Abstract
The antioxidant capacity (AC) of boiled-type coffee brews (CB) and phenolic acids (PA) isolated from them, obtained from the caffeinated and decaffeinated beans of different geographical origins and species and with different roasting degrees, was examined. The AC of PA and CB samples was tested in five antioxidant assays: a total antioxidants reducing capacity assay using a Folin–Ciocalteu reagent (FCR), a ferric ion reducing antioxidant power (FRAP) assay, a DPPH· radical-scavenging activity (DPPH) assay, a metal chelating activity (MCA) assay and a total radical trapping antioxidant parameter (TRAP) assay. In most samples, the total amount of phenolic acids, determined by HPLC, decreased with the increasing degree of roasting the coffee beans, leading to reduced AC. All used methods showed that CB exhibits higher AC compared with the PA samples. Phenolic acids isolated from CB samples have the main contribution (on average over 95 and 84 % in green and roasted coffee extracts, respectively) in AC of the CB samples in FCR, FRAP and TRAP assays, whereas in DPPH and MCA tests, the phenolic acid contribution in AC of CB samples was below 50 % (on average over 36 and 45 % in green and roasted coffee extracts, respectively). Significant differences between the AC values determined for CB and PA samples were noticed only for the MCA and DPPH methods which reflect the different molecular mechanisms underlying each of the assays. Additionally, the statistical methods, including principal component analysis, applied to results of antioxidant capacity obtained with different analytical techniques confirmed their feasibility to distinguish between coffee brews with different degrees of roasting, regardless of coffee origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coffee brew is one of the most frequently consumed beverages in the world, and coffee beans belong to the top trade products with global production of 8.8 million tons in 2012 [1]. The coffee brew is preferred, due to its taste, aroma and stimulating properties. Moreover, compared to other popular beverages, such as black and green tea, cocoa, fruit juice and red and white wine, coffee shows the highest antioxidant capacity (AC) [2, 3]. Therefore, it is not surprising that coffee brews in some countries provide the highest amount of antioxidants in the daily diet [4, 5]. The AC of coffee brews is generally associated with the presence of phenolic compounds, primarily in the form of chlorogenic acids [6]. However, the antioxidant impact of other compounds present in coffee, such as Maillard reaction products, mostly in the form of melanoidins [7–9], tocopherols [10, 11] or caffeine [8, 12], cannot be neglected. Moreover, the profile of the components with potential AC in coffee brews is significantly affected by the degree of roasting, which depends on the parameters of the roasting process [13]. Additionally, the composition of bio-compounds in coffee brews is related to the grinding of beans, their type, and the method of preparation, as well as to the water to coffee bean proportion, temperature, and the time of the infusion [14]. Therefore, the coffee brews can be considered as a non-standardized mixture of bioactive compounds, due to the influence of many factors on final composition, with the potential AC. Whereas, presence in the system of two or more antioxidants with the different physical nature, for instance hydrophobic and hydrophilic, may contribute to occur one of the two opposite phenomena; synergism or antagonism of antioxidants [15].
To indicate similarities and correlations that may exist in complex food matrix between their properties and chemical composition, multivariate analysis has been applied. Principal component analysis has been applied to interpret sensory data in food [16], in milk [17] and also in fermented food products [18]. It was used for evaluating the dependence of the antioxidant potential on the conventional or torrefacto process of roasting coffee beans [19, 20]. PCA is also very useful method applied during screening of adulteration of honey [21], milk [22] and olive oil [23]. PCA has been also used to find correlation between species formed during refining steps of olive oil and its thermal properties [24]. The presented papers indicate that PCA method confirmed its feasibility to retrieve sometimes hidden important correlations giving additional information regarding qualitative and/or quantitative characterization of food products.
To properly estimate antioxidant capacity of food we need reliable, quick and simple method for its determination. However, no single method is capable of evaluating AC of food due to fact that many factors, including type of antioxidant, its concentration, and composition, influence AC value. Some attempt to compare and standardize different assay have been carried out of several beverages including green tea, red wine, fruit juices, and vegetable juices, whereas the coffee beverages were not included in those assays [25–28].
The antioxidant activity of coffee brews was investigated previously [29, 30]; however, to the best of our knowledge, no data exist about the isolation of phenolic acids from coffee brews, with the exception of the report where isolation and purification of the hydroxycinnamic and chlorogenic acids from green Robusta coffee bean by using centrifugal partition chromatography was conducted [31], and comparison of their AC to whole coffee brews from which were separated. Therefore, the aim of the present study is to examine the difference in the AC between isolated phenolic acids from coffee brews obtained from green or roasted beans and the whole coffee brews, i.e., mixtures of bioactive compounds, including phenolic acids, with a potential AC. Since results vary significantly with antioxidant test, and each assay has some advantages and disadvantages, five antioxidant assays were used in this study, based on free radical DPPH· scavenging activity (DPPH), reducing capacity with the Folin–Ciocalteu reagent (FCR), ferric reducing antioxidant power (FRAP), metal chelating activity (MCA), and total radical trapping antioxidant parameter (TRAP) [32, 33].
