Abstract
The international competitiveness of the wine sector and consumer demands for the unique wine styles pose challenges in improving the fermentation process. The basis of proper alcoholic fermentation is knowledge about how individual yeast strains interact with the aroma, taste and color of wine, what results in possibility to select species used as starter cultures. To use the value of non-Saccharomyces yeast strains in wine production and to minimize the possibility of wine deterioration, it is necessary to precisely recognize the yeast cultures present on the fruit of the vine and in grape must, as well as their metabolic properties. The aim of the study was to determine the oenological properties of yeasts isolated from spontaneously fermented grape musts obtained from cool climate grapes. For this purpose, Zweigelt grape must was fermented with yeast monocultures. Alcohol, extract, sugars, glycerol, total acidity and free amine nitrogen were analyzed in the obtained wines. Poor fermentation properties of yeast strains results in obtaining wines with relatively large amounts of residual sugars and low alcohol. A decrease in overall acidity was noted in sets with the participation of M. pulcherrima MG971264, while in other tests the opposite trend was observed. Although some microorganisms have the ability to assimilate organic acids found in wine, they are not able to carry out fermentation or they do it inefficiently. Solution to this problem may, therefore, be use of mixed cultures of noble and non-Saccharomyces yeast, what effectively reduce the concentration of organic acids, while not adversely affecting the organoleptic characteristics of the drink.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important technological achievements in winemaking was inoculation of grape juice with noble cultures of Saccharomyces cerevisiae, what made it possible to control the fermentation process [1,2,3]. There was a conviction that this strain inactivates the growth of native yeast of wine fruit and grains dominance during the transformation of sugar into ethanol. However, numerous studies, prove the widespread presence of non-Saccharomyces yeast in grape must, which persist at various stages of fermentation in the presence of pure S. cerevisiae cultures [4]. The use of only native grape strains in the form of pure cultures as fermentation primers indicates that they exhibit undesirable features, such as production of acetic acid, ethyl acetate, ethanal and acetoin. In addition, most of non-Saccharomyces species have limited fermentation potential and low SO2 resistance [5,6,7,8]. Despite the unfavorable features, a number of studies have been conducted over the past several years regarding the recognition and activity of non-Saccharomyces yeast in grape must. Precise analysis of the stages of their metabolism, fermentation properties and their impact on the complexity of the final aroma of the wine, contributed to update earlier views [9,10,11,12,13,14]. Among the most common yeast microorganisms found in grape must dominate Candida, Issatchenkia, Kluyveromyces, Metschnikowia, Pichia, Torulaspora and in small amounts the Saccharomyces cultures [2, 15,16,17,18,19]. Dekkera, Schizo- and Zygosaccharomyces yeast are much less frequently identified.
The presence of non-Saccharomyces yeast during the wine fermentation process, although it carries a risk of spoilage, is generally considered as desirable [12, 20, 21]. Research conducted by Gila et al. [20] proved the effect of metabolites produced by yeast on the complexity of the taste and aroma of a drink. Glycerol, one of many products of metabolism of these microbes, plays a role in regulating the oxidoreductive potential of cells [22, 23]. In addition, it gives wines a feeling of smoothness and taste complexity [24]. Spontaneously fermented grape musts have glycerol in higher concentration [11, 25], what is the result of the presence of native grape yeast. Candida stellata can be used as an example of yeast species who increase the concentration of glycerol to 10–14 g/L compared to S. cerevisiae wine cultures (4–10.4 g/L). Unfortunately, excessive glycerol production is associated with increased production of acetic acid, which worsens the quality of the final product [22]. Similar properties are shown by the apiculata yeast (Kloeckera apiculata and Hansenniaspora guilliermondi) which can be divided essentially into two groups. The first includes producers of high amount of glycerol (3 g/L) and low ethanol amount (0.9% vol.), while the second includes strains produced low level of glycerin (1 g/L), and much higher amount of alcohol (2.4% vol.) [25]. Similar to C. stellata, increased amount of acetic acid are the result of the presence of these microorganisms in grape must [26]. To the determinants of the final wine bouquet may be also included acetaldehyde, succinic acid, higher alcohols and esters. Microorganisms characterized by high production of these compounds include Pichia anomala and K. apiculata strains [27].
