Abstract
The aim of the study was the physicochemical characterization of wines produced using indigenous yeasts isolated from spontaneously fermented grape musts, obtained from cold climate grapes. Saccharomyces cerevisiae MH020215 and Nakawazaea ishiwadae MG971259 yeast strains were used in this study. The musts obtained from white and red grapes of Johanniter and regent varieties were used as a fermentation raw material. In the produced wines, content of ethyl alcohol, total extract, sugars, free amino nitrogen was analyzed, along with determination of total and volatile acidity and volatile compounds profile. Additionally, organoleptic evaluation was performed. Wines obtained with native S. cerevisiae MH020215 strains were characterized with more favorable enological properties. Synthesis of desirable volatile compounds, especially esters, contributed to the creation of desirable aromatic profile of those wines. Moreover, those beverages contained higher levels of carbonyl compounds (especially acetaldehyde) and lower methanol content. Wines obtained using N. ishiwadae MG971259 cultures were represented by high total acidity level and substantial fusel alcohol content (mainly butanol, propanol), which resulted in an unfavorable sensory profile of the product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wine is a complex product obtained by biological and biochemical transformations by wine microorganisms and during wine aging [1, 2]. The most significant role in the production of this beverage is played by yeasts, which conduct the alcoholic fermentation [3, 4]. In some instances, those microorganisms can exhibit undesirable properties that may contribute to wine deterioration, especially during its maturation and after the beverage has been bottled [5]. Yeasts present during fermentation, aside from pure cultures, mainly come from vine fruit, winery environment and wine equipment. Wine grape must is widely regarded as a nutrient-rich environment for many microorganisms, however it’s low pH and high osmotic pressure, as well as the addition of sulfur dioxide to the grape juice impairs their growth. Aside from Saccharomyces strains, other yeast microorganisms that naturally occur during wine production shows metabolic activity to a greater or lesser extent, and the intermediate compounds they produce have an effect on the final quality of the drink [1, 3, 4, 6, 7].
The international competitiveness and consumer demand for unique wine styles are the challenges in improving the fermentation process. This stage, being crucial for wine production, creates the possibility of utilizing new directions in the usage of the yeasts. Perspective of using non-Saccharomyces strains as well as mixed cultures for grape must fermentation makes the issues of yeast oenology and the metabolic reactions they are carrying out increasingly important in determining the quality of wine. To this day, a lot of research has been done regarding the use of non-Saccharomyces and their mixed cultures in the fermentation process [2, 3, 6,7,8,9,10,11,12,13,14,15,16]. However, there is little experience on this subject when yeast strains, as well as vine varieties from cold climate zones are used. By decision of the Council of the European Union of 20 December 2005, the territory of Poland belongs to the zone A (the coldest) of wine growing zones in Europe. This zone, referred to as ‘cool climate’ is usually characterized by having an average temperature of about 15 °C in the month preceding the harvest. Despite the favorable tendency of increasing temperatures caused by climate changes, in Poland we have much more difficult conditions for growing vines than in traditional wine regions. Due to climatic and soil conditions, the obtained grapes are characterized by a lower content of sugars (usually 17–23%), and thus a low level of alcohol, higher acidity and content of polyphenolic compounds [17]. The climate has an impact on the chemical composition of the grapes, the species diversity of yeasts present on the fruit and grape must, and ultimately on the quality of the wine. Grape varieties currently cultivated in Poland are typical for a cool climate. There are no comprehensive analyses of the microbiological and chemical composition of grape must and wines obtained from grapes from our climate.
The aim of the study was the physicochemical characterization of wines produced using indigenous yeasts isolated from spontaneously fermented grape musts, obtained from cold climate grapes.
Materials and methods
Materials
Yeast strains
Saccharomyces cerevisiae MH020215 and Nakawazaea ishiwadae MG971259 yeast strains isolated from spontaneously fermented grape musts, originating from cold climate zone grapes were used for the research. The isolation and identification of yeast strains was carried out by the Authors at the Department of Fermentation Technology and Microbiology (University of Agriculture in Krakow). Methods for isolation and identifying microorganisms are described in detail in previous publications [18,19,20]. The identification of yeasts was based on the morphological characteristics of the colonies, biochemical tests and molecular techniques (DNA extraction and RAPD-PCR analysis, amplification of the 5.8S-ITS rRNA gene region, PCR-RFLP analysis, 5.8S-ITSrRNA gene region sequencing).
