between 64.58 and 68.10% to 17.30 and 18.44%, respectively. The palmitic, stearic, oleic, linoleic and linolenic (except sonication) acid contents of the chia oils obtained by Soxhlet and sonication systems from roasted seeds were found high compared to the control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chia (Salvia hispanica L.) grown in the area extending from Southern Mexico to northern Guatemala is an annual herbaceous plant belonging to the Labiataeae family [1]. The main factors that make chia seeds a functional food are the essential nutrients they contain [2, 3]. In recent years, there has been an extreme tendency towards the consumption of functional foods that are beneficial for health due to the essential nutrients and phytochemicals [4]. Fiber, one of the components of chia seeds, which has a high carbohydrates (26–41%), lipid (25–40%)and protein (15–24%) content compared to other seeds, has begun to be used in the production of other products as an emulsifier due to its high water retention capacity [5, 6]. Chia seed oils have become important in human health and nutrition in recent years due to their high α-linolenic fatty acid content [7]. Qualitatively different from less common vegetable oils such as flax, chia oil also has a 53.3% α-linolenic acid content and a high content of polyunsaturated fatty acids [8]. Different extraction processes can lead to certain changes in the composition of the oil. Therefore, consumers assume that pure oils are healthier than refined oils. In fact, during the processing of pure oils, the extraction process preserves all fat-soluble components [9]. The ultrasound-assisted extraction method, which increases the efficiency of many processes, is a state-of-the-art, environmentally friendly, highly scalable extraction method for the extraction of oil and other bioactive substances from oilseeds [10]. The ultrasound extraction process, which involves many complex mechanical events including cavitation bubble formation, vibration, mixing and pulverization, weakens the cell wall of the seeds, makes them more permeable and accelerates the mass transfer rate [11, 12]. When ultrasonic waves propagated through liquids, the liquid is exposed to a negative pressure and this pressure causes cavitation in the liquid. The numerous small bubbles formed in this process create shear forces and turbulence as they collapse in the system [11]. The most commonly used extraction type today is the solvent extraction (Soxhlet) type, which uses solvents such as hexane [13]. In the mechanical pressing method, oilseeds are pressed under high temperature and pressure to release crude oil after preliminary processes such as cleaning, roasting and grinding. In this method, the oil content is lower than the solvent extraction type since not all of the oil in the seed was removed [14]. Mechanical pressing has high advantages as it is safer, simpler and involves fewer processing stages than the solvent oil extraction method [15]. In this study, the oil samples were obtained from unroasted and roasted chia seeds (90 °C and 120 °C) by sonication pre-treatment followed by Soxhlet extraction or cold pressing methods. The aim of current work was to investigate the effects of roasted chia seeds at different temperatures (90 and 120 °C) on total phenol, flavonoid, carotenoid contents and antioxidant activities, and the effects of different extraction methods on the phenolic components of chia seeds and chemical properties and the fatty acids of their oils.
Materials and methods
Material
After chia seeds provided from a local market in Konya were brought to the laboratory, the seeds were cleaned of foreign materials such as stones, soil and leaves. It has been reported that chia seeds were harvested when they reached full maturity. Black chia seeds were used as chia seeds and the harvest was done in August 2020. After the seeds were cleaned, they were stored at + 4 °C for a short period of 1 month until analysis.. The oils of seeds were obtained by ultrasonic-assisted extraction, Soxhlet and cold press systems.
Method
Seeds were roasted in a conventional oven (90 °C and 120 °C for 1 h and 30 min, respectively). Raw (control) and roasted chia seeds were ground in a grinder and made ready for analysis.
Moisture
The moisture of chia seeds were measured with the KERN Dbs 60-3 infrared moisture analyzer.
Oil extraction
Chia seed oils were extracted by using sonication extraction, Soxhlet system using petroleum ether and screw cold press.
Soxhlet system
After 10 g of ground chia seeds were placed in the Soxhlet cartridge, the extraction process was carried out at 40 °C in 5.5 h using petroleum ether. The solvent (petroleum ether) in the resulting micelle (oil–solvent mixture) was evaporated via rotary vacuum evaporator at 40 °C. The remaining crude oil was kept until it reached a constant weight, and then the % fat content was determined gravimetrically [16].
Ultrasonic assisted extraction (sonication baths)
After 12 g of powdered chia seeds were weighed into the filter paper cartridge in the sonication bath extraction method, the mouth of the cartridge was closed and put into balloons separately. Then, petroleum ether (200 ml) was added into the flask. After 20 min of sonication treatment, it was placed in the Soxhlet device. The solvent (petroleum ether) in the micelle accumulated in the balloon was removed by the via rotary vacuum evaporator at 40 °C. The amounts of oil in the samples were weighed till constant weight.
Oil extraction by cold press
Cold press (Karaerler NF 80, Turkey) is a screw press that operates at variable speed with a 220–240 V motor and its capacity is to process 1–12 kg of seeds per hour. Chia seeds were slowly placed into the press in the chamber of the device at a certain constant speed. The head was heated with a resistance before extraction and the oil outlet temperature was ensured not to exceed 75–80 °C. After the necessary procedures were carried out, the oils of the chia seeds were extracted by pressing.