To use the value of non-Saccharomyces strains in wine production and to minimize the possibility of wine deterioration, it is necessary to know precisely the yeast cultures present on the fruit of the wine and in grape must, as well as their metabolic properties. The ability to use sugar and their conversion into alcohol, the production of desirable aromas, the production of glycerin and the release of terpene compounds, make non-Saccharomyces yeast enjoying increasing recognition.
The aim of the study was to determine the oenological properties of yeasts isolated from spontaneously fermented grape musts obtained from cool climate grape varieties.
Materials and methods
Yeast
Grapes of two red grape vine varieties (‘Rondo’, ‘Regent’) and four white grape variety (‘Johanniter’, ‘Bianca’, ‘Seyval Blanc’, ‘Hibernal’) from four vineyards located in southern Poland (Srebrna Góra—50° 2′ N, 19° 50′ E; Spotkaniówka—49° 53′ N, 21° 52′ E; Zalipie—50° 14′ N, 20° 51′ E and Zadora—49° 53′ N, 21° 52′ E) during three consecutive vintages (2012 and 2014) were used. Ten bunches of mature grapes were gathered from several grape vines within a sub-area of each vineyard (100 m2). Then, berries were randomly selected (500 g), placed in sterile 500 mL flasks, and pressed until juice has covered the fruits. The flasks were closed with airlocks filled with glycerin. Fermentation was carried out for 28 days at a temperature of 20 °C (each in triplicate). For the quantitative determination of yeast microbiota during spontaneous fermentation of grape musts, Wallerstein Laboratoryagar (WL Agar; Biocorp, Poland) was used. 1 mL samples of the fermenting musts were taken under sterile conditions on the 0, 1st, 2nd, 3rd, 4th, 6th, 9th, 13th, 18th, 24th, and 28th day of fermentation. A serial decimal dilutions in a physiological saline solution (100–10–8) were prepared from the samples taken. Each dilution was inoculated in six replicates on Petri dishes with WL agar or Yeast Nitrogen Base (YNB) medium with l-malic acid (12 g/mL) (Sigma Aldrich). To avoid bacterial growth, 100 mg/L of chloramphenicol was added to the media. The media were incubated at 28 °C for 5 days, which was followed by a macro- and microscopic evaluation of the grown colonies and the determination of their count. Colonies with different morphologies (size, shape, colors) were randomly selected for identification and streaked on Sabouraud Dextrose with Chloramphenicol LAB-AGAR™ (BIOCORP, Poland) to obtain pure cultures. The identification of yeasts was based on the morphological characteristics of the colonies, biochemical tests and molecular techniques (DNA extraction and RAPD-PCR analysis, amplification of the 5.8S-ITS rRNA gene region, PCR–RFLP analysis, 5.8S-ITSrRNA gene region sequencing). Methods for identifying microorganisms are described in detail in previous publications [28, 29].
18 yeast strains isolated from spontaneously fermented grape musts originating from grapes from cold climate zone were used for the research. The following strains were analyzed: Candida railenensis MG971258, Candida oleophila MH020216, Nakawazaea ishiwadae MG971259, Zygoascus meyerae (MG971244, MG970696), Metschnikowia pulcherrima (MG971245, MG971247, MG971249, MG971256, MG971257, MG971264), Hanseniaspora uvarum (MG971252, MG971254, MG971255, MG971265, MG971266), Kluyveromyces lactis MG971263, Saccharomyces cerevisiae MH020215.
Inoculum preparation
Pure yeast cultures were passaged in three stages. In the first stage, the strains were grown on slants with Sabouraud agar (Biocorp, Poland) for 24 h. After that the strains were transported to 10 mL of Sabouraud liquid medium (Biocorp, Poland). Than, after 24 h incubation, dynamic propagation was carried out in 200 mL Sabouraud liquid medium (Biocorp, Poland) for 48 h on a water bath shaker (120 rpm, 28 °C).
After multiplication process, the dry yeast mass was determined on a moisture analyzer (Radwag WPS 210S, Zakład Mechaniki Produkcyjnej, Radom, Poland). Appropriate amount of yeast cake was centrifuged (10 min, 4989 × g/min). The pellet was washed with sterile water, centrifuged again under the same conditions and introduced to the set.