Materials and methods
Solvents, reagents and standards
The phenolic acids standards, caffeic acid, chlorogenic acid, protocatechuic acid and p-coumaric acid, were purchased from Sigma-Aldrich (Steinheim, Germany), and ferulic acid, o-coumaric acid, sinapic acid, vanillic acid, gentisic acid and salicylic acid and also the free radicals 1,1-diphenyl-2-picrylhydrazyl (DPPH·) were obtained from Fluka (Buchs, Switzerland). The methanol and acetonitrile (HPLC grade) were purchased from Merck (Darmstadt, Germany). The Folin–Ciocalteu phenol reagent, 2′,7′-dichlorofluorescein diacetate (DCFH-DA), 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) and all other used reagents and solvents (analytical grade) were obtained from Sigma-Aldrich (Steinheim, Germany).
Coffee bean samples
The green coffee beans, caffeinated and decaffeinated, from several geographical origins (Java, India and Brazil) (Table 1) were roasted separately in a Probat B 3 roaster (Emmerich am Rhein, Germany) at a temperature of 200 °C for 18 min (medium roasted), 20 min (medium–dark roasted) and 22 min (dark roasted). The roasted coffee beans, after cooling to a temperature of 21 ± 2 °C, were hermetically packed into polyethylene bags and delivered on the same day to a chemical laboratory. The samples were ground in an IKA M 20 universal mill with a water cooling system to prevent the overheating of the raw material (Staufen, Germany) until the particles of the coffee beans passed through a 0.5-mm sieve. The ground coffee beans were immediately weighed and directly used for the preparation of brews.
Coffee brews (CB) preparation and pH measurement
Coffee brews (CB) were prepared by solid–liquid extraction using ground coffee (5 g) and boiling water (100 mL). CB were kept for 5 min in a water bath (95 °C) then cooled to 20 °C and finally filtered through filter paper at a medium filtration rate (61–69 g/m2, 10 mL/35 s.). The total extraction time between the addition of boiling water and filtration was 30 min. The pH of each filtered sample was measured with a pH meter, Oyster-10 (Taipei, Taiwan). The scheme of sample preparation is shown in Fig. 1.
Isolation and determination of phenolic acids (PA) from CB
The isolation of PA from CB was carried out as described by Dwiecki et al. [34]. Briefly, the isolation process was conducted using the Chromabond® System (Macherey-Nagle, Germany) together with SPE Bakerbond spe™ columns filled with a quaternary amine (500 mg) (Macherey-Nagle, Germany). The quaternary amine columns were activated using successively with the same volume (10 mL) of three different solvents: methanol, deionized water and 0.15 % NaHCO3. On the activated column, 1 mL of coffee brew sample was placed. Then, the columns were washed by 10 mL of 0.15 % NaHCO3, and finally the phenolic acids were eluted using 10 mL of mixture 0.2 M H3PO4 and methanol, 2:1, v/v. Isolated phenolic acids from 1 mL of coffee brew were supplemented with deionized water to 10 mL. The 1 mL of the CB sample was mixed with 9 mL of deionized water to obtain 10 mL of sample extract, due to the future comparison study.
Determination of phenolic acids was carried out using high-performance liquid chromatography (HPLC) 600 Pump and Controller (Waters, Milford, MA, USA) with the assistance of the column, NovaPak® C18 (3.9 × 150 mm; 5 µm) (Waters, Milford, MA, USA). A mixture of deionized water acidified to pH 2.6 with orthophosphoric acid [A] and acetonitrile/water (50:50, v/v) [B] was used as a mobile phase, with a flow rate of 1 mL/min. Separation was made in the gradient. The concentration of the mobile phase [B] was linearly increased from 0 to 50 % during 50 min. The compounds were detected at the wavelength of λ = 280 nm and λ = 320 nm by UV–Vis detector 2998 (Waters, Milford, MA, USA). Identification and quantitation of phenolic acids was carried out by comparison of the obtained retention time peaks of the examined samples with the retention time of the corresponding standards and by using obtained calibration curves for each standard. The 3- and 4-caffeoylquinic acids were collected in the end of the HPLC capillary and identified by mass spectrometer as was described by Wang et al. [35]. The 3- and 4-caffeoylquinic acids and other unidentified phenolic acids were quantified as 5-caffeoylquinic acid (chlorogenic acid).
Evaluation methods of antioxidant capacity (AC) of PA and CB samples
The Folin–Ciocalteu reagent assay (FCR)
The FCR assay, according to Huang et al. [36], measures sample reducing capacity, which does not reflect the total phenolic content; therefore, it is advised to use the FCR method as a reducing capacity assay [36, 37]. Briefly, to the volumetric flasks (10 mL), 0.1 mL of analyzed sample, 5 mL of water and 0.5 mL of Folin–Ciocalteu reagent were pipetted. After 3 min, 1 mL of saturated sodium carbonate solution was added; the flask was filled with water and incubated for 1 h at 23 ± 2 °C in the dark. Absorption was measured by a Shimadzu UV-1202 spectrophotometer (Kyoto, Japan) at a wavelength of 725 nm in a 1 × 1 cm quartz cuvette. The results were expressed as equivalents of chlorogenic acid (μM).