Fatty acid composition
Seed oil was esterificated according to Multari et al. [17]. The oil (5 mg) was dissolved in hexane (10 ml). The solution (500 μl) was mixed with 1 ml of 1,2,3-triheptadecanoylglycerol as an internal standard, and the mixture was evaporated. The dried sample was dissolved in diethylether (1 ml), and then mixed with methylacetate (25 μl) and sodium methoxide (25 μl). After shaking for 5 min, the addition of acetic acid (6 μl) was applied to stop the reaction, followed by centrifugation at 4500 rpm for 5 min and then evaporation. The residue was dissolved in hexane (1 ml) and injected to gas chromatograph. For that purpose, flame ionization detector (FID) and capillary column (Tecnocroma TR-CN100, 60 m × 0.25 mm, film thickness: 0.20 μm) were used. Based on the area of the peaks, the % fatty acids were recorded.
Extract extraction
For extraction, 5 ml of acidic methanolic water (50:50 v/v; HCl, pH 2) was added onto 0.5 g powdered seed sample weighed into centrifuge tubes, and the solution was stirred in a shaking water bath for 1 h. Then, the samples were centrifuged at 6000 rpm for 10 min. After the methanol extract was filtered, 5 ml of acetone/water (70:30) was added over the remaining residue. It was kept in water bath for 1 h and centrifuged again (6000 rpm for 10 min) and acetone extract was taken. Acetone extract and methanol extract were combined [18].
Antioxidant activity
The antioxidant capacities of chia seeds were determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) [19]. After pretreatments, the absorbance was read at 517 nm. Results expressed in %
Total phenolic contents
Total phenolic results of the chia seed extracts were defined by using Folin Ciocalteu (FC) chemical according to the report stated by Yoo et al., [20]. After pre-analytical tretreatments, absorbance values were recorded at 725 nm. Results are described as mg GAE/100 g.
Total flavonoid contents
Total flavonoid results of chia seed extracts were determined according to the report explained by Dewanto et al. [21]. After preprocessing, absorbance of samples were read at 510 nm. Total flavonoid results of extracts were explained as mg catechol equivalents (CE) per dry weight.
Phenolic compounds
Chromatographic separation of phenolic compounds of chia seed extracts was carried out by HPLC (Shimadzu) equipped with a PDA detector and an Inertsil ODS-3 (5 µm; 4.6 × 250 mm) column. The flow rate of the mobile phase was 1 ml/min at 30 °C and the injection volume was 20 µl. The peaks were recorded at 280 with PDA detector. The gradient program was as follows: 0–0.10 min 8% B; 0.10–2 min 10% B; 2–27 min 30% B; 27–37 min 56% B; 37–37.10 min 8% B; 37.10–45 min 8% B. The total running time per sample was 60 min.
Determination of carotenoid
Total carotenoid results of chia seed extracts were obtained according to the report studied by da Rocha et al. [22]. After the preliminary analytical procedures applied to the samples, the absorbance was read at 450 nm in the spectrophotometer.
Free acidity
Free fatty acidity values of chia oils were anaysed in terms of 5% oleic acid by titration against 0.1 N KOH solution after dissolving in ethanol/diethylether (1:2 v/v) solution [16].
Peroxide determination
For the peroxide number of the oils, the chloroform/acetic acid mixture was added to the Chia seed oil sample. Free iodine released in the dark was determined by titrating against sodium thiosulphate solution in terms of milliequivalentlan gram O2/kg [16]).
Shear rate
Shear rate measurements of oil samples were determined by AND Sine Wave Vibro Viscometer, 0.3 to 10,000 mPa·s, Model SV-10 device.
Statistical analysis
The results of the research were arranged according to the coincidence plots trial scheme. After the analysis results were evaluated by analysis of variance, the changes between the groups were carried out by the Turkey Multiple Comparison Test.
Results and discussion
The moisture amounts and bioactive properties of chia seeds roasted at different temperaures
The moisture contents and bioactive properties of unroasted (raw) and roasted (90 °C and 120 °C) chia seeds are displayed in Table 1. Statistically significant differences were detected in the bioactive properties of chia seeds roasted at different temperatures (p < 0.05). The moisture amount of the control chia sample was determined as 5.24%, while the moisture results of roasted chia seeds (90 °C and 120 °C) are recorded as 2.55% and 2.38%, respectively. The roasting process applied significantly reduced the moisture value of Chia seeds. However, a very small difference was observed between the moisture values of roasted seeds (90 °C and 120 °C). The total phenol content of the control sample was recorded to be 2.55 mg GAE/g, while the total phenol amounts in roasted chia seeds (90 °C and 120 °C) are reported as 2.34 and 2.14 mg GAE/g, respectively. While the highest total phenol result is detected in the control sample, a decrease was monitored in the total phenol values as the roasting temperatures increased. While the flavonoid result of the roasted Chia seeds (90 °C and 120 °C) are established as 13.71 mgCE/g and 12.91 mgCE/g, respectively, the total flavonoid content of the Chia sample belonging to the control group was detected to be 14.61 mg CE/g. In addition, while the highest flavonoid amount are reported in the control sample, a decrease in flavonoid values was detected with the application of the roasting process. The antioxidant capacity result of chia seed (control) was determined to be 65.44%, while the antioxidant capacity results of the roasted Chia seeds (90 and 120 °C) are found to be 80.13 and 75.84%, respectively. The highest antioxidant capacity result of chia seed was reported in Chia sample roasted at 90 °C. A noticeable increase in antioxidant capacity results of Chia seeds was determined with the application of roasting. Antioxidant capacity results of Chia seeds were established to be statistically significant. However, carotenoids could not detected in Chia seeds.