Fermentation of grape musts
The direct raw material for fermentation was the Meinklang Zweigelt grape juice (Austria). The juice was sweetened (sucrose) to 25°Bx and acidified with l-malic acid to total acidity 11 g/L (Table 1). The juice was then pasteurized (100 °C, 10 min) and bottled into 300 mL flasks. The multiplied yeast slurry was introduced to the set in an amount of 0.5 g d.w./L. After tightly closing the flasks and attaching fermentation tubes filled with glycerin, the system was additionally sealed with parafilm. The fermentation process was carried out for 28 days at 20 °C (in triplicate).
Determination of fermentation kinetics
Fermentation kinetics was determined on the basis of weight loss of sets during the process. Weight losses were measured daily. The process was completed at the moment of achieving daily weight losses below 0.01 g/L.
Determination of biomass increase
The yeast pellet after centrifugation was rinsed with distilled water, and dried in moisture analyzer to a constant mass. Dry yeast weight was expressed in g/L.
Determination of ethyl alcohol, extract, total acidity
All the determinations were done according to methods OIV [30].
Determination of organic acid and glycerol content (HPLC)
The content of organic acids (HPLC–UV/Vis) and glycerol (HPLC-RI) was characterized in the obtained wines. Chromatographic separation was carried out with using a Rezex ROA—Organic Acid Aminex HPX—87H column (300 mm, 18 cm × 7.8 mm), mobile phase 0.005 N H2SO4, flow 0.3 mL/min, detection at 210 nm.
Determination of total sugars, reducing sugars and sucrose using 3,5-dinitrosalicylic acid (DNS)
A calibration curve was made on the basis on the extinction value of samples (λ = 520 nm) with the following glucose concentrations: 0 g/L; 0.1 g/L; 0.2 g/L; 0.4 g/L; 0.6 g/l; 0.8 g/L; 1.2 g/L; 2 g/L. With a single-pipette, 25 mL of wine was measured and neutralized with a 10 M sodium hydroxide solution. The neutralized solution was transferred quantitatively into a 100 mL volumetric flask. To remove from material proteins and other compounds that would make it difficult to determine sugars, 5 mL of Carrez I and Carrez II solutions were added successively, followed by mixing, refilled up to the mark with distilled water and filtered through paper filters. In sequence, 5 mL of filtrate was taken up and refilled up to 100 mL with distilled water. Prepared sugar solution was subjected to an inversion process. A 50 mL of the previously prepared sample was pipetted into the conical flask, refilled up with distilled water to approximately 60 mL, than 5 mL of concentrated hydrochloric acid was added. The liquid in the flask was then heated in a water bath to a temperature of 68–70 °C inside the flask, followed by holding the temperature for 5 min. After this time, the fluid was quickly cooled to a temperature of about 20 °C, than three drops of methyl orange were added. Neutralization, indicated by the change of the color of the fluid, was carried out with using 10 M NaOH. The solution was then poured into a 100 mL volumetric flask, brought back to 20 °C and refilled up to the mark with distilled water. After completion of the inversion process, 2 mL of DNS solution and 2 mL of post-inversion samples and sugar stock solution without inversion were pipetted into the tubes. The mixture was boiled for about 5–10 min, than 11 mL of distilled water was added. The differences in the content of reducing sugars were determined spectrophotometrically at wavelength λ = 520 nm.
Statistical analysis of results
The results presented in the study were calculated as averages of three independent repetitions with the determination of the standard deviation. The data were analyzed with the variance analysis (ANOVA) to determine the significance of the tested parameters. Statistically significant differences between the average values were verified with the Duncan’s set using the Statistica 10 statistical software (StatSoft Polska, Cracow).
Results and discussion
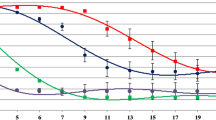
To determine the oenological characteristics of isolated yeast, fermentation of grape musts of the Zweigelt strain (25° Bx), characterized by an increased content of l-malic acid (11 g/L) was carried out. Pasteurized sets were inoculated with monocultures in the amount of 0.5 g/L of dry substance. Based on the conducted research, the kinetics of grape must fermentation was diversified (Figs. 1, 2). Among the analyzed microorganisms, yeasts: M. pulcherrimia MG971264 and S. cerevisiae, were the fastest to ferment. From the 10th day of the fermentation, the mass of sets remained at a similar level until the end of the process. A sugar content above 230 g/L may be a reason of disturbing yeast metabolism and dehydratation caused by high osmotic pressure [31]. In addition, for the proper course of the fermentation process, the presence of nitrogen is necessary, whose primary source is the amino acids and ammonium ions (NH4+) present in the must. If the amount of the nitrogen is limited, what may be a result of excessive must clarification or incomplete ripening of grapes, the function of yeast may be disturbed, fermentation delayed, and production of hydrogen sulfide and mercaptans may be increased [32]. Cultures not belonging to the genus Saccharomyces, including H. uvarum (MG971252, MG971254, MG971255, MG971266) were characterized by weaker fermentation kinetics, which is also confirmed by literature data [33]. These sets were characterized by similar weight loss (from 1.5 to 3%). In the case of other microorganisms, a low degree of fermentation was found, reaching up to 1.5% (Figs. 1, 2).
