Abstract
The non-alcoholic beer (NAB) sector has experienced steady growth in recent years, with breweries continuously seeking new ways to fulfil consumer demands. NAB can be produced by limited fermentation of non-Saccharomyces yeasts; however, beer produced in this manner is often critiqued for its sweet taste and wort-like off-flavours due to high levels of residual sugars and lack of flavour metabolites. The use of Lactobacillus in limited co-fermentation with non-Saccharomyces yeasts is a novel approach to produce NABs with varying flavour and aroma characteristics. In this study, lab-scale fermentations of Lachancea fermentati KBI 12.1 and Cyberlindnera subsufficiens C6.1 with Lactiplantibacillus plantarum FST 1.7 were performed and compared to a brewer’s yeast, Saccharomyces cerevisiae WLP001. Fermentations were monitored for pH, TTA, extract reduction, alcohol production, and microbial cell count. The final beers were analysed for sugar and organic acid concentration, free amino nitrogen content (FAN), glycerol, and levels of volatile metabolites. The inability of the non-Saccharomyces yeasts to utilise maltotriose as an energy source resulted in extended fermentation times compared to S. cerevisiae WLP001. Co-fermentation of yeasts with lactic acid bacteria (LAB) resulted in a decreased pH, higher TTA and increased levels of lactic acid in the final beers. The overall acceptability of the NABs produced by co-fermentation was higher than or similar to that of the beers fermented with the yeasts alone, indicating that LAB fermentation did not negatively impact the sensory attributes of the beer. C. subsufficiens C6.1 and L. plantarum FST 1.7 NAB was characterised as fruity tasting with the significantly higher ester concentrations masking the wort-like flavours resulting from limited fermentation. NAB produced with L. fermentati KBI12.1 and L. plantarum FST1.7 had decreased levels of the undesirable volatile compound diacetyl and was described as ‘fruity’ and ‘acidic’, with the increased sourness masking the sweet, wort-like characteristics of the NAB. Moreover, this NAB was ranked as the most highly acceptable in the sensory evaluation. In conclusion, the limited co-fermentation of non-Saccharomyces yeasts with LAB is a promising strategy for the production of NAB.
Similar content being viewed by others
Introduction
Beer is one of the most widely consumed alcoholic beverages globally; however, despite this, the brewing industry is facing significant challenges. The sector has been severely impacted as a result of the COVID-19 pandemic, with the total volume of beer sold in Europe decreasing by 9% in 2020 [1]. Even prior to this, the beer market had been experiencing a slowdown in overall market growth due to customer demographics, strict legislation, and emerging lifestyle trends [2]. Consumers are becoming increasingly aware of the risks associated with moderate alcohol consumption, such as digestive disorders, cardiovascular disease, cancers, and liver disease [3]; thus, the beer industry is continuously seeking ways to address such consumer demands [2]. Not only does non-alcoholic beer (NAB) allow the consumer to enjoy a beer without the negative effects associated with alcohol consumption, their decreased tax burden and lower production costs offer significant economic opportunities to breweries [4]. In recent years, the global non-alcoholic beer sector has gained popularity in an ever-increasingly health-conscious population and is forecast to grow at a CAGR of 8.5% by 2026 [5]. From 2013 to 2019, the sold production volume of NAB in the EU increased from 0.59 to 1.38 billion litres, with an estimated value of €1.28 billion. In 2019, over 80% of the volume of NAB produced within the EU could be attributed to five countries; Germany was the top producer accounting for 30.5% of the sold production volume, followed by Spain (16.8%), The Netherlands (14.4%), Poland (12%), and Czechia (7.1%) [6].
NAB may be produced using physical or biological processes. Physical processes involve the removal of ethanol from the final beer through the use of membrane-based or thermal technologies, often resulting in the undesired separation or evaporation of flavour or aroma compounds [7]. Biological processes for NAB production include alteration of the mashing process, cold contact fermentation, limited fermentation, or the use of alternative brewing yeasts [8]. Limited fermentation is the most widely used biological process; however, beer produced in this manner is often criticised for its sweet taste due to residual sugars, aldehyde-associated wort-like off-flavours, and compromised flavour profile due to the limited production of esters [9]. Reviewed by Michel et al. [10] and Bellut et al. [2], another commonly used approach is the use of non-Saccharomyces yeasts which are unable to metabolise the sugars in wort, resulting in decreased ethanol production. Moreover, the application of non-Saccharomyces yeasts which produce high levels of flavour compounds is a promising strategy for the enhancement and optimisation of the flavour profile of NAB produced by limited fermentation. The use of Saccharomycodes ludwigii as an alternative brewing yeast has been extensively studied [4, 11,12,13], while other research has investigated the application of non-conventional yeasts from the genera Candida, Cyberlindnera, Lachancea, Pichia, Hanseniaspora, Torulaspora, Brettanomyces, Schizosaccharomyces, and Zygosaccharomyces, to name but a few [10, 12, 14,15,16,17,18].
Non-alcoholic beers produced by limited fermentation or non-Saccharomyces yeasts often lack the desired pH drop which can lead to microbial spoilage. Traditionally, lactic acid bacteria (LAB) were regarded as beer spoilage microorganisms; however, the presence of LAB in beer fermentation results in the production of organic acids and a subsequent decrease in pH, while also improving the flavour complexity of the beverage through the production of organoleptic metabolites, such as alcohols, esters, ketones, aldehydes, and phenolic compounds [19]. Wort acidification by LAB can be performed prior to (pre-fermentation) or in parallel (co-fermentation) with yeast fermentation. LAB pre-fermentation can be carried out in the mashing kettle (mash souring), the brewing kettle (kettle souring) or in the preboiling wort (pre boil wort souring) [19, 20]. Although pre-fermentation eliminates the risk of LAB inhibition by hops or ethanol, the flavour complexity associated with beers produced from mixed fermentations may be lost after boiling [19]. Co-fermentation of LAB and yeast circumvent such an issue, while also shortening beer production times [21]. LAB species which have been investigated for their wort acidification capabilities include Pediococcus acidilactici, Lactiplantibacillus plantarum, Lactobacillus paracasei, Lactobacillus amylovorus, Lactobacillus reuteri, Lactobacillus brevis, Lactobacillus buchneri, and Weisella cibaria [19,20,21,22,23,24,25].
The production of NAB by limited co-fermentation of non-Saccharomyces yeasts with LAB has not yet been explored. In this study, we investigated the co-fermentation of Lachancea fermentati KBI 12.1 and Cyberlindnera subsufficiens C6.1 yeasts with L. plantarum FST 1.7 to produce NAB. The commercially available brewer’s yeast Saccharomyces cerevisiae WLP001 was also included as a control. The fermentation kinetics were monitored at time intervals and the composition and metabolite profiles of the final NABs were analysed.
Materials and methods
Microorganisms and reagents
The microorganisms used in this study are listed in Table 1. All strains were stored frozen at -80 °C in a final concentration of 40% glycerol. L. plantarum FST 1.7 was grown on De Man Rogosa Sharpe (MRS) agar supplemented with 0.05 g/L bromocresol green at 30 °C for 48 h in a microaerophilic environment, while S. cerevisiae WLP001, L. fermentati KBI 12.1, and C. subsufficiens C6.1 were grown on yeast potato dextrose (YPD) agar at 25 °C for 48 − 72 h. All microorganisms were stored at 4 °C and sub-cultured weekly. All reagents used in the study were at least analytical grade from Sigma-Aldrich (St Louis, MO, USA) unless stated otherwise.
Propagation
Wort was prepared by diluting unhopped Bavarian Pilsner liquid malt extract (Weyermann, Bamberg, Germany) with tap water to a specific gravity of 6°P. The diluted wort was sterilised at 121 °C for 15 min and filtered through sterile Grade IV 320 mm Whatman filter paper (Whatman plc, Maidstone, UK) to remove trub precipitates. L. plantarum FST 1.7 was propagated by inoculating a single colony from an MRS agar plate into 10 ml of wort and incubating at 30 °C for 24 h, followed by a 1% sub-culture into 50 ml of fresh wort and incubation for a further 18 h. For propagation of yeast strains, a single colony from a YPD agar plate was transferred into a 250 ml sterile Duran bottle containing 150 ml wort, covered with cotton wool, and incubated for 48 h at 25 °C with an agitation of 100 rpm.