The moisture amount of black Chia seeds originating from Argentina was measured as 6.19%, while the moisture content of white Chia seeds is recorded as 5.71% [23]. The moisture result of Chia seeds was determined to be 7.4%, 6.2% and 6.9% [24]. In a previous study, the moisture contents of chia seeds grown in different locations were close to each other. The moisture amounts of our study were found similar compared to results of studies of Barreto et al. [24], Sargi et al. [25], Tontul et al. [26] and Tunçil and Çelik [23]. The total phenol amounts of raw (unroasted) and roasted chia seeds were determined as 3.07 and 3.43 mg GAE/g, respectively [27]. In another study, Tunçil and Çelik [23] compared white and black Chia seeds from Argentina and determined the total phenol results of white and black chia seeds were recorded as 3.52 ± 0.08 mg GAE/g and 3.42 ± 0.06 mg GAE/g, respectively. In other study, Marineli et al. [28] recorded that the total phenolic amount of Chia seed was found to be 0.94 mg GAE/g. The total phenol results of control and roasted Chia seeds (90 °C and 120 °C) were detectedto be 0.98, 0.91, and 0.88 (mg GAE/100 g), respectively [29]. When looking at the total phenol result of the control seed, similar results were obtained in our total phenol content according to Ghafoor et al. [27] and Tunçil and Çelik [23], and Martínez-Cruz and Paredes-López [30]. These results show that the different seed sources used in the study affected the total phenolic concentration of the seeds. It has been stated that these changes may be due to the phenolic content of the seed being affected by many external factors such as weather and post-harvest conditions. Scapin et al. [31] obtained Chia seed extracts at different temperatures and ethanol concentrations and reported that the total flavonoid amount in the extracts varied between 0.055 and 0.162 g/kg EQ. According to another study, the total flavonoid result of Chia seeds is 84.95 mg/g EQ [32]. The total flavonoid contents of control and roasted Chia seeds (90 °C and 120 °C) were reported as 17.62, 16.87, 15.43 (mg CE/100 g), respectively [29]. The flavonoid content of the control seed was lower than that of Ghafoor et al. [20]. With the application of the heat treatment, the flavonoid contents decreased, and since the heat treatment applied is low, it can be assumed that it is low compared to the control since it can not break the walls with the bound form phenolics. Antioxidant activity values of control and roasted Chia seeds (90 °C and 120 °C) were found to be 88.27%, 85.76%, and 79.57%, respectively [29]. In the study performed by Martínez-Cruz and Paredes-López [30], DPPH radical scavenging activity was found to be 68.83%. In another study, the antioxidant capacity results of Chia extracts were determined between 3.31 (unroasted) and 4.24 μmol trolox/g (seeds roasted at 120 °C) [27]. Differences in antioxidant activity may be due to the chemical composition of the seeds or differences in biochemical pathways and various techniques used for the extraction process [31, 33].When we look at the antioxidant content of the c seed (control) in the studies conducted, similar findings were get in the studies of Ghafoor et al. [29], Martínez-Cruz and Paredes-López [30] and Noshe and Al-Bayyar, [34].
Free fatty acidity, peroxide and share rate values of the oils extracted from chia seeds roasted at different temperaures
Free fatty acidity, shear rate, oil yield and peroxide results of chia oils extracted by different extraction methods applied to the unroasted and roasted chia seeds are displayed in Table 2. The oil content of chia seeds (control) extracted by Soxhlet system was found to be 29.62%, while the oil amounts in the chia seeds roasted at 90 °C and 120 °C are found as 32.65% and 33.85%, respectively. The lipid content of chia seeds was determined based on the dry weight of the samples. While the oil result of the chia seed (control) extracted by sonication system is found to be 28.40%, the oil amounts of chia seeds roasted at 90 °C and 120 °C were determined as 31.73% and 34.68%, respectively. The observed increase in lipid content after roasting could possibly be due to loss of volatile matter and moisture, a phenomenon evident in both control and samples pretreated with ultrasonication. Ghafoor et al. [27] found that the oil results of unroasted and roasted Chia seeds were 35.83% and 37.7%, respectively. The amounts of chia oil obtained by Soxhlet and ultrasonic extraction methods differed depending on the roasting temperature. It was observed that the amount of oil obtained by both extraction methods increased with the increase in roasting temperature compared to the control. These changes in the oil contents are due to the seeds being affected by environmental and genetic factors [35, 36]. Ixtaina et al. [37] observed that the oil quality of Chia seeds was affected by their extraction methods.