The ferric reducing antioxidant power assay (FRAP)
The FRAP assay was carried out as described by Durmaz and Alpaslan [38]. Briefly, 1 mL of sample was mixed with 2.5 mL of phosphate buffer (0.2 M/L, pH 6.6) and 2.5 mL of 1 % (w/v) K3Fe(CN)6 and incubated at 50 °C for 20 min. Then, 2.5 mL of 10 % (w/v) trichloroacetic acid was added and centrifuged (15,000×g, 10 min). After centrifugation, 2.5 mL of the upper layer was taken and mixed with 2.5 mL of water and 0.5 mL of 0.1 % (w/v) FeCl3. The absorbance was measured at a wavelength of 700 nm. Obtained results were expressed as equivalents of chlorogenic acid (μM).
The free radical DPPH· scavenging activity assay (DPPH)
The DPPH assay was performed according to Scherer and Godoy [39]. Briefly, 0.1 mL of analyzed sample was mixed with 3.9 mL of a DPPH· methanolic solution (absorption of the DPPH· solution was 0.670 ± 0.01). Absorbance was measured at λ max of 517 nm by a Shimadzu UV-1202 spectrophotometer (Kyoto, Japan) after 90-min storage at 23 ± 2 °C in the dark. The results were expressed as an antioxidant activity index (AAI) using the following formula:
where IC50 is a concentration providing 50 % inhibition of free radical DPPH·.
The metal chelating activity assay (MCA)
The MCA assay was carried out as described by Zhao et al. [40]. Briefly, 0.5 mL of sample was mixed with 0.05 mL of FeCl2 (2 mM), and, after 5 min, 0.1 mL of ferrozine (5 mM) was added to initiate a reaction. Then, it was supplemented with water to 3 mL, shaken, and incubated (21 °C, 20 min). The absorbance was measured at a wavelength of 562 nm. The results were expressed as equivalents of EDTA (μM).
Total radical trapping antioxidant parameter assay (TRAP)
The TRAP assay was performed according to Nogala-Kałucka et al. [15]. The final concentration of all reagents in the incubated sample (120 min, 37 °C, in the dark) was as follows: DCFH (1 μM), AAPH (100 μM) and phosphate buffer (10 mM, pH 7.4). Fluorescence intensity was measured by a Shimadzu RF 5001 spectrofluorimeter (Kyoto, Japan) at an excitation wavelength of 480 nm and an emission of 520 nm in a 1 × 1 cm quartz cuvette. The results were expressed as equivalents of chlorogenic acid (μM).
Statistical analysis
Results are presented as mean ± standard deviation from three replicates of each experiment separately for coffee brew and extracted phenolic acids. The differences between mean values were determined by analysis of variance (ANOVA). The post hoc analysis was performed using Tukey’s test. The relationship between analyzed variables was assessed by Pearson’s correlation coefficient. Its significance was evaluated by Student’s t test. In case data did not follow normal distribution, alternatively Spearman’s rank correlation coefficient was calculated. All tests were considered significant at p < 0.05.
To retrieve qualitative correlations between methods and show their applicability in AC determination of coffee brews and PA samples, PCA analysis was applied. For this purpose, data matrix X consisting of k variables and n objects was built. The 13 types of coffee samples, the detailed assignments of which are given in Table 1, are considered as objects. The calculated ACs together for both types of samples (CB and PA), using the applied methods, were considered as variables. After standardization of data matrix X, the PCA analysis created new matrices, including principal component scores (P) and principal component loadings (W), which were applied to further analysis. The results are presented as mean ± standard deviation from three replicates of each experiment. All statistical analyses were performed using the statistical package of the Statistica 10.0 program (StatSoft, Tulsa, OK, USA).
Results and discussion
Phenolic acids content
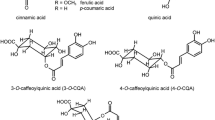
The 3-, 4- and 5-caffeoylquinic acids were predominant forms of phenolic acids identified in the investigated green and roasted coffee samples (Fig. 2). The caffeoylquinic acids as dominant phenolic acids in different type of coffee beans were reported previously [41, 42]. The highest content of phenolic acids was found in green Robusta and the lowest in dark roasted Arabica; both samples originated from India, 208.89 and 33.35 mg/100 mL, respectively. An analogous tendency was observed for the green and roasted coffee samples originating from Java and Brazil (Table 1). The concentration of phenolic acids was higher in Robusta compared to Arabica species. A similar finding was reported previously [43]. The level of phenolic acids in the studied samples decreased significantly (p < 0.05) with increasing the degree of roasting. The concentration of phenolic acids in samples obtained from green CB and PA were twofold to sixfold higher compared with the case with the roasted samples (Table 1). The obtained results are consistent with previous reports [44]. The reduction of phenolic acids content is a result of the thermal instability of phenolic acids during intense roasting and finally their degradation. Simultaneously, chlorogenic acid undergoes isomerization and is partially converted to lactones as a result of dehydration and the formation of intramolecular bonds [14, 45].