The obtained wines were characterized by statistically different alcohol content, amounting from 0.1 to 14.15% vol. The lowest amount of ethanol was observed in sets fermented with M. pulcherrima monocultures (MG971245–MG971257) (Table 2). Perhaps it was a result of too high sugars concentration in the fermentation medium. These compounds are necessary for the proper functioning of microorganisms and the production of ethanol; however, their excess adversely affects on the cells. The high concentration of saccharides in the must leads to osmotic stress in yeast [34]. During the first phase of the stress response, they eliminate water from the cells to thicken the components of the cytoplasm. The undesirable effect on yeast viability is the result of too rapid a process of passive osmoregulation. Damage of the cells membrane, what is a consequence of osmotic stress, manifests itself in the aggregation and denaturation of membrane proteins. In addition, it can lead to the displacement of fatty acids in the lipid bilayer. Additionally, in conditions of too high sugar or salt concentration, yeast synthesizes osmogenic compounds, such as glycerol, whose production and accumulation results in an increase in intracellular osmotic pressure, protect themselves against the external pressure. It is a source of carbon and a precursor of lipids associated with signal transduction [35]. When the sugar concentration is lower than 20°Bx, the fermentation process proceeds with significant speed during the intensive cell proliferation and stationary phase. However, at a high concentration of saccharides, speed of the fermentation process depends mainly on the metabolic activity of non-dividing cells, whose viability decreases. The highest content of ethanol was characterized by sets fermented with the participation of S. cerevisiae yeast (14.15% vol.) Literature data indicate that noble wine yeast S. cerevisiae has very good fermentation properties and highly tolerance to increasing ethanol concentrations [36].
The content of reducing sugars was on a similar level in sets with the strains of C. oleophila, Z. meyerae, N. ishiwadae and most of M. pulcherrima cultures, reaching values from 196.90 to 220.79 g/L (Table 2). A light decrease in the total extract of wine can be explained by the low use of nutrients by yeast for their own metabolic transformations. Microbes show a diverse invertase activity that breaks down sucrose into glucose and fructose, as well as a different resistance of this enzyme to elevated alcohol concentrations in the fermentation medium, which affects the process. A high glycerol content was observed for some wines (Table 2). It is worth noting that this compound has a protective function for cells against adverse environmental conditions, such as alcohol concentration or the presence of cellular metabolites [37]. S. cerevisiae yeast consumed saccharides in the greatest extent (Table 2). Glycerol is a by-product commonly classified as a general extract, which greatly influence the quality of wine by increasing the viscosity and extractivity of aromatic substances. The glycerol content in various types of wine is from 4 to 15 g/L. Glycerol was found to be between 12.7 and 29.05 g/L in the analyzed wines. The amount of this compound in wine depends also on such factors as must pH, yeast breed and temperature of fermentation. In dependent of breed of yeast, an increase in fermentation temperature may increase or decrease the amount of glycerol produced [38]. Analyzing the contents of the real extract, statistically significant differences were found. The lowest values were recorded for S. cerevisiae strains (41.50 g/L), which was directly related to the use of sugars and ethanol production. The content of the real extract below 20°Bx was obtained for the sets inoculated with the H. uvarum yeasts (MG971252, MG971255, MG971266). In this sets, after the process, the alcohol level was about 4% vol. (Table 2). In addition to residual sugars, the general wine extract also includes acids (tartaric and malic), tannins, mineral salts, aromatic, compounds, proteins and non-fermentable sugars, mainly pentose.