The results of free fatty acidity value of oil obtained by cold press of raw (control) and roasted seeds (90 °C) were similar with 3.98% and 3.72%, respectively. The free fatty acidity value decreased to 2.94% by roasting at 120 °C. The free fatty acidity result of the control oil was determined as 4.70% in the oils obtained by the Soxhlet method, while the free fatty acidity values of the oils of roasted chia seeds (90 °C and 120 °C) are determined as 4.20 and 3.61%, respectively. With the application of sonication extraction method, the free fatty acidity result analysed in the control oil was found to be 4.56%, while it is determined as 4.14% and 3.75% at 90 °C and 120 °C, respectively. The highest free fatty acidity value was determined in the oils obtained by the application of Soxhlet and sonication extraction methods of the control group, while the lowest free fatty acidity result is monitored in the cold press method of the oil of chia seeds roasted at 120 °C. In general, free fatty acidity value of Chia oil was observed to be higher in Soxhlet extraction and sonication extraction methods compared to cold press system. The free fatty acidity values decreased due to the increase in roasting temperature. The results of peroxide value in the oil obtained by cold press of control and roasted seeds at 90 °C were reported as 1.50 and 1.50 meqO2/kg, respectively. The peroxide value increased to 2.00 meqO2/kg by roasting at 120 °C. While the peroxide value of the oil obtained from unroasted Chia seed by the application of ultrasonic-assisted extraction method is found to be 3.75, the peroxide values were found as 8.75 meqO2/kg in Chia oil roasted at 90 °C and 3.50 meqO2/kg in Chia oil roasted at 120 °C. However, while no significant difference was observed in the peroxide values of Chia oils roasted at 90 °C with the control in the cold press and Soxhlet method, an increase in the peroxide value of Chia oil roasted at 120 °C was determined. Among the control groups, the highest peroxide value was determined by sonication extraction method. In chia oils roasted at 90 °C, the lowest peroxide value was obtained from the cold press with 1.50 meqO2/kg oil, while the highest peroxide value is determined as 8.75 meqO2/kg oil by the application of sonication extraction method. In general, it has been observed that the peroxide value determined from the cold press in Chia oils was low in the peroxide results of the oils extracted by Soxhlet and sonication extraction. The share rate values of unroasted Chia seed oils extracted by pressing method and roasted at 90 and 120 °C were recorded as 30.70, 30.80 and 31.00 mPa.S (25 °C), respectively. The shear rate value of unroasted chia oil obtained by the Soxhlet method was reported as 31.20 mPa.S (25 °C). Shear rate results of oils extracted by sonication extraction from unroasted and roasted Chia seeds at 120 °C were reported as 31.05 and 31.40 mPa.S (25 °C), respectively [38]. It is thought that the most important factors affecting the shear rate in liquids may vary depending on the ratio of saturated and unsaturated fatty acids in oils.
The free fatty acidity results of the oils of unroasted and roasted Chia seeds extracted by the Soxhlet method were found to be 2.17 and 8.53 mgKOH/g, respectively, while the free fatty acidity values of unroasted and roasted Chia seed oils provided by pressing were recorded as 1.79 and 3.87 mgKOH/g, respectively [39]. In another study, free fatty acidity value of control and roasted chia seeds (90 °C and 120 °C) were found to be 2.85, 5.61, 8.27 (mg KOH/g), respectively [29]. In a study conducted studies on Australian Chia seeds, the free fatty acidity value of Chia oil was found as 2.54 mg KOH/g 38]. The free fatty acidity result of the oil (control) extracted from Chia seed was higher than the free fatty acidity value found by Segura-Campos et al. [2] and Imran et al. [40]. According to the study conducted by Özcan et al. [39], while the roasting temperature causes an increase in the free fatty acidity value, in our study, a decrease was observed in the free fatty acidity value as the roasting temperature increased. While the peroxide values of unroasted and roasted Chia seed oils provided by the Soxhlet system are established as 5.84 and 17.92 meqO2/kg, respectively, the total peroxide values of unroasted and roasted Chia seed oils extractedby pressing were 3.65 and 14.12 meqO2/kg, respectively [39]. In another study, it was determined that the peroxide value of control and roasted chia seeds (90 °C and 120 °C) were 3.21, 8.93, 13.76 (meqO2/kg), respectively [29]. Ixtaina et al. [37] determined the peroxide value in Argentine Chia oil as 1.0 meq O2/kg. While the peroxide value in the control chia oils was lower than the peroxide result obtained by Özcan et al. [39]. it was determined that it was higher than the study conducted by Ixtaina et al. [37]. Our findings showed similar properties by the results of reports carried out by Timilsena et al. [38]. When looking at the determined oil content, free fatty acidity, peroxide and share rate results, these differences amoung the studies may depend on the solvent type, temperature, extraction method and time applied during oil extraction.