Chromatograms of phenolic acids separation in extracts obtained from green (a) medium (b) and dark (c) roasted coffee beans Arabica (India). 1 protocatechuic acid, 2 gentisic acid, 3 3-caffeoylquinic acid, 4 vanillic acid, 5 caffeic acid, 6 5-caffeoylquinic acid (chlorogenic acid), 7 4-caffeoylquinic acid, 8 salicylic acid, 9 p-coumaric acid, 10 ferulic acid, 11 o-coumaric acid, 12 sinapic acid, U1, 2, 3 unknown compounds 1,2,3
pH and AC
The pH of CB prepared from green beans showed higher values (5.41–5.90) compared with those of their roasted counterparts (4.95–5.39) (Table 1). However, the pH values of CB from light roasted coffee beans were lower than those obtained from dark roasted coffee beans. The same observation was reported by Daglia et al. [46]. It can be concluded that the increased degree of roasting of coffee beans contributes to the higher pH of CB; nevertheless, it is still lower compared with the case with CB samples obtained from green coffee beans. It was reported that the pH of the medium can influences the free radical-scavenging action of the phenolic compounds [47]; in the present study, such a relation was not found, due to significant differences with the content of total phenolic acids in the investigated samples with various pH, especially for green and dark roasted coffee.
The application of the Folin–Ciocalteu reagent for the determination of phenol compounds in foodstuffs by Singleton et al. [48] was rapidly popularized in the science community. The proposed method, despite certain limitations, as the Folin–Ciocalteu reagent gives positive reactions with amino acids as also the case with several organic substances without a phenolic hydroxyl group, such as aromatic amines, ascorbic acid, fructose or EDTA, but also with inorganic substances, such as some salts of iron (II) or sodium [49], is very often applied by scientists for the determination of total phenolic compounds. However, in the present study, the method was used to assess the reducing capacity of the samples. Considering the PA samples, the highest values were noted for the extracts isolated from green CB, and lowest values were noted for the extracts from dark roasted CB (Fig. 3a). A similar tendency was observed for other CB samples. However, the reducing capacity of medium roasted CB was slightly higher when compared with that of green CB (Fig. 3A). An analogous observation for CB was reported by Sacchetti et al. [50]. The Maillard reaction products, formed during roasting of coffee bean, give a positive result with the Folin–Ciocalteu reagent [51], and their impact cannot be neglected when the Folin–Ciocalteu reagent is used, whereas at beginning of the thermal process, a smaller loss of phenolic acids occurs for medium roasted CB, compared to the dark roasted coffee samples (Table 1). Additionally, roasting can release functional groups which are not available in the green beans, mainly hydroxyl, and/or lead to an increase the recovery of some bioactive compound, for instance tocopherols [11], giving positive reactions with the Folin–Ciocalteu reagent. CB samples exhibited slightly higher values of reducing capacity (average of 4 %) for green and significantly higher values (average by 22 %) for roasted coffee, compared to PA samples. The amino acids, which give a positive reaction with the Folin–Ciocalteu reagent [49], are present in green coffee extracts, however, during roasting they are destroyed to a very high degree with roast [52]. These reports may explain the slightly higher values of the reducing capacity observed in green CB samples compared with their counterparts in PA samples. In roasted CB, the observed significantly higher values compared with PA samples are associated with the presence of melanoidins in light, medium and dark roasted coffee beans, and their antioxidant activity [53]. Presented results of the FCR assay for both CB and PA samples showed that applying the Folin–Ciocalteu reagent for the purpose of determining total phenol compounds, especially in roasted coffee samples, is prone to a high measurement error. Therefore, it can be concluded that the name of the method “FCR, total content of phenolic compounds” is not a correct one, and that a more appropriate name is “FCR, reducing capacity assay,” as was suggested by Huang et al. [36] and used in a previous study [24].
AC obtained in the different antioxidant assays in CB and PA samples. a The Folin–Ciocalteu reagent assay (FCR), b the ferric reducing antioxidant power assay (FRAP), c the free radical DPPH· scavenging activity assay (DPPH), d the metal chelating activity assay (MCA), e total radical trapping antioxidant parameter assay (TRAP). AC antioxidant capacity, CB coffee brew samples, PA phenolic acids isolated from coffee brew samples; 1GA, 1RA, 2GA, 2MRA, 2DRA, 3GR, 3RR, 4GA, 4MRA, 4DRA, 5GA, 5MRA, 5DRA the sample codes provided in Table 1
In the FRAP assay, for both CB and PA samples, the highest values were noted for extracts obtained from green coffee and the lowest values were noted for dark roasted coffee (Fig. 3b). The value of the reducing power was decreased with the increasing degree of roasting of the coffee samples. Moreover, higher values of reducing power were recorded for Robusta compared to Arabica coffee samples. Similar observations were reported by Richelle et al. [6] and Moreira et al. [43]. The reducing power of CB samples was higher, compared to PA samples, by an average of 3 % and 14 % for green and roasted coffee, respectively. Recorded differences arose from the presence in roasted CB samples of phenolic acids and also new compounds formed during coffee roasting, for instance, melanoidins, which exhibit reduction properties [54]. The results from the FRAP assay are similar to those obtained in the FCR assay.