Yeast strains used in the article were characterized by favorable oenological properties [21].
Grape musts
The musts obtained from red and white grapes of Regent and Johanniter varieties respectively, deriving from Srebrna Góra vineyard (Cracow, Poland), were used as the fermentation raw material.
Inoculum preparation
Pure yeast cultures were passaged in three stages. In the first stage, the strains were propagated on Sabouraud slants (Biocorp, Poland) for 24 h, then subsequently transferred to 10 ml of liquid Sabouraud medium (Biocorp, Poland). After another 24 h, dynamic propagation was conducted in 200 ml of liquid Sabouraud medium (Biocorp, Poland) during the next 48 h on a water bath shaker (120 rpm, 28 °C).
Upon completion of the yeast multiplication process, their dry weight was determined with a moisture analyzer, and then the adequate amount of yeast slurry was centrifuged (10 min, 4 989 × g/min). The precipitate was washed with sterile water, centrifuged again under the same conditions and set into the grape must.
Grape musts fermentation
The raw material used for fermentation were musts from white and red grapes of the Johanniter and Regent varieties. The grapes were pressed on a basket press, and the obtained must was pasteurized. When cooled down, potassium pyrosulfite was added (100 mg SO2/L). The multiplied yeast slurry was introduced to the set in an amount of 0.05 g d.w./100 mL. Fermentation was conducted in 2 L flasks, containing 1 L of the must. After carefully closing the fermentation flasks and fixing fermentation tubes filled with glycerin, the setup was additionally sealed with a parafilm. The fermentation process was carried out for 28 days at 20 °C (in three independent repetitions).
Methods
Determination of fermentation kinetics
Fermentation kinetics were determined based on the weight losses of the setups during the process. For the first 7 days, weight losses were measured daily, and then every 2 days, until the end of fermentation. The process was completed at the moment of achieving daily weight losses below 0.01 g/L.
Determination of biomass growth yield
Yeast pellet after centrifugation was washed with distilled water, and then dried on moisture analyzer to a constant mass. Yeast dry mass was presented in g/L.
Determination of ethyl alcohol content, total extract, total acidity and volatile acidity
Analyses conducted according to OIV (2012) methodologies [22].
Alcohol concentration in finished wine was determined using the pycnometric method. For this purpose, the distillation of samples after fermentation was performed. The obtained distillate was filled up to 100 mL with distilled water, its density was determined and the concentration of ethanol was read from the adequate tables.
In order to determine the real extract content, the distillation residues were quantitatively transferred to a 100 mL volumetric flask, filled up to 100 mL with distilled water and the procedure was analogous.
The potentiometric method was applied to determine general acidity, titrating a sample with 0.1 M NaOH solution to obtain pH 7.
Volatile acids were separated from the wine by steam distillation and titrated using standard sodium hydroxide.
Determination of wine volatile compounds composition (GC-SPME)
1 mL of water and analyzed wines, along with a standard solution (4-methyl-2-pentanol) at a concentration of 50.7 mg/L and 0.9 g of NaCl were added to a 15 mL vial. The vials were closed with a screw cap fitted with a Teflon seal, and placed in a laboratory thermostat at 40 ºC, mixing the vials content using a magnetic stirrer. The SPME fiber (PDMS, 100 μm, Supelco) was placed in a gas phase above the surface of the sample for 35 min. The adsorbed analytes were desorbed in an injector of a gas chromatograph for 3 min. Then calibration curves were made—functions of the relationship of a chemical compound concentration on the peak area, for the tested chemical components (acetaldehyde, acetone, methanol, butanol, amyl alcohols, isobutanol, phenylethyl alcohol, ethyl acetate, isoamyl acetate, phenethyl acetate and ethyl caproate).
Operational conditions: capillary column HP-INNOVAX (30 m; 0.53 mm; 1,0 μm); injector and detector temperature: 250 °C; programmed column temperature: maintained at 35 °C for 5 min, and then raised to 110 °C at the rate of 5 °C/min, then at a rate of 40 °C/min to 220 °C, and maintained a stable temperature for 3 min; carrier gas: helium; carrier gas flow rate: 20 mL/min; helium flow rate: 33 mL/min; air flow rate: 400 mL/min.