Fermentation
Fermentation trials were carried out in sterile 2L Duran bottles filled with 1600 ml of wort and fitted with an airlock. LAB and yeast cells were harvested by centrifugation at 5000×g for 5 min and washed with sterile tap water to avoid carryover of sugars from the propagation wort. LAB cells were inoculated at a final concentration of 1 × 107 CFU/ml, while yeast cells were pitched at 1 × 106 CFU/ml. Fermentations were performed at 25 °C with each of the three yeast species alone and in combination with L. plantarum FST 1.7 (Fig. 1). The NAB was then filled into 330 ml brown glass bottles and pasteurised, with successful pasteurisation confirmed by plating on agar plates. The produced NAB was stored in the dark at 2 °C for further analysis. Fermentations were performed in triplicate.
Beer analyses
At each sampling point, the fermentation bottles were gently shaken to homogenise microbial cells settled at the bottle base into suspension and a 50 ml sample was withdrawn. Cell counts of L. plantarum FST 1.7 were determined using MRS agar plates supplemented with 0.05 g/L bromocresol green and 50 µg/ml cycloheximide to suppress yeast growth. Yeast cell counts were determined using YPD agar plates supplemented with 30 µg/ml chloramphenicol to inhibit bacterial growth. pH was measured using a digital pH meter (Mettler Toledo, Columbus, Ohio, USA). Total titratable acidity (TTA) was determined via titration of 10 mL sample against 0.1 M NaOH until pH 7 using an EasyPlus Titrator Easy pH (Mettler Toledo, Columbus, Ohio, USA). Cells were harvested by centrifugation, and the ethanol content (% v/v) and specific gravity (°P) of the supernatant were analysed using density meter DMA 4500 M with Alcolyzer Beer ME (Anton-Paar GmbH, Graz, Austria).
The final beers were analysed as follows. After appropriate dilution and filtration through 0.2 µm syringe-driven filters, organic acids (lactic, acetic, citric, and succinic) were quantified on a Dionex Ultimate 3000 HPLC system (Thermo Fisher Scientific, Waltham, MA, USA), with ultraviolet light/diode array detection (UV/DAD; Thermo Fisher Scientific) and quantification at 210 nm. Analytes were separated on a Hi-Plex H column (8 µm, 7.7 mm × 300 mm; Agilent Technologies, Santa Clara CA, USA) with isocratic elution with 5 mM sulfuric acid and a flow rate of 0.5 mL/min at 60 °C. The compounds were quantified using an external calibration (0.03–6 g/L). After dilution and filtration of the beer samples, sucrose, maltose, glucose, and fructose were quantified via high-performance anion exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD) on a Dionex ICS-5000 + system (Sunnyvale, CA, USA) equipped with a Dionex CarboPac PA200 column (3 mm × 250 mm) and the corresponding guard column (3 mm × 50 mm; Thermo Fisher Scientific, Waltham, MA, USA) as described by Ispiryan et al. [27]. External standard calibrations were used for each of the compounds between 0.05–1 mg/L and 1–20 mg/L. A 50 mg/L sodium azide solution was used for all sample and reference standard dilutions.
Free vicinal diketones were analysed by MS-Omics. Briefly, samples were mixed with methyl tert-butyl ether (MTBE) 1:1 and in GC–MS vials mixed thoroughly for 2 min, followed by centrifugation for 2 min. All samples were analysed in a randomized order. Analysis was performed as described by Pinu and Villas-Boas [28] with an Agilent 7890B gas chromatograph coupled to an Agilent 5977B quadrupole detector (Agilent Technologies, CA, USA). The system was controlled by ChemStation (Agilent Technologies, CA, USA). Raw data were converted to netCDF format using ChemStation, before the data were imported and processed in Matlab R2014b (Mathworks, Inc.) using tailormade in-house scripts and the PARADISe software described by Johnsen et al. [29]. Free amino nitrogen (FAN) was measured according to MEBAK 2.6.4.1, a ninhydrin-based photometric method where absorbance is measured at 570 nm against glycine. Glycerol was determined via a glucokinase enzymatic assay using a commercial kit (K-GCROLGK, Megazyme International, Wicklow, Ireland).
Sensory evaluation
An experienced sensory panel of 8 individuals (5 female and 3 male, age: 23–33) collected descriptors that characterised the aroma and flavour of the NABs. Following this, the panel evaluated the intensity of the flavour attributes ‘sour’, ‘honey’, and ‘fruity’ and the aroma attributes ‘sweet’ and ‘fruity’ on a scale from 0, ‘not present’ to 10, ‘extremely’. 50 ml samples were provided at ambient temperature (20 °C) in clear glasses labelled with a three-digit code. Sensory evaluations were performed in duplicate under white light and evaluators were instructed to use still water provided to cleanse their palates between samples.
Statistical analysis
Fermentations and analyses were performed in triplicate, unless stated otherwise. Values are presented as means ± standard deviation. Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS v2.5). Data were analysed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. Where equal variances were not assumed, data were analysed by Welch’s ANOVA followed by Games–Howell for pairwise comparison. The level of statistical significance for all tests was set at p ≤ 0.05.
Results
Fermentation kinetics
In this study, wort was fermented with S. cerevisiae WLP001, L. fermentati KBI 12.1, and C. subsufficiens C6.1 yeasts, singly and in combination with L. plantarum FST 1.7 to produce non-alcoholic beer (NAB). Pre-trials were performed to determine the appropriate fermentation cut-off points to ensure that the ethanol level did not exceed the threshold of 0.5%. Fermentations with S. cerevisiae WLP001 and L. fermentati KBI 12.1 yeast were stopped after 17 h and 24 h, respectively. As C. subsufficiens C6.1 produced low amounts of ethanol which did not exceed the ethanol threshold, fermentations with this yeast strain were stopped after 96 h, prior to the development of an unpleasant solvent-like flavour.
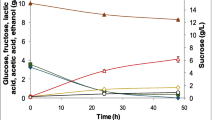
The characterisation of the fermentation wort is shown in Table 2. pH, TTA, alcohol, and extract values were measured continuously at time intervals throughout the fermentations (Fig. 1). In the case of WLP001 and WLP001 + FST 1.7, no change in pH and TTA was observed during the first 8 h of fermentation. After 14 h of fermentation, the pH of WLP001 had decreased to 4.60, while a significantly lower pH of 3.89 was observed for WLP001 + FST 1.7. Only minor changes in pH were observed during the remaining fermentation period, with final values of 4.43 and 3.79 determined for WLP001 and WLP001 + FST 1.7, respectively, after 17 h. The TTA values of WLP001 and WLP001 + FST 1.7 were comparable for the first 14 h of fermentation, after which differences in acidity became evident, with the final TTA of WLP001 + FST 1.7 (2.19 ml) being significantly higher than that of WLP001 (1.26 ml). Drop in extract was comparable for both conditions, with a decrease of approx. 0.81°P observed at the end of fermentation. Similarly, no difference in alcohol production between WLP001 and WLP001 + FST 1.7 was observed, with both fermentations reaching an ethanol level of 0.47–0.49% prior to cut-off.