The DPPH free radical-scavenging activity is basis applied in common assay to characterize the AC of biological materials, and this method has been constantly revised until the present day [55]. The highest antioxidant activity index (AAI) in cases of the CB and PA samples was noted for green, and the lowest value was noted for dark roasted coffee samples (Fig. 3c). The PA compared to their counterparts of CB samples, exhibited 2.5-fold lower AAI values for the green coffee and more than twofold lower values for the dark roasted coffee. It was found, for CB and PA samples, that, with increasing degree of coffee bean roasting, the AAI index decreased. In the DPPH assay, significantly higher differences between PA and CB samples, for both green and roasted coffee compared to the other used methods, were obtained. Delgado-Andrade et al. [56] reported that the contribution of melanoidins to the total AC of CB determined by the DPPH assay can be even to up of 20 %.
The TRAP assay presented the ability of the sample to scavenge peroxyl and alkoxyl radicals. The highest values in the TRAP assay were noted for CB and PA obtained from green CB, and the lowest values were obtained for dark roasted coffee samples (Fig. 3e). Significant higher protection of green coffee extracts, compared to roasted coffee extracts, against oxidation of low-density lipoprotein in the system where oxidation was mediated by AAPH was reported previously [57]. The observed differences in the TRAP value between the CB and PA samples were proportional to the degree to which the coffee beans have been roasted (Fig. 3e). The calculated differences between the CB and PA samples were 6 % for green, 10 % for medium roasted and 20 % for dark roasted coffee beans. The growing difference in the AC between the CB and PA samples extracted from green to dark roasted coffee beans shows that the impact of Maillard reaction products on the AC, in relation to phenolic acids, increases with the degree of roast. Such explanation is obvious, since during roasting the amount of phenolic acids is decreased (Table 1), but Maillard reaction products, present in CB of roasted coffee beans interacting with peroxyl radicals [58].
The ability of the bio-compounds present in foodstuffs to chelate transition metal can lead to an impaired balance between the iron consumed and expelled in the human organism. It was calculated that coffee may reduce the absorption of iron consumed with a meal by up to even 50 % [59]. Approximately one-third higher values for green compared with roasted CB samples were noted. The chelating activity of all PA extracts showed similar values, opposite to those of the CB samples in which a significant difference between green and roasted coffee was obtained (Fig. 3d). The recorded value of chelating activity for CB samples was about threefold and twofold higher for green and roasted coffee, respectively, compared to the values obtained for PA extracts. Phenolic acids form only a temporary complex with Fe+2 [60] which may result in the weak chelating activity of PA samples. Wen et al. [61] demonstrated that an increased degree of roasting coffee beans in coffee extracts is related with the reduced activity of the chelation of zinc ions; however, a similar effect was not observed in the presence of iron ions. Higher chelating activity values measured for CB arise from its complex chemical composition since the number of compounds capable to chelate transition metal ions is much higher than in PA samples. For instance, the chelating activity was reported for the low and high molecular wight fraction of Maillard reaction products [62].
All used methods showed high AC variability depending on the applied test, thus reflecting the molecular mechanisms underlying each of the used assays. In FCR, FRAP and TRAP assays, in green coffee extracts, the isolated phenolic acids from CB samples represented the highest percentage in AC of CB samples with the range between 93.7–99.1 % (96.1 % on average), 96.1–98.9 % (97.4 % on average) and 91.9–97.9 % (94.7 % on average), respectively, whereas the roasting process decreased the phenolic acids contribution in AC of CB samples by 74.5–83.7 % (78.2 % on average), 80.3–91.3 % (85.9 % on average) and 81.4–92.8 % (87.1 % on average), respectively. However, in DPPH and MCA assays, the phenolic acid contribution in AC of CB samples was below 50 %. Moreover, the opposite phenomenon in DPPH and MCA tests, higher percentage in roasted coffee extracts compared with green coffee extracts (39.5–47.4 % (44.1 % on average), 42.0–56.4 % (46.1 % on average) and 37.1–40.3 % (38.6 % on average), 30.8–36.6 % (33.0 % on average), respectively), to the FCR, FRAP and TRAP assays was observed. Those results show a significant impact of the applied assays for AC determination. A smaller difference between the CB and PA samples was obtained in FCR, FRAP and TRAP assays, whereas significant differences were recorded using DPPH and MCA tests. Additionally, it appears that the nature of the tested sample (green vs. roasted) has a significant impact since greater differences between the CB and counterparts of PA samples were obtained for roasted than for green coffee samples in all study assays.