A deficiency of nutrients, in particular nitrogen in ammonium from, may result in fermentation stopping. Usually, their addition is not necessary for red wines, unlike white ones. Based on the conducted tests, the highest consumption of ammonium nitrogen was found in sets fermented with the participation of S. cerevisiae yeast (Table 2). These strains utilized over 75% of low-molecular amino acids. In the case of K. lactis, C. oleophila, Z. meyerae cultures and some strains of H. uvarum and M. pulcherrima, the loss of short-molecular proteins during fermentation was comparable and ranged from 60 to 70% on average (Table 2).
After the end of the 4-week fermentation, the wine was separated from the yeast biomass, the quantity of which was determined by means on a moisture analyzer (Table 2). A high dry substance content was noted in the sets inoculated with M. pulcherrima MG971264, C. oleophila MH020216 and Z. meyerae MG970696. In contrast, significantly lower biomass content was observed in wines inoculated with S. cerevisiae. Species that do not belong to the genus Saccharomyces are aerobic cultures, characterized by significant cell growth, in contrast to noble wine yeast mainly focused on the fermentation process.
The basic parameters, conditioning the process of wine making and affecting their quality and harmonized taste, includes sugar content and acidity. Already in the fermentation process, for the proper operation of yeast, it is required to provide the right amount of saccharides and acidity at an optimal level [39]. The total acidity of the obtained wines ranged from 10.25 to 11.6 g/L, calculated as malic acid. A decrease in this value was noted in sets inoculated with M. pulcherrima MG971264 (Table 2). A slight increase in acidity in the remaining tests could have been the result of the synthesis of organic acids by yeasts during the process. The degree of must fermentation may also indicate the ability of strains to function in an environment with increased acidity. According to the literature data, the S. cerevisiae yeast have the ability to break down l-malate in up to 3 g/L [40], what depends primarily on the different level of expression of the gene encoding the apple enzyme, what in turn is affected by the surrounding medium [41]. The highest total acidity was observed for wines inoculated with M. pulcherrima MG971256 (11.48 g/L), Z. meyerae MG970696 (11.5 g/L), H. uvarum MG971254, K. lactis MG971263 (11.6 g/L) and C. oleophila MH020216 (11.58 g/L). Most of the analyzed yeasts belonged to group K (+). These microorganisms are susceptible to the phenomenon of glucose repression. If sugar is predominantly present in the substrate, yeast will use it first to create biomass [42], as observed in the experiment (Table 2). Although some microorganisms have the ability to assimilate organic acids found in wine, they are not able to carry out fermentation by themselves or they do this inefficiently. The solution to this problem may, therefore, be the use of mixed cultures of noble and non-Saccharomyces yeast, which effectively reduce the concentration of organic acids, while not adversely affecting the organoleptic characteristics of the drink.
Conclusion
Grape must is a rich environment for various species of yeast. Knowledge of the kinetics of their growth and metabolism is the basis for understanding the impact of these microorganisms on the quality of wine. Poor fermentation properties of yeast strains results in obtaining wines with relatively large amounts of residual sugars and low alcohol. A decrease in overall acidity was noted in sets with the participation of M. pulcherrima MG971264, while in other tests the opposite trend was observed. Despite the limited fermentation capacity of some non-Saccharomyces yeast strains and the production of low alcohol concentrations, numerous literature reports prove the applicability of these microorganisms and mixed cultures in the process of wine fermentation.