Similar trends were observed with KBI 12.1 and KBI 12.1 + FST 1.7, with little change in pH or TTA observed during the first 8 h of fermentation. Subsequently, a sharp decrease in pH was observed in both conditions, with KBI 12.1 + FST 1.7 expectedly reaching a lower pH (3.54) than KBI 12.1 (3.89) at the end of fermentation. A corresponding increase in TTA was observed, with NaOH volumes of 2.96 and 3.65 ml required for KBI 12.1 and KBI 12.1 + FST 1.7, respectively. Extract decreased in a linearly in both fermentations, with only minor differences observed in the final extract values for KBI 12.1 (5.43°P) and KBI 12.1 + FST 1.7 (5.38°P). Due to the lower rate of ethanol production by KBI 12.1, the fermentations proceeded for 24 h compared to 17 h for WLP001 fermentations. No significant differences in ethanol production were observed between both fermentations, with final values of 0.42% (KBI 12.1) and 0.45% (KBI 12.1 + FST 1.7) determined.
Despite the longest fermentation time, the highest pH value was observed with C6.1, decreasing to just 4.60 after 96 h, while a final TTA value of 1.47 ml was recorded. In contrast, co-fermentation of C6.1 with FST 1.7 resulted in the lowest pH (3.21) and highest TTA (3.77 ml) values of any of the fermentations performed. Extract values of C6.1 and C6.1 + FST 1.7 decreased by just 0.28°P and 0.38°P, respectively, during fermentation with final values of 5.76°P and 5.61°P measured after 96 h. At the end of fermentation, C6.1 had produced 0.26% ethanol, while a slightly higher value of 0.32% was recorded for C6.1 + FST 1.7.
Microbial cell growth during fermentations is shown in Fig. 2. Yeasts were pitched at 1 × 106 CFU/ml, while L. plantarum FST 1.7 was inoculated in co-fermentations at a concentration of 1 × 107 CFU/ml. Results showed that regardless of whether the selected yeasts were fermented singly or in co-fermentation with L. plantarum FST 1.7, significant differences in yeast cell numbers were not observed. Cell counts of WLP001 increased by approx. 1 log CFU/ml after 14 h of fermentation and remained relatively stable thereafter, with final cell numbers of 6.93 log CFU/ml and 6.83 log CFU/ml recorded for S. cerevisiae WLP001 alone and in co-fermentation, respectively (Fig. 2A). A similar trend was observed for KBI 12.1, whereby cell counts increased by 1 log CFU/ml, reaching peak numbers of 7.12–7.16 log CFU/ml after 20 h fermentation and decreasing slightly thereafter (Fig. 2B). Growth of C. subsufficiens C6.1 was slower in comparison to the other yeasts, with increases of 0.5 log CFU/ml and 0.8 log CFU/ml recorded after 48 h and 96 h fermentation, respectively (Fig. 2C) Growth of L. plantarum FST 1.7 was comparable in all co-fermentations, increasing by approx. 1 log CFU/ml with peak cell numbers of 8.10–8.25 log CFU/ml determined.
Analysis of final beers
Sugars and organic acids
The attributes of the final beers are shown in Table 3. WLP001 and WLP001 + FST 1.7 showed similar sugar consumption patterns, with sucrose and fructose completely depleted in both cases, and low levels of fructose remaining (0.54–0.64 g/L). While sucrose was completely depleted during fermentation with L. fermentati KBI 12.1, residual amounts of glucose (0.41 g/L) were detected at the end of the 24 h fermentation period. In contrast, both sucrose and glucose were fully depleted when L. fermentati KBI 12.1 was co-fermented with L. plantarum FST 1.7. Data showed that sucrose was fully depleted after fermentation with C6.1, with high levels of residual glucose (2.47 g/L) detected after 96 h fermentation. In comparison, co-fermentation of C6.1 with L. plantarum FST 1.7 resulted in increased glucose consumption, with a lower level of 1.52 g/L measured in the final beer. Fructose was not fully depleted in any of the fermentations, with levels ranging from 0.5 to 0.9 g/L in the beer samples. The highest levels of maltose depletion were visible in the WLP001 (21.41 g/L) and WLP001 + FST 1.7 (20.14 g/L) fermentations, with approx. 3.15 g/L and 4.42 g/L of this sugar metabolised, respectively. In contrast, residual levels of maltose in the non-Saccharomyces yeast fermentations were within the range of 22–24 g/L. With regards to maltotriose, levels in the final beers were comparable across all samples, with values of 6.2–7.2 g/L detected. Lactic acid was not detected in the NAB beers fermented with S. cerevisiae WLP001 or C. subsufficiens C6.1 alone, while 0.41 g/L of lactic acid was measured in the L. fermentati KBI 12.1 NAB. As expected, the NABs produced as a result of yeast and L. plantarum FST 1.7 co-fermentation had significantly higher levels of lactic acid present than those produced by yeast fermentation alone. Fermentation of WLP001 + FST 1.7 for 17 h resulted in the production of 0.62 g/L lactic acid, while 1.13 g/L of lactic acid was produced after 24 h fermentation of KBI 12.1 + FST 1.7. The highest level of lactic acid was produced by co-fermentation of C6.1 + FST 1.7, with 2.94 g/L present in the final beer. Citric acid present in the initial wort (0.11 g/L) was not utilised by S. cerevisiae WLP001 and was depleted in varying amounts by WLP001 + FST1.7, KBI 12.1, and KBI 12.1 + FST 1.7, while the acid was fully metabolised by C. subsufficiens C6.1 and C6.1 + FST 1.7. Similarly, more than half (57%) of the acetic acid naturally present in the starting wort was depleted at the end of fermentation with C6.1 and C6.1 + FST 1.7.
Nitrogen metabolism and glycerol
S. cerevisiae WLP001 utilised approx. 25% of the FAN content of the fermentation wort, while WLP001 + FST 1.7 had a slightly higher consumption level of approx. 27%. A similar trend was observed for KBI 12.1 and KBI 12.1 + FST 1.7 fermentations whereby around 22% and 31% of the initial FAN content was depleted by the end of each respective fermentation. The FAN content of the C. subsufficiens C6.1 NAB (83.42 mg/L) was significantly higher than that of the other samples, with just over 3% of the initial amount utilised during the 96 h fermentation period; FAN consumption increased to approx. 25% when C. subsufficiens C6.1 was co-fermented with FST 1.7. Fermentations with S. cerevisiae WLP001 and L. fermentati KBI 12.1 yeasts alone resulted in the production of 0.50 g/L and 0.52 g/L glycerol, respectively, while lower levels of glycerol were present when the yeasts were co-fermented with L. plantarum FST 1.7 (0.40 g/L and 0.42 g/L, respectively). Beers fermented with C6.1 and C6.1 + FST 1.7 had significantly lower levels of glycerol than the other samples, with values of 0.16 and 0.14 g/L determined in the final beers, respectively.