Determination of total sugars, reducing sugars and sucrose content using 3,5-dinitrosalicylic acid (DNS)
The calibration curve was made, testing the extinction (λ = 520 nm) of the following glucose samples: 0 g/L; 0.1 g/L; 0.2 g/L; 0.4 g/L; 0.6 g/L; 0.8 g/L; 1.2 g/L and 2 g/L. With a volumetric pipette 25 mL of wine was measured, which was later neutralized with a 10 M sodium hydroxide solution, using a pH meter to control solution’s pH. The neutralized solution was quantitatively transferred to 100 mL volumetric flask. In order to free material from proteins and other compounds that may hinder the sugar determination, 5 mL of Carrez I and Carrez II solutions were added consecutively, followed by mixing, making up to the mark with the distilled water and filtering through paper. 5 mL of that filtrate was taken up and made up to 100 mL in a volumetric flask using distilled water. After obtaining the sugar solution, it was subjected to the inversion process. 50 mL of previously prepared solution was transferred to a Erlenmeyer flask with a pipette, made up to approximately 60 mL using distilled water and 5 mL of concentrated hydrochloric acid was added. The flasks were then heated in a water bath to a temperature inside a flask of 68–70 °C, followed by holding the temperature for 5 min. After this time the flasks were quickly cooled to about 20 °C, three drops of methyl orange were added and solution was neutralized with 10 M NaOH, until the fluid changed its color. The solution was then quantitatively transferred to 100 mL volumetric flask, brought back to 20 °C and made up to the mark with distilled water. Upon the inversion completion, 2 mL of DNS, 2 mL of post-inversion sample and stock sugar solution without inversion were poured into test tubes. The mixture was boiled for about 5–10 min, after which 11 mL of distilled water was added. Differences in the content of reducing and non-reducing sugars were determined spectrophotometrically, at a light’s wavelength of λ = 520 nm.
Organoleptic evaluation
Four sensory attributes of wine were assessed (namely taste, aroma, color and clarity). An appropriate point value was assigned to individual attributes—taste 0–12 pt., aroma 0–4 pt., color 0–2 pt. and clarity 0–2 pt. Wine analyses were done in three repetitions, and the values of individual attributes were averaged and added together. Evaluation was done by a trained sensory panel of five people. The end result is presented as the average number of points for each wine, in the range of 0–20 pt.
Statistical analysis
The results presented in the study were the means of three independent repetitions with the determination of the standard deviation. The data were analyzed with the variance analysis (ANOVA) in order to establish the significance of the tested parameters. Statistically significant differences between the means were verified with the Duncan’s set using the Statistica 10 statistical software (StatSoft Polska, Cracow).
Results and discussion
Physiochemical analysis
For fermentation, musts obtained from Regent and Johanniter grape varieties were used. The chemical composition of grape juices is presented in Table 1. The juices were characterized by a relatively high content of reducing sugars (188.5 and 189.6 g/L) and a small amount of sucrose (14.0–14.2 g/L). According to the literature, glucose is used as first during the fermentation, while sucrose and fructose prolong the duration of the process [23]. The acidity reached 7.4 and 6.3 g/L for white and red musts, accordingly. Two yeast strains were used for the study—S. cerevisiae MH020215 and N. ishiwadae MG971259. The obtained wines were subjected to analyses in order to determine the content of alcohol, residual sugars, free amino nitrogen, volatile compound profile along with total and volatile acidity.
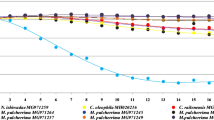
Differences in grape musts fermentation kinetics were found, depending on yeast culture used. S. cerevisiae MH020215 strains started the process just 24 h after the pitching (Fig. 1). In case of the must obtained from Johanniter variety, there was a 2 day delay in fermentation’s initiation, which could have been caused by the less favorable juice composition. N. ishwadae MG971259 yeasts showed significantly worse results in this aspect. Wines pitched with this culture were indicated by substantially slower weight loss, which was directly related to sugar utilization. Decrease in fermentation rate was observed on days 6 and 15 in musts pitched with S. cerevisiae MH020215 and N. ishiwadae MG971259 respectively. The presence of amino acids and ammonium ions is an essential condition for the proper course of the fermentation process. Deficiency of these elements along with incomplete ripeness of the vine fruit can lead to delay in the onset of fermentation, as well as increased production of hydrogen sulfide and mercaptans [24]. Juice obtained from the fruits of Johanniter variety was characterized by a lower content of amine nitrogen (73.70 mg/L) compared to fresh Regent must (92.61 mg/L), which may explain the yeasts prolonged onset of the fermentation.