References
Comitini F, Gobbi M, Domizio P et al (2011) Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol 28:873–882. https://doi.org/10.1016/j.fm.2010.12.001
Fleet GH (2008) Wine yeasts for the future. FEMS Yeast Res 8:979–995. https://doi.org/10.1111/j.1567-1364.2008.00427.x
Rainieri S, Pretorius IS (2000) Selection and improvement of wine yeasts. Ann Microbiol 50:15–32
Mora J, Barbas JI, Mulet A (1990) Growth of yeast dpecies furing the fermentation of musts inoculated with kluyveromyces thermotolerans and Saccharomyces cerevisiae. Am J Enol Vitic 41:156–159
Farkas G, Rezessy-Szabó JM, Zákány F, Hoschke Á (2005) Interaction of Saccharomyces and non-Saccharomyces yeast strains in an alcoholic fermentation process. Acta Aliment 34:81–90. https://doi.org/10.1556/AAlim.34.2005.1.11
Howell KS, Cozzolino D, Bartowsky EJ et al (2006) Metabolic profiling as a tool for revealing Saccharomyces interactions during wine fermentation. FEMS Yeast Res 6:91–101. https://doi.org/10.1111/j.1567-1364.2005.00010.x
Jolly NP, Augustyn OPH, Pretorius IS (2006) The role and use of Non-Saccharomyces yeasts in wine production. S Afr J Enol Vitic 27:15–38. https://doi.org/10.21548/27-1-1475
Mendes Ferreira A, Clímaco MC, Faia AM (2001) The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components—a preliminary study. J Appl Microbiol 91:67–71. https://doi.org/10.1046/j.1365-2672.2001.01348.x
Domizio P, Lencioni L, Ciani M et al (2007) Spontaneous and inoculated yeast populations dynamics and their effect on organoleptic characters of Vinsanto wine under different process conditions. Int J Food Microbiol 115:281–289. https://doi.org/10.1016/j.ijfoodmicro.2006.10.052
Egli CM, Edinger WD, Mitrakul CM, Henick-Kling T (1998) Dynamics of indigenous and inoculated yeast populations and their effect on the sensory character of Riesling and Chardonnay wines. J Appl Microbiol 85:779–789. https://doi.org/10.1046/j.1365-2672.1998.00521.x
Henick-Kling T, Edinger W, Daniel P, Monk P (1998) Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of wine. J Appl Microbiol 84:865–876. https://doi.org/10.1046/j.1365-2672.1998.00423.x
Lema C, Garcia-Jares C, Orriols I, Angulo L (1996) Contribution of Saccharomyces and non-Saccharomyces populations to the production of some components of Albarino wine aroma. Am J Enol Vitic 47:206–216
Rojas V, Gil JV, Piñaga F, Manzanares P (2001) Studies on acetate ester production by non-Saccharomyces wine yeasts. Int J Food Microbiol 70:283–289. https://doi.org/10.1016/S0168-1605(01)00552-9
Zohre DE, Erten H (2002) The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem 38:319–324. https://doi.org/10.1016/S0032-9592(02)00086-9
Hierro N, González Á, Mas A, Guillamón JM (2006) Diversity and evolution of non-Saccharomyces yeast populations during wine fermentation: effect of grape ripeness and cold maceration. FEMS Yeast Res 6:102–111. https://doi.org/10.1111/j.1567-1364.2005.00014.x
Mercado L, Dalcero A, Masuelli R, Combina M (2007) Diversity of Saccharomyces strains on grapes and winery surfaces: analysis of their contribution to fermentative flora of Malbec wine from Mendoza (Argentina) during two consecutive years. Food Microbiol 24:403–412. https://doi.org/10.1016/j.fm.2006.06.005
Ocón E, Gutiérrez AR, Garijo P et al (2010) Quantitative and qualitative analysis of non-Saccharomyces yeasts in spontaneous alcoholic fermentations. Eur Food Res Technol 230:885–891. https://doi.org/10.1007/s00217-010-1233-7
Romancino DP, Di Maio S, Muriella R, Oliva D (2008) Analysis of non-Saccharomyces yeast populations isolated from grape musts from Sicily (Italy). J Appl Microbiol 105:2248–2254. https://doi.org/10.1111/j.1365-2672.2008.03894.x
Zott K, Miot-Sertier C, Claisse O et al (2008) Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int J Food Microbiol 125:197–203. https://doi.org/10.1016/j.ijfoodmicro.2008.04.001
Gil JV, Mateo JJ, Jiménez M et al (1996) Aroma compounds in wine as influenced by apiculate yeasts. J Food Sci 61:1247–1250. https://doi.org/10.1111/j.1365-2621.1996.tb10971.x
Soden A, Francis IL, Oakey H, Henschke PA (2000) Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust J Grape Wine Res 6:21–30. https://doi.org/10.1111/j.1755-0238.2000.tb00158.x
Prior BA, Toh TH, Jolly N et al (2000) Impact of yeast breeding for elevated glycerol production on fermentative activity and metabolite formation in Chardonnay wine. S Afr J Enol Vitic 21:92–99. https://doi.org/10.21548/21-2-2218