Volatile compounds
The final beers were analysed for fermentation metabolites (Table 4). In terms of higher alcohols, isobutanol concentrations ranged from 1.07 g/L (KBI 12.1) to 1.41 g/L (C6.1 + FST 1.7). C6.1 and C6.1 + FST 1.7 produced significantly lower concentrations of 2-methyl-1-butanol at 0.68 and 0.79 g/L, respectively, than the other samples (1.04–1.22 g/L). Both WLP001 and WLP001 + FST 1.7 produced approx. 9 mg/L of isoamyl alcohol, significantly more than that of the fermentations with non-Saccharomyces yeasts (2.2–6.4 mg/L). The highest concentrations of 2-phenyl ethanol were produced by KBI 12.1 (3.50 mg/L) and KBI 12.1 + FST 1.7 (5.21 mg/L), values almost sevenfold and tenfold higher, respectively, than the concentrations determined in beers produced using C. subsufficiens C6.1 yeast. Levels of total higher alcohols in beers fermented with S. cerevisiae WLP001 and L. fermentati KBI 12.1 yeasts (alone and in co-fermentation with L. plantarum FST 1.7) ranged from 10.40 to 13.96 mg/L, significantly higher concentrations than detected in C6.1 (4.60 mg/L) and C6.1 + FST 1.7 (5.18 mg/L) beers. Total levels of higher alcohols were higher in the beers co-fermented with L. plantarum FST 1.7; however, this difference was not statistically significant. Regarding ester production, C6.1 + FST 1.7 produced relatively high amounts of ethyl acetate at 19.42 mg/L, almost twofold more than C6.1 alone (9.98 mg/L) and 50–80 times more than WLP001 (0.24 mg/L), WLP001 + FST 1.7 (0.22 mg/L) and KBI 12.1 + FST 1.7 (0.35 mg/L). Similarly, levels of isoamyl acetate produced by C6.1 and C6.1 + FST 1.7 were significantly higher than in the other beers. Ethyl hexanoate was present in small amounts (0.06 mg/L) in beers fermented with WLP001 and WLP001 + FST 1.7 and was determined in the other beers, but was present in levels below the limit of detection of 0.05 mg/L. Ethyl octanoate was detected in all samples in small amounts, with concentrations ranging from 0.22–0.36 mg/L. Levels of 2-phenylethyl acetate were below the limit of detection in beer fermented with S. cerevisiae WLP001 alone, while concentrations in the other beer samples were within the range of 0.13–0.18 mg/L. Overall, C6.1 and C6.1 + FST 1.7 produced significantly higher amounts of esters at concentrations of 10.88 mg/L and 20.47 mg/L, respectively, in comparison to the relatively low levels detected in beers fermented with S. cerevisiae WLP001 (0.67–0.75 mg/L) or L. fermentati KBI 12.1 (0.93–0.96 mg/L) yeasts singly or with L. plantarum FST 1.7. Diacetyl was the only compound detected in levels exceeding its sensory threshold of 0.1 mg/L, with concentrations of 0.35, 0.29, and 0.14 mg/L produced by WLP001, WLP001 + FST 1.7 and KBI 12.1, respectively. In comparison, just 0.03 and 0.02 mg/L were determined in the beers fermented with KBI 12.1 + FST 1.7 and C6.1 + FST 1.7, respectively, while levels of the compound were below the limit of detection in beer produced by C. subsufficiens C6.1 alone. Co-fermentation of S. cerevisiae WLP001 with L. plantarum FST 1.7 resulted in an increase in acetoin levels from 2.36 to 14.41 mg/L, while a twofold increase from 2.69 to 6.19 mg/L was observed in the case of L. fermentati KBI 12.1 co-fermentation. In contrast, acetoin concentrations did not differ significantly between C6.1 and C6.1 + FST 1.7 NABs. Acetaldehyde contents in all beers were well below the flavour threshold of 10 mg/L, with concentrations ranging from 2.26 mg/L (C6.1) to 3.27 mg/L (KBI 12.1).
Sensory evaluation
For evaluation of the NABs, a sensory trial was conducted with 8 trained panelists, whereby they were first asked to describe the samples in their own words, followed by an assessment of the intensity of different attributes. The results of the sensory evaluation are shown in Fig. 3. In the descriptive analysis, attributes such as ‘sweet’, ‘malty’, and ‘wort-like’ were used to describe the taste and aroma of the beer produced by S. cerevisiae WLP001, while the beer was also perceived as having ‘honey’ and ‘caramel’ flavours. Although WLP001 + FST 1.7 NAB was still described as having wort-like aroma and taste characteristics, it was also perceived as ‘fruity’ and citrus’ in aroma and taste. Similiarly, L. fermentati KBI 12.1 NAB was described as having ‘honey’, ‘malty’, and ‘wort-like’ flavour attributes, while co-fermentation with L. plantarum FST 1.7 resulted in the beer being perceived as ‘fruity’ and ‘citrus’ in aroma and flavour, along with a ‘sour’ and ‘acidic’ taste. In intensity rankings, WLP001 + FST 1.7 and KBI 12.1 + FST 1.7 beers were evaluated as less sweet smelling with average values of 4.9 and 3.8 out of 10, respectively, compared to values of 5.6 and 4.5 out of 10 for S. cerevisiae WLP001 and L. fermentati KBI 12.1 fermented alone; however, the difference was not statistically significant. In terms of a ‘honey’ flavour, S. cerevisiae WLP001 was ranked the highest with an average rating of 6.1 out of 10, followed by L. fermentati KBI 12.1 with an average rating of 5.9 out of 10. Interestingly, co-fermentation decreased the perceived intensity of this ‘honey’ flavour, with ratings of 5.2 and 4.9 out of 10 recorded for WLP001 + FST 1.7 and KBI 12.1 + FST 1.7, respectively (p < 0.05). Notably, the WLP001 + FST 1.7 and KBI 12.1 + FST 1.7 NABs produced as a result of co-fermentation were evaluated as being sourer than their WLP001 and KBI 12.1 counterparts, however, not significantly. With regards to beers produced with C. subsufficiens yeast, 66% and 75% of panelists described C6.1 NAB as having a ‘fruity’ aroma and flavour, with notes of pineapple, pear and melon perceived. Similar ‘fruity’ and ‘citrus’ flavours and aromas were described for C6.1 + FST 1.7 beer. Correspondingly, the average intensities of a ‘fruity’ flavour (6.8–7.0 out of 10) and ‘fruity’ aroma (6.5–7.3 out of 10) in both C6.1 and C6.1 + FST 1.7 beers were higher than that of all the other beers (3.1–5.3 out of 10), significantly so (p < 0.05) in the case of WLP001 and WLP001 + FST 1.7. Over 75% of the panelists described C6.1 + FST 1.7 as ‘sour’, with an average intensity of 8.4 out of 10 recorded for this attribute, significantly higher than the other beers (p < 0.01). Consequently, this beer was also perceived as significantly less ‘sweet’ tasting than the other samples (p < 0.05). Regarding overall acceptability, KBI 12.1 + FST 1.7 was ranked the highest with an average rating of 7.0 out of 10, followed closely by L. fermentati KBI 12.1 (6.8 out of 10) and C. subsufficiens C6.1 (6.6 out of 10). C6.1 + FST 1.7 had a slightly lower acceptability at 6.0 out of 10, with several panelists commenting that the beer was ‘too sour’. WPL001 and WLP001 + FST 1.7 had comparable acceptability scores of 6.8 and 6.0 out of 10, respectively. However, it should be noted that differences in overall acceptability scores were not statistically significant.
Discussion
Limited fermentation and the use of non-Saccharomyces yeasts are techniques that can be used for the production of NAB; however, such processes often result in a sweet-tasting beer with off-flavours, and which is susceptible to microbial spoilage. One approach to address this is the co-fermentation of yeast with LAB during the beer production process. To date, research on the production of beer by co-fermentation of yeast and LAB is limited, with most studies in the literature relating to sour beer production. For example, Dysvik et al. [19] produced sour beer by pre-acidification of wort with L. buchneri for 24 h and subsequent fermentation with S. cerevisiae, resulting in sour beer with ethanol contents of 3–4%. The same authors also produced alcoholic sour beer by co-fermentation of L. brevis, L. plantarum, or L. buchneri with S. cerevisiae [21]. Others have also exploited the acid-producing nature of LAB for the production of sour beer [24, 25, 30,31,32]; however, to our knowledge, co-fermentation of LAB and yeast for the production of NAB has not yet been investigated. The aim of the current study was not to produce a sour beer, but to investigate if lactic acid and other metabolite production by LAB could potentially negate the unfavourable effects of limited fermentation with non-Saccharomyces yeasts, thus producing an NAB with improved analytical and sensory attributes.