When fermentation ended, the amount of yeast biomass was determined using a moisture analyzer (Table 2). N. ishiwadae cultures exhibit aerobic metabolism and show weaker fermentation capabilities. Research by Ruiz et al. [25] proves that N. ishiwadae in single inoculation was unable to complete the entire fermentation, leaving more than the 40% of initial glucose and fructose concentration. In the case of a mixed culture of N. ishiwadae and S. cerevisiae, the fermentation process was not fully completed. In turn, Boynton and Greig [26] defined N. ishiwadae as a keystone species in wine fermentations, which can dominate or determine the microbiological dynamics of wine fermentation, even in relatively small amounts. It is therefore no surprise that sugars were used to the greater extent by S. cerevisiae MH020215. The wine’s real extract consists of compounds remaining after simple distillation and is a good indicator of the sugars content and their degree of attenuation. The value of this parameter is directly related to the amount of ethyl alcohol and residual sugars. The higher the ethanol concentration, the fewer sugars remaining after fermentation, and thus the smaller the real extract. The real extract of wines obtained with S. cerevisiae MH020215 ranged from 24.5 to 27.0 g/L (Table 2). Slightly lower values were achieved in N. ishiwadae MG971259 inoculated wines. The highest amount of total and reducing sugars was recorded in the wines from the Johanniter variety, fermented with N. ishiwadae MG971259 culture (Table 2).
N. ishiwadae MG971259 cultures produced slightly less ethanol, as compared to S. cerevisiae MH020215 (Table 2). According to the literature, S. cerevisiae yeasts distinguish themselves with a good fermentation capacity, ability to easily colonize the environment and tolerate elevated alcohol content [25, 27].
The total acidity of the fermented samples, expressed as a tartaric acid, was in the range of 6.76–8.28 g/L (Table 2). This parameter is one of the differentiators which are responsible for the harmonized taste of the wine. In the finished beverage, the value of this parameter should not exceed 10 g/L, wherein the most desirable value is between 4 and 6 g/L [28, 29]. The lower acidity was observed in wines fermented with S. cerevisiae MH020215 yeast (6.76 and 8.24 g/L). N. ishiwadae MG971259 yeast has acidified wines to a higher degree. The obtained total acidity level in the white wines was the result of a higher acidity in fresh Johanniter must, as compared to Regent juice (Tables 1, 2).
Volatile acidity in wines is generally expressed in conversion to acetic acid, which is formed as a result of the transformation of ethyl alcohol into acetaldehyde, followed by its oxidation with the participation of aldehyde dehydrogenase. It should not exceed 1.3 g/L, and usually amounts from 0.2 to 0.8 g/L [29]. The level of volatile acidity in the alcoholic beverages depends on the yeast strain, fermentation temperature and sugar content in the must. Higher saccharide concentration in the wine determines a higher amount of acids. The volatile acidity of the analyzed wines was in the range from 0.10 to 0.11 g/L (Table 2).
FAN (Free Amino Nitrogen) describes the content of amino acids and short chain proteins which are found within the wine that can be used by yeast as a building material. This parameter is a measure of the free nitrogen that can be assimilated by yeast cells. The FAN content in wines depends on many factors, such as: wine grape variety, soil quality, winegrowing practices and the use of plant protection products [30]. The content of nitrogen compounds in the obtained wines was in the range of 29.13–43.04 mg/L. Taking into the consideration the concentration of these compounds in fresh grape juices (Table 1), it was found that the yeast examined in this study used low-molecular amino acids in the amount of 31.45 – 46.47%. In the fermented musts obtained from Regent variety, the lowest level of nitrogen compounds was recorded for N. ishiwadae MG971259 cultures (29.13 mg/L). In the samples of the Johanniter variety, the smallest amount was observed in juices pitched with S. cerevisiae MH020215 yeasts (32.03 mg/L).
Volatile compounds
Wine is one of the products with the most complex flavor profile, which is influenced by the grape variety, their growing conditions and the course of the fermentation, maturation and aging processes [31].