Correlations between results obtained with different methods
To explore relations between antioxidant assays and the total amount of phenolic acids in CB and PA samples, a correlation matrix was calculated (Table 2). This shows a higher (0.79–0.98) correlation between the data obtained from different assays for the PA extracts compared to the CB samples (0.15–0.96). This observation indicates that a more complex composition makes extracts (CB) less predictable in different antioxidant systems. The lowest correlation coefficient, 0.15, was found between the MCA and FCR data. This can be expected to result from reaction of different types of antioxidants in CB in these two assays. The results of the FCR test indicate a strong impact of PA on the AC of CB, whereas in the MCA assay the effect of other antioxidants present in CB is more significant (Fig. 3a, d). The MCA results for CB are rather poorly correlated with those obtained in the other assays. Also in the case of PA samples the lowest correlation coefficients are found between the MCA assay and the other test, which is consistent with the previous observation that the PA have a rather poor activity in MCA compared to the other assays. These results confirm the conclusion obtained from experimental data that the observed difference is related to a chelation mechanism, compared to other assays based on reducing or free radical mechanisms. Significant correlations between three antioxidant assays DCA, FRAP and ABTS of CB samples were also reported previously [56]. Present study shows that all applied antioxidant assays were highly correlated; however, it the case of the MCA test some exemption of this rule were noted.
Principal component analysis (PCA)
Principal component analysis (PCA) and correlation methods were applied to estimate the contribution of different chemical compounds in coffee brews which may be related to observed antioxidant capacity [19, 63]. Statistical analyses showed significant correlations between browned compounds, trigonelline, 5-caffeoylquinic acid and cafeic acid contents with the antioxidant activity measured by both DPPH and redox potential methods.
During our studies on AC values obtained with different techniques in different type of samples, a lot of data have been created. To retrieve existing correlations between the properties and applied assays, the PCA method was applied. The obtained results presented in Fig. 4 showed that first two PCs describe 95 % of the initial variability. PC1 explains 85 % and PC2 10 % of the observed variability. Both components were highly correlated with the AC results obtained from the applied methods. The variables on the plot (Fig. 4a) lying close to each other have similar characteristics; thus, we may assume that these methods are highly positively correlated. These include results from DPPH, FCR, TRAP, FRAP (for both CB and PA samples) and MCA (only for PA samples) method measurements. This shows that the PC1 component is related to the results of the used methods, with medium correlations to “MCA_CB.” Second, the PC2 component accounts for 10 % of the total variance and is built from results left after the first factor was formed. Figure 4a shows that the PC2 component is highly positively correlated with “MCA_CB”.
Based on plots from Fig. 4a, we may conclude that results obtained for PA and CB samples using all methods, except results for “MCA_CB,” are highly correlated. The low correlation of MCA results measured in the CB sample with other results probably arises from the specificity of this method. This statement is strongly supported by fact that “MCA_CB” results are not correlated with the results from other methods and samples. The MCA method is based on the ability of compounds to chelate transition metal ions. It seems obvious that the number of compounds in CB with chelating activity is much higher compared with that of the PA samples. The relation obtained from PCA between methods are very similar to the results presented in correlation matrix, Table 2, where correlations coefficients were calculated separately for one type of samples, i.e., coffee brew or extracted phenolic acids. This seems not surprising since both methods are based on correlation matrices. However, similarity between the results from both approaches indicates that antioxidant capacity in both types of samples arises from these same components.
Another output from the PCA method is a matrix of factor scores, values that specify the position of each product along each of the PC components. The calculated score plot, Fig. 4b, shows the position of the investigated coffee samples in the multivariate space of first two PCs. It illustrates that calculated scores arranged coffee bean properties in three clusters of the Arabica type and two single locations of Robusta in this space. Such a clear separation between the sample points and differences in physical and chemical parameters related to the investigated coffee. The score plot for PC1/PC2 shows a cluster composed from “5GA,” “4GA” and “2GA,” which includes green beans, cluster “5DRA,” “4DRA” and “2DRA,” which is assembled from dark roasted beans, and cluster “5MRA,” “4MRA” and “2MRA,” which refers to medium roasted beans. In each cluster, the beans were from a different origin, the sample numbers 2, 4, 5 referring to Java, Brazil and India, respectively. We may notice that decaffeinated green coffee bean “1GA” is located close to the cluster of green coffee beans. Similarly, “1RA,” the dark roasted decaffeinated bean is close to the dark roasted beans. Since the first two PC components are related to AC results, this clearly shows that all used methods may easily differentiate between Arabica types of coffee beans, regardless of their origin, based on measured AC. Relatively highest scores obtained for Robusta and separation from Arabica suggest its easy distinction from the Arabica type during AC measurements. Based on the presented results, we may conclude that during AC measurements, each of the applied method can easily differentiate, with a statistically significant difference, between types of coffee beans, whereas the origin and decaffeinated properties of “1GA” and “1RA” do not influence the results of AC determination.