The inability of L. fermentati KBI 12.1 and C. subsufficiens C6.1 to ferment maltotriose [14, 15, 33] resulted in higher extract values and extended fermentation times of 24 h and 96 h, respectively, compared to 17 h with S. cerevisiae WLP001. Bellut et al. [33] had a similar approach in which the authors prematurely halted fermentation with L. fermentati KBI 12.1 to produce a low alcohol beer. In that case, the fermentation was allowed to proceed for a longer time period of 36 h; however, the final ethanol concentration was 1.26%, exceeding that of the 0.5% ethanol cut-off level for NAB in the current study. As expected, the pH values of the NABs in which yeasts were co-fermented with L. plantarum FST 1.7 were lower than the NABs produced with the single yeasts. The pH value of the NAB produced by L. fermentati KBI 12.1 singly was significantly lower than that of the NABs produced by S. cerevisiae WLP001 or C. subsufficiens C6.1 yeasts, due to the unusual lactic acid-producing nature of the Lachancea genus [33]. To date, the factors influencing lactic acid production by L. fermentati are poorly understood. A study by Bellut et al. [34] investigated the suitability of alternative substrates for reduced-alcohol beer production, with results showing that lactic acid production by L. fermentati KBI 12.1 differed significantly between the various substrates, even with the same fermentation parameters (time, temperature, and pitching rate) and similar sugar composition and amino acid profiles. Osburn et al. [35] reported a lactate production of 0.90 g/L by a L. fermentati strain after 1 month of fermentation in 11.4°P wort, while Domizio et al. [36] determined 0.24 g/L lactic acid in beer after 10 day fermentation with a L. thermotolerans strain. In contrast, another study utilising L. thermotolerans for beer production reported fourfold less lactic acid produced (0.06 g/L) after 14 day fermentation [37]. Despite the differing fermentation parameters in each study which are known to significantly affect lactic acid production (pitching rate, temperature, and substrate), the production of 0.41 g/L lactic acid by L. fermentati KBI 12.1 in the current study after just 24 h fermentation is remarkable. Interestingly, co-fermentation of L. fermentati KBI 12.1 with L. plantarum FST 1.7 for 24 h resulted in a lactic acid concentration similar to that obtained by Bellut et al. [15] following fermentation of L. fermentati KBI 12.1 alone for 7 days, highlighting the efficacy of LAB-yeast co-fermentation for lactic acid production. Notably, the concentration of lactic acid in the C6.1 + FST 1.7 NAB was within the range of that typically reported for alcoholic sour beers in the literature (2–9 g/L) [4, 24, 25, 38, 39]. Indeed, according to Tonsmeire [40], any beer with a pH < 3.9 is considered a sour beer; thus, all the NABs in the current study with the exception of S. cerevisiae WLP001 and C. subsufficiens C6.1 fermented samples would be classed as such. Although not the purpose of the current study, limited co-fermentation of non-Saccharomyces yeasts with L. plantarum FST 1.7, or other LAB, could be a promising strategy for the production of NAB sour beer, a research area that remains underexplored.
Reduced pH and the presence of lactic acid did not appear to impact the fermentation kinetics of the yeasts, with no significant differences observed in extract reduction, alcohol production or microbial growth between single fermentation and co-fermentations. Similar observations have been made by Chan et al. [25] and Carvalho et al. [41]; however, this is believed to be highly strain-dependent [42]. Dysvik et al. [19] investigated a range of LAB pre-fermentation methods and reported that the growth kinetics of S. cerevisiae were impaired only when the strain was inoculated into beer containing viable L. buchneri cells. This in turn lead the authors to believe that it was not the pH, lactic acid concentration or the presence of LAB which negatively impacted yeast growth, but rather the viability of the LAB at the time of yeast inoculation [19]. The production of antifungal compounds by the LAB should also be considered as a possible reason for yeast growth impairment in the study [43, 44]. The yeasts used in this study had previously been characterised as relatively acid-tolerant, with no growth impairment reported at pH values as low as 3.5 for S. cerevisiae WLP001 and L. fermentati KBI 12.1 and pH 4 for C. subsufficiens C6.1 [14, 33]. Microbial growth of L. plantarum FST 1.7 also remained stable throughout the co-fermentations. Previous work by Peyer et al. [22] established the ability of L. plantarum FST 1.7 to grow well in barley malt extract, reaching the highest cell numbers and lowest pH of the 4 LAB species tested. L. plantarum FST 1.7 was also able to better withstand lower pH levels with growth continuing until pH 3.0 was reached, while the other species were inhibited at pH 3.5–4.0, highlighting the suitability of this strain for LAB-yeast co-fermentation to produce NAB.
Sugar consumption by yeast and LAB cultures can majorly influence the sensory attributes of the beer, as fructose is perceived as twofold and threefold sweeter tasting than glucose and maltose, respectively. Thus, not only is the perceived sweetness of the beer influenced by the total level of residual sugars, but also by the ratio in which those sugars the present. In this study, maltotriose was not utilised by the non-Saccharomyces yeasts and maltose utilisation was negligible, resulting in slower ethanol production and higher extract values in the NABs. The previously reported glucophilic nature of the yeast strains and L. plantarum FST 1.7 were observed [14, 15, 22, 33], with glucose being the preferred sugar source in each fermentation. Interestingly, glucose was fully depleted in the WLP001 + FST 1.7 and KBI 12.1 + FST 1.7 NABs after 17 and 24 h fermentation; however, over 30% of the initial glucose content remained in the C6.1 + FST 1.7 NAB after 96 h fermentation. Fructose levels remained relatively unchanged compared to initial wort levels, with no significant consumption of the sugar observed during any of the fermentations, even when glucose was fully depleted. This is in contrast to observations by Bellut et al. [15], whereby fructose was fully depleted along with glucose after 24 h fermentation with S. cerevisiae WLP001 or L. fermentati KBI 12.1, although it should be noted that the limit of quantification was much higher at 90 mg/L compared to 0.5 mg/L in the current study. In another study, the authors reported that fructose levels after 24 h fermentation with L. fermentati KBI 12.1 were similar to the starting concentration in the wort, and fully depleted after 36 h fermentation; hence, it is likely that the short fermentation time in the current study interrupted assimilation of the sugar. Additionally, it has been shown that substrate utilisation by yeast is highly dependent on fermentation parameters, such as temperature, pitching rate, wort extract, and so on [14, 33]. In the case of C6.1 and C6.1 + FST 1.7 NABs, fructose was not utilised due to the continued presence of glucose until the end of the fermentation; similar trends have been observed in other studies of Cyberlindnera spp. fermentation [14, 45]. It should also be considered that fermentation is highly depended on the sugar composition of wort, which can be altered to achieve different levels of fermentable and unfermentable sugars. Endres et al. [46] recently developed a single-step mashing procedure at 72 °C, a temperature at which β-amylase is denatured, but the heat-stable enzyme α-amylase retains its activity, resulting in a wort with decreased glucose and maltose contents and increased levels of the unfermentable sugars maltotetraose and maltoheptaose. Using this, a reduced fermentation time of 6 days and a final ethanol concentration of 3.4% were obtained in comparison to a 10–11 day fermentation and an ethanol content of 4.4% with conventional wort. Bellut et al. [34] observed limited ethanol production during fermentation of wort produced from alternative substrates (pulses, cereals, and pseudocereals), due to the low levels of fermentable sugars. Such strategies could be promising for the production of NAB by limited co-fermentation of LAB and yeast, allowing for longer fermentation times and decreased ethanol production.
Free amino nitrogen (FAN) is a measure of the nitrogenous compounds in beer, comprising amino acids, ammonia, small peptides, and individual alpha-acids. A minimum FAN content of 100–130 mg/L in fermentation wort is recommended, while 200–250 mg/L regarded as the optimum level, with insufficient amounts resulting in decreased fermentation rates and limited attenuation [47, 48]. Although the FAN content of the wort in the current study was lower than the recommended value, it is clear from high residual FAN contents of the NABs that the nitrogenous compounds were present in sufficient amounts for the limited time the fermentations were allowed to proceed. FAN content did not differ significantly between NABs fermented with S. cerevisiae WLP001 or L. fermentati KBI 12.1, regardless of whether L. plantarum FST 1.7 was present. The lowest level of FAN assimilation was evident in the C. subsufficiens C6.1 NAB with just 3% of the initial amount consumed during the 96 h fermentation. A similar scenario was observed by Bellut et al. [14] with just 10% of wort FAN consumed after 13 days of fermentation with the same strain. In the current study, FAN consumption increased to 25% when C. subsufficiens C6.1 was co-fermented with L. plantarum FST 1.7 indicating that most of the FAN utilised during this fermentation could be attributed to LAB metabolism. L. plantarum has previously been shown to consume between 15 and 32% FAN when fermented for 24–120 h, with consumption highly dependent on the substrate [22, 23, 49]. FAN consumption by L. fermentati KBI 12.1 has been reported to be relatively low at around 20–30% utilisation after 7–13 day fermentation [15, 33]. Approx. 22% of FAN was consumed by the strain in the current study after 24 h, indicating that the majority of nitrogen metabolism may occur during the early stages of the fermentation.