One of the main groups of compounds synthesized by yeasts are higher alcohols, also referred to as fusels. Their molecules contain more than two carbon atoms and are characterized by a higher molecular weight along with a higher boiling point in relation to ethanol. They are produced during the fermentation process, and their concentration reaches approx. 150–550 mg/L. In the chemical terms, they can be divided into aliphatic and aromatic alcohols. The first group includes propanol, isobutanol and amyl alcohols. The second, consists among others of 2-phenylethanol, tyrosol or tryptophol. Fusel alcohols are characterized by an intense aroma that plays an important role in forming a wine bouquet. At low concentration (below 300 mg/L) they positively affect its aroma, whereas their higher content masks the proper bouquet of the beverage [29]. Considering fusels, the obtained drinks were characterized by a relatively low concentration of amyl alcohols, the amount of which was in the range of 22.6–45.5 mg/L (Table 3). The highest levels of these compounds were found in wines pitched with S.cerevisiae MH020215 yeasts. The methanol content was comparable for samples obtained from both vine varieties. Wines obtained from Regent grapes fermented with the N. ishiwadae MG971259 cultures were the exception, because the amount of the compound in question was nearly twice as high. A similar trend was found for propanol (71.3 mg/L) and isobutanol (32.0 mg/L). Interestingly, an opposite trend was observed in white wines. Research by Ruiz et al. [25] also show that N. ishiwadae yeast produces high levels of isobutanol in wines. Larger amounts of the analyzed compounds were synthesized by S. cerevisiae MH020215 strains. According to the literature, these cultures produce several times less isobutanol than amyl alcohols [32], which coincides with conducted analysis.
Esters were another group of the compounds, playing an important role in shaping the sensory traits of the drink. One of the factors affecting the amount and type of these compounds are yeasts, because depending on the strain, there is a possibility of obtaining a product with a varied content of these substances. Moreover, ester concentration also depends on the fermentation temperature and the presence of nitrogen compounds in the must [33]. The highest amount of ethyl acetate was found in wines made with N. ishiwadae MG971259 yeast (26.7 and 52.1 mg/L) (Table 3). As stated in the literature, cultures that do not belong to the Saccharomyces genus are responsible for a higher levels of this component, as well as other aromatic compounds [34]. Comparable values for all wines were noted for isoamyl acetate (Table 3). Sources indicate higher production rate of this ester by the S. cerevisiae yeast, in relation to other Saccharomyces cultures [35].
Acetaldehyde may be formed due to the oxidation of ethyl alcohol, but also during the fermentation of the must in the presence of sulfurous acid, which binds acetaldehyde arose by decarboxylation of pyruvic acid, what prevents its reduction to ethanol. Thereby, the ethanal concentration in a heavily sulphated wine may exceed 100 mg/L [29]. In the analyzed wines, the levels of the acetaldehyde reached from 17.0 to 33.7 mg/L (Table 3). Its lowest content was found in fermented musts obtained from the Johanniter variety with the use of N. ishiwadae MG971259 yeast. Obtained results confirm the literature data, according to which these strains are not good producers of this compound, however, in the mixed cultures with S. cerevisiae¸ they are able to synthesize a higher amounts of acetaldehyde [36].
Organoleptic evaluation
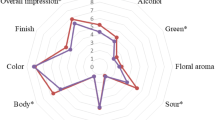
The obtained grape wines were subjected to organoleptic evaluation using the Buxbaum method [37]. After the completion of the analysis, the highest score was obtained by samples fermented with S. cerevisiae MH020215 yeasts (Table 4). Those samples were distinguished by high clarity, proper color and harmonized taste. Much lower values were reported for wines pitched with N. ishiwadae MG971259 monoculture. Interestingly, fermented musts of the Regent variety achieved lower scores, whose taste, being the most influential factor of the end result, was rated quite low. Low taste scores were probably caused by a high acidity, which reached 8.24–8.34 g/L (Table 1).
Conclusion
Saccharomyces cerevisiae MH020215 yeasts were characterized by conducting a fermentation process at a fast pace and expressing favorable oenological properties. The synthesis of desirable volatile compounds, particularly esters, contributed to the formation of a proper aromatic profile of wine, which translated into its assessment during sensory analysis. Samples obtained using these yeast cultures were characterized by higher levels of carbonyl compounds (especially acetaldehyde) and lower methanol content. N. ishiwadae MG971259 cultures conducted the fermentation process less efficiently than species described above, showing a 2 day delay in its initiation. Despite they were characterized by high alcohol production and the sugar utilization, a significant level of total acidity probably contributed to decrease the taste values of the analyzed wines. Drinks obtained using N. ishiwadae MG971259 cultures were characterized by higher, in relation to S. cerevisiae MH020215 content of fusel alcohols (mainly propanol), a larger amount of which could also result in an unfavorable sensory profile of the beverage.