Conclusions
The presented study showed that the boiled-type CB possesses higher AC compared with the extracts of PA isolated from corresponding CB samples. Nevertheless, isolated PA from CB samples had the main contribution (on average over 95 and 84 % in green and roasted coffee extracts, respectively) in AC of the CB samples, in three (FCR, FRAP and TRAP assays) from five applied antioxidant tests. In DPPH and MCA assays, the phenolic acids contribution in AC of CB samples was below 50 % (on average over 36 and 45 % in green and roasted coffee extracts, respectively). The green CB samples exhibited the highest AC, decreasing with the rise in the degree of coffee bean roasting, which was associated with a diminishing total amount of phenolic acids. Obtained results demonstrate some differences and similarities between antioxidant tests underlining the specific molecular mechanisms of each applied assay.
The applied statistical calculations evidenced that all used methods are equally suitable to determine the AC property of PA and CB samples. The results from the MCA method are poorly correlated with the other methods, most likely due to a completely different molecular mechanism involved during antioxidant capacity measurement. PCA was applied to evidence graphically the feasibility of the methods to distinguish between coffee brews with different degrees of roasting, regardless of coffee origin.
Abbreviations
- AAPH:
-
2,2′-Azobis (2-amidinopropane) dihydrochloride
- AC:
-
Antioxidant capacity
- CB:
-
Coffee brew samples
- DCFH:
-
2′,7′-Dichlorofluorescein
- DCFH-DA:
-
2′,7′-Dichlorofluorescein diacetate
- DPPH· :
-
1,1-Diphenyl-2-picrylhydrazyl
- DPPH:
-
Free radical DPPH· scavenging activity assay
- EDTA:
-
Ethylenediaminetetraacetic acid
- FCR:
-
Folin–Ciocalteu reagent assay
- FRAP:
-
Ferric reducing antioxidant power assay
- MCA:
-
Metal chelating activity assay
- PA:
-
Phenolic acid samples isolated from coffee brew extracts
- PCA:
-
Principal component analysis
- RP-HPLC:
-
Reverse-phase high-performance liquid chromatography
- TRAP:
-
Total radical trapping antioxidant parameter assay
References
FAOSTAT (2014) FAO Statistical Database. [cited 10 Dec 2014]. http://www.fao.org
Jalil AMM, Azlan A, Ismail A (2007) Assessment of antioxidant capacity and phenolic content of selected commercial beverages. Malays J Nutr 13:149–159
Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M, Brighenti F (2003) Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr 133:2812–2819
Pulido R, Hernandez-Garcia M, Saura-Calixto F (2003) Contribution of beverages to the intake of lipophilic and hydrophilic antioxidants in the Spanish diet. Eur J Clin Nutr 57:1275–1282
Svilaas A, Sakhi AK, Andersen LF, Svilaas T, Strom EC, Jacobs DR, Ose L Jr, Blomhoff R (2004) Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr 134:562–567
Richelle M, Tavazzi I, Offord E (2001) Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J Agric Food Chem 49:3438–3442
Delgado-Andrade C, Morales FJ (2005) Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J Agric Food Chem 53:1403–1407
Liu Y, Kitts DD (2011) Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res Int 44:2418–2424
Rufián-Henares JA, de la Cueva SP (2009) Antimicrobial activity of coffee melanoidins—a study of their metal-chelating properties. J Agric Food Chem 57:432–438
Górnaś P, Siger A, Polewski K, Pugajeva I, Waśkiewicz A (2014) Factors affecting tocopherol contents in coffee brews: NP-HPLC/FLD, RP-UPLC-ESI/MSn and spectroscopic study. Eur Food Res Technol 238:259–264
Górnaś P, Siger A, Pugajeva I, Czubinski J, Waśkiewicz A, Polewski K (2014) New insights regarding tocopherols in Arabica and Robusta species coffee beans: RP-UPLC-ESI/MSn and NP-HPLC/FLD study. J Food Compost Anal 36:117–123
Brezová V, Šlebodová A, Staško A (2009) Coffee as a source of antioxidants: an EPR study. Food Chem 114:859–868
Baggenstoss J, Poisson L, Kaegi R, Perren R, Escher F (2008) Coffee roasting and aroma formation: application of different time–temperature conditions. J Agric Food Chem 56:5836–5846
Clifford M (2000) Chlorogenic acids and other cinnamates— nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric 80:1033–1043
Nogala-Kałucka M, Dwiecki K, Siger A, Górnaś P, Polewski K, Ciosek S (2013) Antioxidant synergism and antagonism between tocotrienols, quercetin and rutin in model system. Acta Aliment 42:360–370
Luciano G, Tormod N (2009) Interpreting sensory data by combining principal component analysis and analysis of variance. Food Qual Prefer 20:167–175