Phenolic profiles of chia seeds roasted at different temperaures
Phenolic compounds and their quantitative results of unroasted and roasted-chia seeds are depicted in Table 3. The contents of quercetin, catechin, resveratrol, kaempferol and izorhamnetin were found to be the main components, while the contents of other phenolic components such as syringic acid, p-coumaric acid, caffeic acid and gallic acid are found to be quite low (Fig. 1). Statistically significant differences were detected in the phenolic composition of chia seeds roasted at different temperatures (p < 0.05). The gallic acid result of the chia seed samples were recorded to be between 1.02 and 4.67 mg/100 g. The chia seed sample (control) had the lowest gallic acid result(1.02 mg/100 g). The gallic acid result of chia seeds roasted at 90 °C was reported as 1.06 mg/100 g and the highest gallic acid value was observed with 4.67 mg/100 g by roasting at 120 °C. The caffeic acid result of the control was established to be 1.52 mg/100 g, while the caffeic acid content of chia seeds are determined as 3.13 mg/100 g and 3.20 mg/100 g by roasting at 90 °C and 120 °C, respectively. The 1,2-dihydroxybenzene result of the control was 9.95 mg/100 g, while the 1,2-dihydroxybenzene amounts of the roasted seed samples (90 °C and 120 °C) are reported as 12.69 and 13.90 mg/100 g, respectively. There was an increase in the values of gallic acid, 1,2-dihydroxybenzene and caffeic acid in Chia seeds in parallel with the roasting temperature. While the (+)-catechin content of the control is determined as 14.75 mg/100 g, the catechin contents of roasted chia seeds (90 °C and 120 °C) were monitored as 7.94 and 10.19 mg/100 g, respectively. While the syringic result of the control Chia sample is found to be 3.18 mg/100 g, the syringic acid results of the samples roasted at 90 °C and 120 °C were reported as 5.37 and 4.50 mg/100 g, respectively. The quercetin result of the control was determined as 10.85 mg/100 g, while this value decreased to 9.07 and 8.34 mg/100 g, with roasting at 90 °C and 120 °C, respectively. While the resveratrol results of raw (control) and Chia seeds roasted at 90 °C are recorded as 35.77 and 30.99 mg/100 g, respectively, the resveratrol results of Chia seeds roasted at 120 °C decreased to 2.21 mg/100 g. Differences between resveratrol content in Chia seeds were established to be statistically significant (p < 0.01). Quercetin and resveratrol contents of Chia seeds decreased due to the increase in roasting temperature. In addition, kaempferol content of the control sample was establishedto be 7.82 mg/100 g, while this value increase from 8.44 mg/100 g to 9.06 mg/100 g, with the application of 90 °C and 120 °C temperatures, respectively. The isorhamnetin amount of the unroasted chia sample was 38.74 mg/100 g, while the isorhamnetin result of chia seeds roasted at 90 °C and 120 °C are determined as 39.46 mg/100 g and 39.50 mg/100 g, respectively. Although the temperature application part caused differences in the value of isorhamnetin, no significant change was observed. In a previous study, the most common phenolics found in raw and roasted Chia seeds at 120 °C were caffeic acid, (+)-catechin, vanillic acid and coumaric acid, and it was reported that roasting partially increased the contents of phenolic components. Caffeic acid and (+)-catechin results of raw and roasted seeds were to be as 3.11 and 3.54 mg/100 g to 1.47 and 1.92 mg/100 g, respectively. Raw and roasted Chia seeds were established to contain 1.02 and 1.04 mg/100 g of vanillic, 0.41 and 0.54 mg/100 g of coumaric and 0.21 and 0.29 mg/100 g of ferulic acids, respectively. The phenolic components of chia seeds differ depending on the roasting process [27]. The caffeic acid amount of Chia seeds was found to be 3.089 mg/100 g [5]. In another study, the gallic acid and caffeic acid amounts of Chia seeds were determined as 1.15 mg/100 g and 2.74 mg/100 g, respectively [30]. Ghafoor et al. [29] examined the phenolic components of roasted chia seeds (90, 120, 150 and 180 °C) and statistically significant changes were reported amoung the phenolic constituents. In general, they found that the phenolic component values decreased with increasing temperatures. Few differences were monitored compared to literature values. These differences in the phenolic components were estimated to vary depending on the different conditions in which the Chia seeds are grown. At the same time, it is seen that the temperature applied to the seed affects the results.
Fatty acid profiles of the oils extracted from chia seeds roasted at different temperaures
Fatty acid compositions of the oils of unroasted and roasted chia seeds are represented in Table 4. Statistically significant differences were detected in the fatty acid composition of oils obtained by different extraction methods from chia seeds roasted at different temperatures (p < 0.05). The linolenic acid value in the control Chia oil was found to be 64.81%. This value increased to 67.41% and 67.37% in seed oils at which roasting temperatures of 90 °C and 120 °C applied, respectively. An increase in linolenic acid values were monitored in oils extracted by cold press depending on the temperature (Fig. 2). The linolenic acid results of the control were rocorded to be higher than the press with 67.685% and 68.10% in oils obtained by Soxthlet and Sonication systems, respectively. According to these two extraction methods, the value of linolenic acid decreased in seed oils that were applied temperature. While there is a significant difference among the chia oil samples in terms of palmitic, stearic, oleic, linoleic and linolenic acid, no significant changes were found in terms of arachidonic and behenic acid (Fig. 2). Arachidic, arachidonic and behenic acid values were identified below < 1%. In general, it has been determined that the highest fatty acid detected in Chia oils was linolenic acid. It is seen that the linolenic acid value in Chia oils varied between 64.58 and 68.10%. The difference between roasting temperatures did not show a significant change in linolenic acid values. The amount of linoleic acid in Chia oils is much higher than the amount of palmitic, stearic and oleic acid. The linoleic acid value in the obtained Chia oils varies between 17.30 and 18.44%. While the linoleic acid value of control Chia oil is determined as 17.30% in oils extracted by cold press, the linoleic acid value of the oil obtained as a result of roasting the seeds increased to 17.88%. However, the difference between the roasting temperatures of the seeds in the oils obtained by the press method did not affect the linoleic acid content. Similarly, the linoleic amount increased when the roasting temperature was applied in the oils extracted with Soxhlet system. While the linoleic acid result of Chia oil (control) applied with sonication extraction system is to be 17.56%, this value increased to 18.44% in the seed oil applied at 90 °C, but the linoleic acid value decreased again to 17.64 in the seed oil applied at 120 °C. This may be due to the oxidation of linoleic acid at high temperatures. The oleic acid results of Chia oils varied to be between 5.03 and 7.74%. The highest amount of oleic acid was identified as 7.74% in the oil provided by cold press of the control, and this value decreased to 5.38% and 5.29% with 90 °C and 120 °C roasting process, respectively. While the oleic acid content of the control sample is recorded as 5.59% with Soxhlet application, it was determined as 5.82% and 6.63% with 90 °C and 120 °C roasting process, respectively. The oleic acid result of the oils extracted with Soxhlet increased depending on the temperature. The lowest oleic acid value (5.03%) was the control chia oil obtained by ultrasonic-assisted extraction. In the sonication extraction system, a value of 5.91% oleic acid was found in chia oil roasted at 90 °C, and this value was established to be higher compared to the control. The oleic acid value was determined as 5.37% at 120 °C temperature application and it was observed that the results were similar.