References
Fleet GH (1993) Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, Switzerland
Romano P, Fiore C, Paraggio M et al (2003) Function of yeast species and strains in wine flavour. Int J Food Microbiol 86:169–180. https://doi.org/10.1016/S0168-1605(03)00290-3
Swiegers JH, Pretorius IS (2005) Yeast modulation of wine flavor. Adv Appl Microbiol 57:131–175. https://doi.org/10.1016/S0065-2164(05)57005-9
Swiegers JH, Bartowsky EJ, Henschke PA, Pretorius IS (2005) Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine Res 11:139–173. https://doi.org/10.1111/j.1755-0238.2005.tb00285.x
Loureiro V, Malfeito-Ferreira M (2003) Spoilage yeasts in the wine industry. Int J Food Microbiol 86:23–50. https://doi.org/10.1016/S0168-1605(03)00246-0
Fleet GH (2003) Yeast interactions and wine flavour. Int J Food Microbiol 86:11–22. https://doi.org/10.1016/S0168-1605(03)00245-9
Lambrechts MG, Pretorius IS (2000) Yeast and its importance to wine aroma-a review. S Afr J Enol Vitic 21:97–129. https://doi.org/10.21548/21-1-3560
Jolly NP, Augustyn OPH, Pretorius IS (2006) The role and use of Non-Saccharomyces yeasts in wine production. S Afr J Enol Vitic 27:15–38. https://doi.org/10.21548/27-1-1475
Moreira N, Mendes F, Guedes de Pinho P et al (2008) Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as pure and mixed cultures in grape must. Int J Food Microbiol 124:231–238. https://doi.org/10.1016/j.ijfoodmicro.2008.03.025
Clemente-Jimenez JM, Mingorance-Cazorla L, Martínez-Rodríguez S et al (2005) Influence of sequential yeast mixtures on wine fermentation. Int J Food Microbiol 98:301–308. https://doi.org/10.1016/j.ijfoodmicro.2004.06.007
Jolly NP, Augustyn OPR, Pretorius IS (2003) The effect of non-Saccharomyces yeasts on fermentation and wine quality. S Afr J Enol Vitic 24:55–62. https://doi.org/10.21548/24-2-2638
Hernández-Orte P, Cersosimo M, Loscos N et al (2008) The development of varietal aroma from non-floral grapes by yeasts of different genera. Food Chem 107:1064–1077. https://doi.org/10.1016/J.FOODCHEM.2007.09.032
Comitini F, Gobbi M, Domizio P et al (2011) Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol 28:873–882. https://doi.org/10.1016/j.fm.2010.12.001
Contreras A, Hidalgo C, Henschke PA et al (2014) Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl Environ Microbiol 80:1670–1678. https://doi.org/10.1128/AEM.03780-13
Medina K, Boido E, Fariña L et al (2013) Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem 141:2513–2521. https://doi.org/10.1016/j.foodchem.2013.04.056
Domizio P, Romani C, Lencioni L et al (2011) Outlining a future for non-Saccharomyces yeasts: selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int J Food Microbiol 147:170–180. https://doi.org/10.1016/j.ijfoodmicro.2011.03.020
Izajasz-Parchanska M, Cioch M, Tuszynski T (2014) Monitoring parametrów dojrzałości technologicznej winogron na terenie małopolskiej Winnicy Srebrna Góra, w sezonie wegetacyjnym 2012. Acta Agrophysica 21:263–278
Cioch-Skoneczny M, Satora P, Skoneczny S, Skotniczny M (2019) Yeasts associated with the spontaneously fermented grape musts obtained from cool climate white grape varieties. J Food Nutr Res 58:295–306
Cioch-Skoneczny M, Satora P, Skotniczny M, Skoneczny S (2018) Quantitative and qualitative composition of yeast microbiota in spontaneously fermented grape musts obtained from cool climate grape varieties “Rondo” and “Regent”. FEMS Yeast Res 18:foy089
Cioch-Skoneczny M, Satora P, Skoneczny S, Skotniczny M (2020) Biodiversity of yeasts isolated during spontaneous fermentation of cool climate grape musts. Arch Microbiol. https://doi.org/10.1007/s00203-020-02014-7
Cioch-Skoneczny M, Satora P, Skoneczny S, Pater A (2020) Determination of the oenological properties of yeast strains isolated from spontaneously fermented grape musts obtained from cool climate grape varieties. Eur Food Res Technol. https://doi.org/10.1007/s00217-020-03574-0