Higher alcohol concentrations in the WLP001 and KB1 12.1 NABs were similar, with slight increases observed when co-fermentation with L. plantarum FST 1.7 occurred; however, this increase was not statistically significant. In contrast, C6.1 and C6.1 + FST 1.7 demonstrated less than half the amount of higher alcohols, mainly due to lower production of isoamyl alcohol (banana, alcoholic flavour) and 2-phenyl ethanol (rose flavour). The trends in isoamyl alcohol production by the yeasts are concurrent with the literature, with S. cerevisiae WLP001 previously shown to produce more of the compound than L. fermentati KBI 12.1 and C. subsufficiens C6.1 [14, 15, 33]. Low production of 2-phenylethyl alcohol by Cyberlindnera spp. has also been documented [45, 50]. Overall, the levels of higher alcohols in each NAB were well below their respective thresholds. Cyberlindnera spp. have been well characterised in terms of their ester-producing abilities [14, 50, 51] with C. subsufficiens C6.1 producing significantly higher levels than the other yeasts in the current study, mainly ethyl acetate. Ethyl acetate is described as having a fruity flavour but also solvent-like, especially in high concentrations [14]. This solvent-like taste was particularly evident in pre-trials following fermentation with C. subsufficiens C6.1 for > 96 h (data not shown), where ethyl acetate likely reached levels exceeding its flavour threshold of 33 mg/L. Interestingly, the concentration of ethyl acetate in the C6.1 + FST 1.7 NAB was almost double that of the C. subsufficiens C6.1 NAB, indicating that co-fermentation had a significant impact on the production of this ester. Depletion of more than half of the acetic acid present in the fermentation wort by C6.1 and C6.1 + FST1.7 suggests that the high levels of acetate esters in these NABs may have been produced as a result of the esterification of acetate with ethanol by the yeast and/or LAB species [52,53,54]. Alternatively, it has been hypothesised that suboptimal growth conditions (pH, temperature, and limited substrate) may reduce the flux of acetyl-CoA into the yeast tricarboxylic acid cycle (TCA), resulting in an accumulation of acetyl-CoA which reacts with ethanol to form ethyl acetate [52]. Isoamyl acetate, with a threshold of 1.6 mg/L, is described as having a banana aroma. Concentrations produced by C6.1 and C6.1 + FST 1.7 were also higher than in the other NABs; however, they were lower than values reported in the literature for Cyberlindnera spp. (0.70–7.5 mg/L), likely due to the shorter fermentation period [2, 50, 51]. Similar to the higher alcohols, all esters were below their sensory thresholds. However, it should be considered that the reported thresholds are for alcoholic beer. As NAB is substantially lower in volatile compounds and ethanol which have masking activities, it is possible that sensory thresholds for non-alcoholic beverages may be lower [50, 55]. Additionally, synergistic effects between compounds may also result in an impact on flavour, even below the threshold [19]. The concentration of diacetyl, a compound which is regarded as undesirable in beer due to its buttery flavour, was above the threshold of 0.1 mg/L in three NABs. Interestingly, the diacetyl levels in L. fermentati KBI 12.1 NAB exceeded the threshold; however, levels were reduced almost threefold and decreased below the threshold after co-fermentation with L. plantarum FST 1.7. Significantly higher levels of acetoin were determined when S. cerevisiae WLP001 and L. fermentati KBI 12.1 were co-fermented with L. plantarum FST 1.7. Diacetyl, acetoin, and butanediol are by-products of yeast fermentation and can also be produced from LAB citrate metabolism [56,57,58], an organic compound which was naturally present in the wort and utilised during fermentation. α-Acetolactate is a metabolite produced by both yeast and LAB, which once synthesised is unstable and is decarboxylated to acetoin by α-acetolactase decarboxylase (α-ALD) or can be non-enzymatically decarboxylated to diacetyl (under aerobic conditions). Acetoin may also be produced from diacetyl by diacetyl reductase (DAR) [56,57,58]. Studies have shown that α-acetolactate synthesis is enhanced at acidic pH [59, 60], a possible explanation as to why acetoin concentrations were increased in the LAB co-fermented beers. After primary fermentation, yeasts have the capability to reabsorb diacetyl and produce 2,3-butanediol via acetoin [47]. From the results, it is clear that this reaction was not complete in the case of WLP001 + FST 1.7 and KBI 12.1 + FST 1.7 NABs; however, the lower levels of both diacetyl and acetoin in the C6.1 + FST 1.7 NAB suggested that the longer fermentation time allowed completion of this conversion.
Although below their respective thresholds, the increased ester production by C. subsufficiens C6.1 was clearly detected in the sensory evaluation, with C6.1 and C6.1 + FST 1.7 NABs ranking highest in fruity taste and aroma, and assigned descriptions, such as ‘pear’, ‘melon’, and ‘pineapple’. Despite having the highest levels of residual sugars, C6.1 NAB was not evaluated as significantly sweeter smelling or tasting than the other samples, indicating that the fruity flavour and aroma masked the wort-like off flavour. Additionally, the main residual sugar in this sample was maltotriose which is significantly less sweet tasting than the other carbohydrates in the beer. Due to the intense acidity of the C6.1 + FST 1.7 beer, it was ranked as the least sweet tasting; however, the acceptability of this sample was decreased compared to C6.1 NAB, with some panelists considering it ‘too sour’. However, it should be considered that a decreased co-fermentation time of less than 96 h could potentially result in an NAB with a fruity flavour and a balanced ratio of acidity and sweetness, without being perceived as overly sour. Alternatively, the intense sourness of this sample could be exploited to produce a sour NAB, research on which is sparse. Of note is the significant reduction in the perceived honey flavour of the WLP001 and KBI 12.1 beers which were co-fermented with L. plantarum FST 1.7, underlining the previously highlighted contribution of LAB fermentation to the sensory properties of beer [61,62,63]. KBI 12.1 + FST 1.7 was ranked as the most acceptable beer in the sensory evaluation. The lactic acid content of 1.13 g/L in the NAB far exceeded the sensory threshold of 80 mg/L; this was perceived in the sensory evaluation with this beer being ranked as sourer than the non-co-fermented beer; however, the difference was not statistically significant. Additionally, the increased sourness did not appear to be off-putting to the panellists and did not negatively affect the acceptability of the product.
Conclusion
In summary, co-fermentation of L. fermentati KBI 12.1 and C. subsufficiens C6.1 with L. plantarum FST 1.7 was found to be a promising strategy for the production of NAB (< 0.5% ethanol). Due to the limited fermentative capacity of the yeasts and subsequently lower ethanol production, fermentation time could be extended beyond that of which was possible with the control yeast, S. cerevisiae WLP001. Short co-fermentation of L. fermentati KBI 12.1 with L. plantarum FST 1.7 produced an NAB with a relatively high concentration of lactic acid, lower levels of residual sugars, and a reduction in the amount of diacetyl to below the sensory threshold. Moreover, the beer was rated the most acceptable in the sensory evaluation. NAB produced by C6.1 + FST 1.7 was described as having a fruity taste and aroma which masked the wort-like flavours/aromas due to the high levels of residual sugars. The high concentration of lactic acid in this sample resulted in it being evaluated as too sour by some panelists; however, this could be exploited to produce an NAB sour beer or alternatively, future work could investigate the alteration of the fermentation parameters to produce a fruity beer with a more balanced acidity. Additionally, further work could explore the impact of different LAB species and strains on the fermentation kinetics and sensory attributes of NABs. Overall, this study highlights the potential of limited co-fermentation of non-Saccharomyces yeasts and LAB for application in non-alcoholic beer brewing.