The palmitic acid result of the Chia oil samples provided by cold press ranged from 5.97% to 6.99%. The palmitic acid content of Chia oil obtained by cold pressing varies depending on the applied temperature. Palmitic acid was identified as 5.97% in Chia oil (control) obtained by Soxhlet and sonication extraction systems. It was determined that this value increased a little with the roasting temperature in both extraction methods. The highest palmitic acid value was determined as 6.99% in the oil extracted by Soxhlet system of Chia seeds roasted at 120 °C. The lowest palmitic acid result (5.97%) was the oil of the control group provided by Soxhlet and sonication extraction. The stearic acid amounts of the Chia oil samples had close values between 2.25 and 2.72% and there was no visible difference with the application of heat. These findings are in line with those reported in previous studies. The fatty acid profile of Chia seeds of Argentina origin was determined as palmitic (6.89%), stearic acid (2.36%), oleic acid (6.73%), linoleic acid (22.5%), and linolenic acid (60.35%), respectively [41]. Segura-Campos et al. [3] determined palmitic (7.47%), stearic acid (0.29), arachidic acid (0.15%), behenic acid (0.06%), oleic acid (2.43%), linoleic acid (20.40%), linolenic acid (68.52%) and arachidonic acid (0.13%) in the oil obtained from Mexico originated Chia seeds with the Soxhlet method. The most abundant fatty acids obtained from chia seeds by both cold press and Soxhlet systems were linolenic acid [39]. Linolenic acid results of unroasted and roasted Chia oils provided by pressing and Soxhlet were determined between 66.24% and 67.84% to 64.98% and 66.75%, respectively. While the linoleic acid results of unroasted and roasted Chia oils obtained by pressing vary between 18.34 and 19.61%, the linoleic acid results of unroasted and roasted Chia oils provided by the Soxhlet method were reported between 17.25% and 18.97% [39]. Ghafoor et al. [29] examined the fatty acid composition of the oils extracted from roasted chia seeds (90, 120, 150 and 180 °C) and observed that the levels of fatty acids in general decreased with increasing temperatures. In this study, chia oil contained 6.48–7.08% palmitic, 2.69–2.97% stearic, 9.94–10.09% oleic, 19.47–20.57% linoleic and 58.64–59.84% linolenic acids [29]. Other fatty acids were detected at minor levels (< 0.17%). In another study where chia oil was obtained by cold press, the fatty acid composition was determined as palmitic 7.07%, stearic 3.36%, oleic 7.04%, linoleic 18.23% and linolenic 62.80%, respectively [28]. Ghafoor et al. [27] observed that the oleic and linolenic acid values increased with increasing of the roasting temperature. At the same time, stearic and palmitic acid values decreased as the roasting temperature increased [27]. Results obtained were supported by the study values of Ayerza and Coates [41], Ghafoor et al. [27], Ghafoor et al. [29], Ixtaina et al. [37], Marineli et al. [28] and Segura-Campos et al. [2]. In the study carried out by Ixtaina et al. [37], they determined that the fatty acid profiles of Chia oils provided by Soxhlet and press methods were different from each other. According to this result, it is seen that the extraction method has an effect on values of fatty acids. Several factors may have caused the differences in fatty acid concentration in Chia seeds. It is estimated that these differences are due to some analytical factors, environmental differences, climatic changes, growing year or soil conditions.
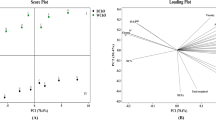
Principal component analysis (PCA)
Principal component analysis (PCA) was presented to evaluate the effect of roasting process on bioactive compounds of chia seeds, which are given in Fig. 3. The first two PCs explained 75.818% and 24.182% for chia seed, respectively. The main variables of PC1 were found as 3,4-dihydroxybenzoic acid (0.996), 1,2-dihydroxybenzene (0.996), caffeic acid (0.939), coumaric acid (0.971), apigenin-7-glucoside (0.957), kaempferol (0.997) and isorhamnetin (0.942). PC2 had positive correlation with rutin (0.935) (Table 5). Most of the phenolic compounds were detected in samples roasted at 90 °C and 120 °C, which were located in the positive area of PC1. The control sample was located in the negative area of both PC1 and PC2 and contained catechin and quercetin at the maximum level which were placed in the same area.
Conclusion
It has been determined that chia oil contains high amounts of Omega-3 fatty acids.
-
Roasting at 120 °C is recommended due to the increase in oil yield and the decrease in acidity due to the increase in temperature.
-
Since no heating process is applied in the cold press method, it is thought that the components of the oil are preserved.
-
Positive results were obtained in the analysis of oils extracted by cold press and ultrasonic assisted extraction.
-
According to the findings, it has been revealed that the ultrasonic assisted extraction system is more efficient than cold press.