OIV (2012) Compendium of international methods of wine and must analysis. Organisation Internationale de la Vigne et du Vin, Paris
Wang D, Xu Y, Hu J, Zhao G (2004) Fermentation kinetics of different sugars by apple wine yeast Saccharomyces cerevisiae. J Inst Brew 110:340–346. https://doi.org/10.1002/j.2050-0416.2004.tb00630.x
Julien A, Roustan J-L, Dulau L, Sablayrolles J-M (2000) Comparison of nitrogen and oxygen demands of enological yeasts: technological consequences. Am J Enol Vitic 51:215–222
Ruiz J, Ortega N, Martín-Santamaría M et al (2019) Occurrence and enological properties of two new non-conventional yeasts (Nakazawaea ishiwadae and Lodderomyces elongisporus) in wine fermentations. Int J Food Microbiol 305:1–9. https://doi.org/10.1016/j.ijfoodmicro.2019.108255
Boynton PJ, Greig D (2016) Species richness influences wine ecosystem function through a dominant species. Fungal Ecol 22:61–72. https://doi.org/10.1016/j.funeco.2016.04.008
Masneuf-Pomarède I, Bely M, Marullo P et al (2010) Reassessment of phenotypic traits for Saccharomyces bayanus var. uvarum wine yeast strains. Int J Food Microbiol 139:79–86. https://doi.org/10.1016/j.ijfoodmicro.2010.01.038
Volschenk H, Viljoen-Bloom M, Subden RE, Van Vuuren HJJ (2001) Malo-ethanolic fermentation in grape must by recombinant strains of Saccharomyces cerevisiae. Yeast 18:963–970. https://doi.org/10.1002/yea.743
Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A (2006) Handbook of enology. In: The microbiology of wine and vinifications, vol 1. Wiley, Chichester, England
Fugelsang K, Edwards C (2010) Wine microbiology (ed.). Springer, New york
Chen S, Xu Y (2010) The influence of yeast strains on the volatile flavour compounds of Chinese rice wine. J Inst Brew 116:190–196. https://doi.org/10.1002/j.2050-0416.2010.tb00417.x
Vidrih R, Hribar J (1999) Synthesis of higher alcohols during cider processing. Food Chem 67:287–294. https://doi.org/10.1016/S0308-8146(99)00136-3
Torrea D, Fraile P, Garde T, Ancı́n C (2003) Production of volatile compounds in the fermentation of chardonnay musts inoculated with two strains of Saccharomyces cerevisiae with different nitrogen demands. Food Control 14:565–571. https://doi.org/10.1016/S0956-7135(02)00146-9
Bisson LF, Karpel JE (2010) Genetics of yeast impacting wine quality. Annu Rev Food Sci Technol 1:139–162. https://doi.org/10.1146/annurev.food.080708.100734
Orlic S, Redzepovic S, Jeromel A et al (2007) Influence of indigenous Saccharomyces paradoxus strains on Chardonnay wine fermentation aroma. Int J food Sci Technol 42:95–101. https://doi.org/10.1111/j.1365-2621.2006.01217.x
Morata A, Gómez-Cordovés MC, Calderón F, Suárez JA (2006) Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int J Food Microbiol 106:123–129. https://doi.org/10.1016/j.ijfoodmicro.2005.05.019
Kovačević Ganić K, Staver M, Peršurić Đ et al (2003) Influence of blending on the aroma of Malvasia istriana wine. Food Technol Biotechnol 41:305–314
Author information
Authors and Affiliations
Contributions
Conceptualization, MC-S and PS; methodology, MC-S and PS; software, SS; validation, MC-S and PS; formal analysis, MC-S; investigation, MC-S; resources, MC-S; writing—original draft preparation, MC-S; writing—review and editing, MC-S; SS, KK and PS; visualization, MC-S and S.S.; supervision, MC-S; and PS; project administration, MC-S; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cioch-Skoneczny, M., Satora, P., Skoneczny, S. et al. Physicochemical characterization of wines produced using indigenous yeasts from cold climate grapes. Eur Food Res Technol 247, 201–209 (2021). https://doi.org/10.1007/s00217-020-03618-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03618-5