Cell counts of a S. cerevisiae WLP001 singly (black line) and S. cerevisiae WLP001 in co-fermentation (CF; white bar) with L. plantarum FST 1.7 (grey bar) b L. fermentati KBI 12.1 singly (black line) and L. fermentati KBI 12.1 in CF (white bar) with L. plantarum FST 1.7 (grey bar) c C. subsufficiens C6.1 singly (black line) and C. subsufficiens C6.1 in CF (white bar) with L. plantarum FST 1.7 (grey bar)
References
Europe Economics (2021) Economic report to assess the impact of Covid-19 on the brewing sector in Europe 2020. https://brewersofeurope.org/uploads/mycms-files/documents/publications/2021/covid-impact-report-final.pdf. Accessed 05 May 2022
Bellut K, Arendt EK (2019) Chance and challenge: non-Saccharomyces yeasts in nonalcoholic and low alcohol beer brewing—a review. J Am Soc Brew Chem 77:77–91. https://doi.org/10.1080/03610470.2019.1569452
World Health Organisation (2018) Global status report on alcohol and health 2018. https://apps.who.int/iris/bitstream/handle/10665/274603/9789241565639-eng.pdf?ua=1. Accessed 02 Mar 2022
Adamenko K, Kawa-Rygielska J, Kucharska AZ (2020) Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem 312:125968. https://doi.org/10.1016/j.foodchem.2019.125968
The Business Research Company (2022) Non-alcoholic beer global market report 2022 - market size, trends and global forecast 2022–2026. https://www.thebusinessresearchcompany.com/report/nonalcoholic-beer-global-market-report. Accessed 24 Feb 2022
Kokole D, Jané Llopis E, Anderson P (2022) Non-alcoholic beer in the European Union and UK: Availability and apparent consumption. Drug Alcohol Rev 41:550–560. https://doi.org/10.1111/dar.13429
Müller M, Bellut K, Tippmann J, Becker T (2017) Physical methods for dealcoholization of beverage matrices and their impact on quality attributes. ChemBioEng Rev 4:310–326. https://doi.org/10.1002/cben.201700010
Salanță LC, Coldea TE, Ignat MV et al (2020) Non-alcoholic and craft beer production and challenges. Processes 8:1–22. https://doi.org/10.3390/pr8111382
Kozłowski R, Dziedziński M, Stachowiak B, Kobus-Cisowska J (2021) Non- and low-alcoholic beer – popularity and manufacturing techniques. Acta Sci Pol Technol Aliment 20:347–357. https://doi.org/10.17306/j.afs.0961
Michel M, Meier-Dörnberg T, Jacob F et al (2016) Review: Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J Inst Brew 122:569–587. https://doi.org/10.1002/jib.381
De Francesco G, Turchetti B, Sileoni V et al (2015) Screening of new strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to produce low-alcohol beer. J Inst Brew 121:113–121. https://doi.org/10.1002/jib.185
Bellut K, Michel M, Zarnkow M, et al (2018) Application of non-Saccharomyces yeasts isolated from kombucha in the production of alcohol-free beer. Fermentation 4:. https://doi.org/10.3390/fermentation4030066
Jiang Z, Yang B, Liu X et al (2017) A novel approach for the production of a non-alcohol beer (≤0.5% abv) by a combination of limited fermentation and vacuum distillation. J Inst Brew 123:533–536. https://doi.org/10.1002/jib.465
Bellut K, Michel M, Zarnkow M et al (2019) Screening and application of Cyberlindnera yeasts to produce a fruity, non-alcoholic beer. Fermentation. https://doi.org/10.3390/fermentation5040103
Bellut K, Michel M, Hutzler M et al (2019) Investigation into the potential of Lachancea fermentati strain KBI 12.1 for low alcohol beer brewing. J Am Soc Brew Chem 77:157–169. https://doi.org/10.1080/03610470.2019.1629227
Callejo MJ, García Navas JJ, Alba R et al (2019) Wort fermentation and beer conditioning with selected non-Saccharomyces yeasts in craft beers. Eur Food Res Technol 245:1229–1238. https://doi.org/10.1007/s00217-019-03244-w
Vaštík P, Šmogrovičová D, Kafková V, et al (2020) Production and characterisation of non-alcoholic beer using special yeast. Kvas Prum 66:336–344. https://doi.org/10.18832/kp2019.66.336
Estela-Escalante WD, Rosales-Mendoza S, Moscosa-Santillán M, González-Ramírez JE (2016) Evaluation of the fermentative potential of Candida zemplinina yeasts for craft beer fermentation. J Inst Brew 122:530–535. https://doi.org/10.1002/jib.354
Dysvik A, Liland KH, Myhrer KS et al (2019) Pre-fermentation with lactic acid bacteria in sour beer production. J Inst Brew 125:342–356. https://doi.org/10.1002/jib.569
Peyer LC, Zarnkow M, Jacob F et al (2017) Sour brewing: Impact of Lactobacillus amylovorus FST2.11 on technological and quality attributes of acid beers. J Am Soc Brew Chem 75:207–216. https://doi.org/10.1094/ASBCJ-2017-3861-01
Dysvik A, La Rosa SL, Liland KH et al (2020) Co-fermentation involving Saccharomyces cerevisiae and Lactobacillus species tolerant to brewing-related stress factors for controlled and rapid production of sour beer. Front Microbiol 11:1–16. https://doi.org/10.3389/fmicb.2020.00279
Peyer LC, Zannini E, Jacob F, Arendt EK (2015) Growth study, metabolite development, and organoleptic profile of a malt-based substrate fermented by lactic acid bacteria. J Am Soc Brew Chem 73:303–313. https://doi.org/10.1094/ASBCJ-2015-0811-01
Peyer LC, Bellut K, Lynch KM et al (2017) Impact of buffering capacity on the acidification of wort by brewing-relevant lactic acid bacteria. J Inst Brew 123:497–505. https://doi.org/10.1002/jib.447
Mahanta S, Sivakumar PS, Parhi P et al (2022) Sour beer production in India using a coculture of Saccharomyces pastorianus and Lactobacillus plantarum: optimization, microbiological, and biochemical profiling. Brazilian J Microbiol. https://doi.org/10.1007/s42770-022-00691-8
Chan AMZ, Chua JY, Toh M, Liu SQ (2019) Survival of probiotic strain Lactobacillus paracasei L26 during co-fermentation with S. cerevisiae for the development of a novel beer beverage. Food Microbiol 82:541–550. https://doi.org/10.1016/j.fm.2019.04.001
Dal Bello F, Clarke CI, Ryan LAM et al (2007) Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J Cereal Sci 45:309–318. https://doi.org/10.1016/j.jcs.2006.09.004
Ispiryan L, Heitmann M, Hoehnel A et al (2019) Optimization and validation of an HPAEC-PAD method for the quantification of FODMAPs in cereals and cereal-based products. J Agric Food Chem 67:4384–4392. https://doi.org/10.1021/acs.jafc.9b00382
Pinu FR, Villas-Boas S (2017) Rapid quantification of major volatile metabolites in fermented food and beverages using gas chromatography-mass spectrometry. Metabolites. https://doi.org/10.3390/metabo7030037
Johnsen LG, Skou PB, Khakimov B, Bro R (2017) Gas chromatography—mass spectrometry data processing made easy. J Chromatogr A 1503:57–64. https://doi.org/10.1016/j.chroma.2017.04.052
Ciosek A, Rusiecka I, Poreda A (2020) Sour beer production: impact of pitching sequence of yeast and lactic acid bacteria. J Inst Brew 126:53–58. https://doi.org/10.1002/jib.590
Ciosek A, Fulara K, Hrabia O et al (2020) Chemical composition of sour beer resulting from supplementation the fermentation medium with magnesium and zinc ions. Biomolecules 10:1–14. https://doi.org/10.3390/biom10121599
Dysvik A, La Rosa SL, Buffetto F et al (2020) Secondary lactic acid bacteria fermentation with wood-derived xylooligosaccharides as a tool to expedite sour beer production. J Agric Food Chem 68:301–314. https://doi.org/10.1021/acs.jafc.9b05459