-
When looking at the phenolic component analysis results of chia seeds, the results of quercetin, (+)-catechin, resveratrol and isorhamnet were recorded as the main components.
-
The fatty acids found in significant amounts in chia oil are linoleic, linolenic, oleic and palmitic acids.
-
Roasting of oils extracted with the Soxhlet system and ultrasonic assisted extraction appear to increase the fatty acid profile of the seed oil.
Data availability
Data will be made available on request.
References
E. Ergene, E.B. Bingöl, Diyet Lif İçeriği Yüksek Bazı Gıdalar ve Beslenme Üzerindeki Etkileri. Adnan Menderes Univ. Sağlık Bil. Fak. Derg. 3(1), 70–78 (2019)
M.R. Segura-Campos, N. Ciau-Solís, G. Rosado-Rubio, L. Chel-Guerrero, D. Betancur-Ancona, Chemical and functional properties of chia seed (Salvia hispanica L.) gum. Int. J. Food Sci. 2014, 241053 (2014)
M.R. Segura-Campos, N. Ciau-Solís, G. Rosado-Rubio, L. Chel-Guerrero, D. Betancur-Ancona, Physicochemical characterization of chia (Salvia hispanica) seed oil from Yucatán, México. Agric. Sci. 05(03), 220–226 (2014)
S.H. Al-Sheraji, A. Ismail, M.Y. Manap, S. Mustafa, R.M. Yusof, F.A. Hassan, Prebiotics as functional foods: a review. J. Func. Foods 5(4), 1542–1553 (2013)
M.S. Coelho, M. Salas-Mellado, Chemical characterization of chia (Salvia hispanica L.) for use in food products. J. Food Nutr. Res. 2(5), 263–269 (2014)
D. Orona-Tamayo, M. Valverde, O. Paredes-López, Chia-the new golden seed for the 21st century: nutraceutical properties and technological uses. Sustain. Protein Sour. 2017, 265–281 (2017)
W. Coates, Production potential of chia in northwestern Argentina. Ind. Crops Prod. 5(3), 229–233 (1996)
R. Bhatty, Further compositional analyses of flax: mucilage, trypsin inhibitors and hydrocyanic acid. J. Am. Oil Chem. Soc. 70(9), 899–904 (1993)
B. Matthäus, F. Spener, What we know and what we should know about virgin oils—a general introduction. Eur. J. Lipid Sci. Technol. 110(7), 597–601 (2008)
P.W. Mwaurah, S. Kumar, N. Kumar, A.K. Attkan, A. Panghal, V.K. Singh, M.K. Garg, Novel oil extraction technologies: process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 19, 3–20 (2020)
J.A. Cárcel, J.V. García-Pérez, J. Benedito, A. Mulet, Food process innovation through new technologies: use of ultrasound. J. Food Eng. 110(2), 200–207 (2012)
A. Mushtaq, U. Roobab, G.I. Denoya, M. Inam-Ur-Raheem, B. Gullón, J.M. Lorenzo, R.M. Aadil, Advances in green processing of seed oils using ultrasound-assisted extraction: a review. J. Food Process. Preserv. 44(10), e14740 (2020)
I.A. Kartika, P.Y. Pontalier, L. Rigal, Twin-screw extruder for oil processing of sunflower seeds: thermo-mechanical pressing and solvent extraction in a single step. Ind. Crop Prod. 32, 297–304 (2010)
P.C. Bargale, Prediction of oil expression by uniaxial compression using time-varying oil seed properties. J. Agr. Eng. Res. 77, 171–181 (2000)
A. Oyinlola, A. Ojo, L.O. Adekoya, Development of a laboratory model screw pres for peanut oil expression. J. Food Eng. 64, 221–227 (2004)
AOAC,Official Methods of Analysis, 15 th edn. Association of Official Analytical Chemists, Washington, DC.(1990).