Scanes KT, Hohrnann S, Prior BA (1998) Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: a review. S Afr J Enol Vitic 19:17–24. https://doi.org/10.21548/19-1-2239
Ciani M, Maccarelli F (1997) Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J Microbiol Biotechnol 14:199–203. https://doi.org/10.1023/A:1008825928354
Romano P, Suzzi G, Comi G et al (1997) Glycerol and other fermentation products of apiculate wine yeasts. J Appl Microbiol 82:615–618
Ciani M, Picciotti G (1995) The growth kinetics and fermentation behaviour of some non-Saccharomyces yeasts associated with wine-making. Biotechnol Lett 17:1247–1250. https://doi.org/10.1007/BF00128395
Clemente-Jimenez JM, Mingorance-Cazorla L, Martínez-Rodríguez S et al (2004) Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol 21:149–155. https://doi.org/10.1016/S0740-0020(03)00063-7
Cioch-Skoneczny M, Satora P, Skoneczny S, Skotniczny M (2019) Yeasts associated with the spontaneously fermented grape musts obtained from cool climate white grape varieties. J Food Nutr Res 58:295–306
Cioch-Skoneczny M, Satora P, Skotniczny M, Skoneczny S (2018) Quantitative and qualitative composition of yeast microbiota in spontaneously fermented grape musts obtained from cool climate grape varieties Rondo and Regent. FEMS Yeast Res. https://doi.org/10.1093/femsyr/foy089
OIV (2012) Compendium of international methods of wine and must analysis. Organisation Internationale de la Vigne et du Vin, Paris
Moreno-Arribas MV, Polo MC (2009) Wine chemistry and biochemistry, 1st edn. Springer, New York
Julien A, Roustan J-L, Dulau L, Sablayrolles J-M (2000) Comparison of nitrogen and oxygen demands of enological yeasts: technological consequences. Am J Enol Vitic 51:215–222
Barata A, Pais A, Malfeito-Ferreira M, Loureiro V (2011) Influence of sour rotten grapes on the chemical composition and quality of grape must and wine. Eur Food Res Technol 233:183–194. https://doi.org/10.1007/s00217-011-1505-x
Jiménez-Martí E, Zuzuarregui A, Gomar-Alba M et al (2011) Molecular response of Saccharomyces cerevisiae wine and laboratory strains to high sugar stress conditions. Int J Food Microbiol 145:211–220. https://doi.org/10.1016/j.ijfoodmicro.2010.12.023
Brown CM, Johnson B (1970) Influence of the concentration of glucose and galactose on the physiology of Saccharomyces cerevisiae in continuous culture. Microbiology 64:279–287. https://doi.org/10.1099/00221287-64-3-279
Masneuf-Pomarède I, Bely M, Marullo P et al (2010) Reassessment of phenotypic traits for Saccharomyces bayanus var. uvarum wine yeast strains. Int J Food Microbiol 139:79–86. https://doi.org/10.1016/j.ijfoodmicro.2010.01.038
Omori T, Ogawa K, Umemoto Y et al (1996) Enhancement of glycerol production by brewing yeast (Saccharomyces cerevisiae) with heat shock treatment. J Ferment Bioeng 82:187–190. https://doi.org/10.1016/0922-338X(96)85048-3
Wang Z, Zhuge J, Fang H, Prior BA (2001) Glycerol production by microbial fermentation: a review. Biotechnol Adv 19:201–223. https://doi.org/10.1016/S0734-9750(01)00060-X
Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A (2006) Handbook of enology, Volume 1: the microbiology of wine and vinifications. Wiley, New York
Redzepovic S, Orlic S, Majdak A et al (2003) Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int J Food Microbiol 83:49–61. https://doi.org/10.1016/S0168-1605(02)00320-3
Coloretti F, Zambonelli C, Castellari L et al (2002) The effect of dl-malic acid on the metabolism of l-malic acid during wine alcoholic fermentation. Food Technol Biotechnol 40:317–320
Saayman M, Viljoen-Bloom M (2006) The biochemistry of malic acid metabolism by wine yeasts—a review. S Afr J Enol Vitic 27:113–122. https://doi.org/10.21548/27-2-1612
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization, MC-S and PS; methodology, MC-S and PS; software, SS; validation, MC-S and PS; formal analysis, MC-S; investigation, MC-S; resources, MC-S; writing—original draft preparation, MC-S; writing—review and editing, MC-S, SS, AP and PS; visualization, MC-S and SS; supervision, MC-S and PS; project administration, MC-S; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cioch-Skoneczny, M., Satora, P., Skoneczny, S. et al. Determination of the oenological properties of yeast strains isolated from spontaneously fermented grape musts obtained from cool climate grape varieties. Eur Food Res Technol 246, 2299–2307 (2020). https://doi.org/10.1007/s00217-020-03574-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03574-0