Bellut K, Krogerus K, Arendt EK (2020) Lachancea fermentati strains isolated from kombucha: fundamental insights, and practical application in low alcohol beer brewing. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.00764
Bellut K, Michel M, Zarnkow M et al (2019) On the suitability of alternative cereals, pseudocereals and pulses in the production of alcohol-reduced beers by non-conventional yeasts. Eur Food Res Technol 245:2549–2564. https://doi.org/10.1007/s00217-019-03372-3
Osburn K, Amaral J, Metcalf SR et al (2018) Primary souring: A novel bacteria-free method for sour beer production. Food Microbiol 70:76–84. https://doi.org/10.1016/j.fm.2017.09.007
Domizio P, House JF, Joseph CML et al (2016) Lachancea thermotolerans as an alternative yeast for the production of beer. J Inst Brew 122:599–604. https://doi.org/10.1002/jib.362
Zdaniewicz M, Satora P, Pater A, Bogacz S (2020) Low lactic acid-producing strain of Lachancea thermotolerans as a new starter for beer production. Biomolecules 10. https://doi.org/10.3390/biom10020256
Tipler A, Reuter WM, Chadwick LR (2015) The analysis of lactic and acetic acids in sour beers by HPLC with PDA detection
Witrick KA (2012) Characterization of aroma and flavor compounds present in lambic (gueuze) beer. Virginia Polytechnic Institute and State University
Tonsmeire M (2014) American sour beers: innovative techniques for mixed fermentation. Brewers Publications
Carvalho FP, Duarte WF, Dias DR et al (2015) Interaction of Saccharomyces cerevisiae and Lactococcus lactis in the fermentation and quality of artisanal cachaca. Acta Sci—Agron 37:51–60. https://doi.org/10.4025/actasciagron.v37i1.18397
Rogers CM, Veatch D, Covey A et al (2016) Terminal acidic shock inhibits sour beer bottle conditioning by Saccharomyces cerevisiae. Food Microbiol 57:151–158. https://doi.org/10.1016/j.fm.2016.02.012
Nasrollahzadeh A, Mokhtari S, Khomeiri M, Saris PEJ (2022) Antifungal preservation of food by lactic acid bacteria. Foods 11:1–18. 10.3390oods11030395
Chen H, Yan X, Du G, et al (2021) Recent developments in antifungal lactic acid bacteria: Application, screening methods, separation, purification of antifungal compounds and antifungal mechanisms. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1977610
Liu SQ, Quek AYH (2016) Evaluation of beer fermentation with a novel yeast Williopsis saturnus. Food Technol Biotechnol 54:403–412. https://doi.org/10.17113/ftb.54.04.16.4440
Endres F, Prowald A, Fittschen UEA et al (2022) Constant temperature mashing at 72 °C for the production of beers with a reduced alcohol content in micro brewing systems. Eur Food Res Technol. https://doi.org/10.1007/s00217-022-03968-2
Krogerus K, Gibson BR (2013) 125th anniversary review: Diacetyl and its control during brewery fermentation. J Inst Brew 119:86–97. https://doi.org/10.1002/jib.84
Hill AE, Stewart GG (2019) Free amino nitrogen in brewing. Fermentation 5. https://doi.org/10.3390/fermentation5010022
Byakika S, Mukisa IM, Byaruhanga YB (2020) Sorghum malt extract as a growth medium for lactic acid bacteria cultures: a case of Lactobacillus plantarum MNC 21. Int J Microbiol 2020. https://doi.org/10.1155/2020/6622207
Methner Y, Dancker P, Maier R et al (2022) Influence of varying fermentation parameters of the yeast strain Cyberlindnera saturnus on the concentrations of selected flavor components in non-alcoholic beer focusing on (E)-β-Damascenone. Foods 11:1–24
Methner Y, Hutzler M, Zarnkow M et al (2021) Investigation of non-Saccharomyces yeast strains for their suitability for the production of non-alcoholic beers with novel flavor profiles. J Am Soc Brew Chem 1–15. https://doi.org/10.1080/03610470.2021.2012747
Löser C, Urit T, Keil P, Bley T (2015) Studies on the mechanism of synthesis of ethyl acetate in Kluyveromyces marxianus DSM 5422. Appl Microbiol Biotechnol 99:1131–1144. https://doi.org/10.1007/s00253-014-6098-4
Lu H, Huang C, Yu K, Liu Z (2022) Effects of mixed inoculation of Leuconostoc citreum and Lactobacillus plantarum on suansun (sour bamboo shoot) fermentation. Food Biosci 47:101688. https://doi.org/10.1016/j.fbio.2022.101688
Yi C, Li Y, Zhu H et al (2021) Effect of Lactobacillus plantarum fermentation on the volatile flavors of mung beans. LWT - Food Sci Technol 146:111434. https://doi.org/10.1016/j.lwt.2021.111434
Perpète P, Collin S (2000) Influence of beer ethanol content on the wort flavour perception. Food Chem 71:379–385. https://doi.org/10.1016/S0308-8146(00)00179-5
Laëtitia G, Pascal D, Yann D (2014) The citrate metabolism in homo- and heterofermentative LAB: a selective means of becoming dominant over other microorganisms in complex ecosystems. Food Nutr Sci 05:953–969. https://doi.org/10.4236/fns.2014.510106
Quintans NG, Blancato V, Repizo G et al (2008) Citrate metabolism and aroma compound production in lactic acid bacteria. Mol Asp Lact Acid Bact Tradit New Appl 37(661):65–88
Wang Y, Wu J, Lv M et al (2021) Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front Bioeng Biotechnol 9:1–19. https://doi.org/10.3389/fbioe.2021.612285
Cogan TM, Dowd MO (1981) Effects of pH and sugar on acetoin production from citrate by Leuconostoc lactis. Appl Environ Microbiol 41:1–8
Mcfall SM, Montville TJ (1988) pH-mediated regulation of pyruvate catabolism in Lactobacillus plantarum chemostat cultures. 4:335–340
Salmerón I, Loeza-Serrano S, Pérez-Vega S, Pandiella SS (2015) Headspace gas chromatography (HS-GC) analysis of imperative flavor compounds in Lactobacilli-fermented barley and malt substrates. Food Sci Biotechnol 24:1363–1371. https://doi.org/10.1007/s10068-015-0175-z
Nsogning Dongmo S, Procopio S, Sacher B, Becker T (2016) Flavor of lactic acid fermented malt based beverages: Current status and perspectives. Trends Food Sci Technol 54:37–51. https://doi.org/10.1016/j.tifs.2016.05.017
Mukisa IM, Byaruhanga YB, Muyanja CMBK et al (2017) Production of organic flavor compounds by dominant lactic acid bacteria and yeasts from Obushera, a traditional sorghum malt fermented beverage. Food Sci Nutr 5:702–712. https://doi.org/10.1002/fsn3.450
Acknowledgements
The authors would like to thank Luk Daenan and David P. De Schutter for their support of this manuscript. The authors would also like to thank Tom Hannon for his technical support and Dr. Lilit Ispiryan for her assistance in performing the HPLC and IC analyses.
Funding
This work was supported by the Baillet Latour Fund within the framework of a scholarship for doctoral students.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Compliance with ethics requirement
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contribution for the Special Issue: The chemistry behind malt and beer production—from raw material to product quality.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nyhan, L., Sahin, A.W. & Arendt, E.K. Co-fermentation of non-Saccharomyces yeasts with Lactiplantibacillus plantarum FST 1.7 for the production of non-alcoholic beer. Eur Food Res Technol 249, 167–181 (2023). https://doi.org/10.1007/s00217-022-04142-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04142-4