S. Multari, A. Marsol-Vall, P. Heponiemi, J.-P. Suomela, B. Yang, Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 122, 318–329 (2019)
F. Abderrahim, E. Huanatico, R. Segura, S. Arribas, M.C. Gonzalez, L. Condezo-Hoyos, Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd) from Peruvian Altiplano. Food Chem. 183, 83–90 (2015)
S.K. Lee, Z.H. Mbwambo, H.S. Chung, L. Luyengi, E.J.C. Games, R.G. Mehta, Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1, 35–46 (1998)
K.M. Yoo, K.W. Lee, J.B. Park, H.J. Lee, I.K. Hwang, Variation in major antioxidants and total antioxidant activity of Yuzu (Citrusjunos SiebexTanaka) during maturation and between cultivars. J. Agric. Food Chem. 52, 5907–5913 (2004)
V. Dewanto, X. Wu, K.K. Adom, R.H. Liu, Thermal processing Enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50(10), 3010–3014 (2002)
S.A. da Rocha, E.K. Rocha, L.M. Alves, B. Amaral de Moraes, T. Carvalho de Castro, N. Albarello, C. Simoes-Gurgel, Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae). J. Plant Biochem. Biotechnol. (2013). https://doi.org/10.1007/s13562-013-0241-7
Y.E. Tunçil, Ö.F. Çelik, Total phenolic contents, antioxidant and antibacterial activities of chia seeds (Salvia hispanica L.) having different coat color. Akademik Zir. Derg. 8(1), 113–120 (2019)
A.D. Barreto, E.M. Gutierrez, M.R. Silva, F.O. Silva, N.O. Silva, I.C. Lacerda, I.R.L. Araújo, Characterization and bioaccessibility of minerals in seeds of Salvia hispanica L. Am. J. Plant Sci. 7(15), 2323–2337 (2016)
S.C. Sargi, B.C. Silva, H.M.C. Santos, P.F. Montanher, J.S. Boeing, O.O. Santos Júnior, N.E. Souza, J.V. Visentainer, Antioxidant capacity and chemical composition in seeds rich in omega-3: chia, flax, and perilla. Food Sci. Technol. 33(3), 541–548 (2013)
S.A. Tontul, C. Mutlu, A. Koç, M. Erbaş, Çiya Tohumundan Ultrason Destekli Yağ Ekstraksiyonunun Optimizasyonu. GIDA/J. FOOD (2018). https://doi.org/10.15237/gida.GD18013
K. Ghafoor, F. Aljuhaimi, M.M. Özcan, N. Uslu, S. Hussain, E.E. Babiker, G. Fadimu, Effects of roasting on bioactive compounds, fatty acid, and mineral composition of chia seed and oil. J. Food Process. Preserv. (2018). https://doi.org/10.1111/jfpp.13710
R. Marineli, E.A. Moraes, S.A. Lenquiste, A.T. Godoy, M.N. Eberlin, M.R. Maróstica Jr., Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT Food Sci. Technol. 59(2), 1304–1310 (2014)
K. Ghafoor, I.A.M. Ahmed, M.M. Özcan, F.Y. Al-Juhaimi, E.E. Babiker, I.U. Azmi, An evaluation of bioactive compounds, fatty acid composition and oil quality of chia (Salvia hispanica L.) seed roasted at different temperatures. Food Chem. 333, 127531 (2020)
O. Martínez-Cruz, O. Paredes-López, Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra high performance liquid chromatography. J. Chromatogr. A 1346, 43–48 (2014)
G. Scapin, M. Schmidt, R. Prestes, C. Rosa, Phenolics compounds, flavonoids and antioxidant activity of chia seed extracts (Salvia hispanica) obtained by different extraction conditions. Int. Food Res. J. 23(6), 2341 (2016)
V. Sarasvathi, J.N.D. Many, Chia Seed (Salvia hispanica)—an antioxidant potent to pharmacological activities. Int. J. Sci. Res. Manag. 5(7), 5949–5952 (2017)
Y. Okada, M. Okada, Y. Sagesaka, Screening of dried plant seed extracts for adiponectin production activity and tumor necrosis factor-alpha inhibitory activity on 3T3-L1 adipocytes. Plant Foods Hum. Nutr. 65(3), 225–232 (2010)
A.S. Noshe, A.H. Al-Bayyar, Effect of extraction method of chia seeds oil on its content of fatty acids and antioxidants. Int. Res. J. Eng. Technol. 4, 545–551 (2017)
R. Ayerza, Effects of seed color and growing locations on fatty acid content and composition of two chia (Salvia hispanica L.) genotypes. J. Am. Oil Chem. Soc. 87(10), 1161–1165 (2010)
M.L. Martínez, M.A. Marín, C.M.S. Faller, J. Revol, M.C. Penci, P.D. Ribotta, Chia (Salvia hispanica L.) oil extraction: study of processing parameters. LWT Food Sci. Technol. 47(1), 78–82 (2012)
V.Y. Ixtaina, M.L. Martínez, V. Spotorno, C.M. Mateo, D.M. Maestri, B.W. Diehl, M.C. Tomás, Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Com. Anal. 24(2), 166–174 (2011)
Y.P. Timilsena, J. Vongsvivut, R. Adhikari, B. Adhikari, Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 228, 394–402 (2017)
M.M. Özcan, F.Y. Al-Juhaimi, I.A.M. Ahmed, M.A. Osman, M.A. Gassem, Effect of soxhlet and cold press extractions on the physico-chemical characteristics of roasted and non-roasted chia seed oils. J. Food Measur. Character. 13(1), 648–655 (2019)
M. Imran, M. Nadeem, M.F. Manzoor, A. Javed, Z. Ali, M.N. Akhtar, Y. Hussain, Fatty acids characterization, oxidative perspectives and consumer acceptability of oil extracted from pre-treated chia (Salvia hispanica L.) seeds. Lipids Health Dis. 15(1), 162 (2016)
A.R. Yerza, W. Coates, Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind. Crops Prod. 34, 1366–1371 (2011)
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project Number (Grant No. RSP2024R083), King Saud University, Riyadh, Saudi Arabia.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Fahad AlJuhaimi: software, methodology, investigation, editing; Ayşenur Erdem: formal analysis, statistical analysis; Isam A. Mohamed Ahmed: editing; validation; Nurhan Uslu: methodology, formal analysis; Mehmet Musa Özcan: Supervision, formal analysis, methodology, investigation, writing-reviewing, editing; Oladipupo Adiamo: investigation, editing.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Ethical approval
No ethical approval required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Juhaimi, F., Erdem, A., Ahmed, I.A.M. et al. Effect of roasting temperature on bioactive compounds, antioxidant activity, phenolic profile, chemical properties, and oil extraction method on fatty acids composition of chia (Salvia hispanica L.) seeds and oil. Food Measure 18, 3806–3819 (2024). https://doi.org/10.1007/s11694-024-02455-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02455-4