Chapman KW, Lawless HT, Boor KJ (2001) Quantitative descriptive analysis and principal component analysis for sensory characterization of ultrapasteurized milk. J Dairy Sci 84:12–20
Ghosh D, Chattopadhyay P (2012) Application of principal component analysis (PCA) as a sensory assessment tool for fermented food products. J Food Sci Technol 49:328–334
Lopez-Galilea I, De Pena MP, Cid C (2007) Correlation of selected constituents with the total antioxidant capacity of coffee beverages: influence of the brewing procedure. J Agric Food Chem 55:6110–6117
Lopez-Galilea I, de Pena MP, Cid C (2008) Application of multivariate analysis to investigate potential antioxidants in conventional and torrefacto roasted coffee. Eur Food Res Technol 227:141–149
Subari N, Saleh JM, Shakaff AYM, Zakaria A (2012) A hybrid sensing approach for pure and adulterated honey classification. Sensors 12:14022–14040
Santos PM, Pereira-Filho ER, Rodriguez-Saona LE (2013) Rapid detection and quantification of milk adulteration using infrared microspectroscopy and chemometrics analysis. Food Chem 138:19–24
Dourtoglou VG, Dourtoglou TH, Antonopoulos A, Stefanou E, Lalas S, Poulos C (2003) Detection of olive oil adulteration using principal component analysis applied on total and regio FA content. JAOCS 80:203–208
Caponio F, Chiavaro E, Paradiso VM, Paciulli M, Summo C, Cerretani L, Gomes T (2013) Chemical and thermal evaluation of olive oil refining at different oxidative levels. Eur J Lipid Sci Technol 115:1146–1154
Zulueta A, Esteve MJ, Frígola A (2009) ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem 114:310–316
Tabart J, Kevers C, Pincemail J, Defraigne J-O, Dommesa J (2009) Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem 113:1226–1233
Leopoldini M, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125:288–306
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835
Cämmerer B, Kroh LW (2006) Antioxidant activity of coffee brews. Eur Food Res Technol 223:469–474
del Castillo MD, Ames JM, Gordon MH (2002) Effect of roasting on the antioxidant activity of coffee brews. J Agric Food Chem 50:3698–3703
Budryn G, Pałecz B, Rachwał-Rosiak D, Oracz J, Zaczyńska D, Belica S, Navarro-González I, Meseguer JMV, Pérez-Sánchez H (2015) Effect of inclusion of hydroxycinnamic and chlorogenic acids from green coffee bean in β-cyclodextrin on their interactions with whey, egg white and soy protein isolates. Food Chem 168:276–287
Karadag A, Ozcelik B, Saner S (2009) Review of methods to determine antioxidant capacities. Food Anal Methods 2:41–60
Moon J-K, Shibamoto T (2009) Antioxidant assays for plant and food components. J Agric Food Chem 57:1655–1666
Dwiecki K, Siger A, Czubiński J, Nogala-Kałucka M, Lampart-Szczapa E (2012) The interactions between rapeseed lipoxygenase and native polyphenolic compounds in a model system. J Am Oil Chem Soc 89:379–387
Wang Y, Wen J, Zheng W, Zhao L, Fu X, Wang Z, Xiong Z, Li F, Xiao W (2015) Simultaneous determination of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid and geniposide in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study after administration of reduning injection. Biomed Chromatogr 29:68–74
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Górnaś P, Šnē E, Siger A, Segliņa D (2014) Sea buckthorn (Hippophae rhamnoides L.) leaves as valuable source of lipophilic antioxidants: the effect of harvest time, sex, drying and extraction methods. Ind Crops Prod 60:1–7
Durmaz G, Alpaslan M (2007) Antioxidant properties of roasted apricot (Prunus armeniaca L.) kernel. Food Chem 100:1177–1181
Scherer R, Godoy HT (2009) Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem 112:654–658
Zhao H, Fan W, Dong J, Lu J, Chen J, Shan L, Lin Y, Kong W (2008) Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem 107:296–304
Farah A, Donangelo CM (2006) Phenolic compounds in coffee. Braz J Plant Physiol 18:23–36
Nebesny E, Budryn G (2003) Antioxidative activity of green and roasted coffee beans as influenced by convection and microwave roasting methods and content of certain compounds. Eur Food Res Technol 217:157–163
Moreira DP, Monteiro MC, Ribeiro-Alves M, Donangelo CM, Trugo LC (2005) Contribution of chlorogenic acids to the iron-reducing activity of coffee beverages. J Agric Food Chem 53:1399–1402
Bekedam EK, Loots MJ, Schols HA, Van Boekel MA, Smit G (2008) Roasting effects on formation mechanisms of coffee brew melanoidins. J Agric Food Chem 56:7138–7145
Farah A, de Paulis T, Trugo LC, Martin PR (2005) Effect of roasting on the formation of chlorogenic acid lactones in coffee. J Agric Food Chem 53:1505–1513
Daglia M, Papetti A, Gregotti C, Berte F, Gazzani G (2000) In vitro antioxidant and ex vivo protective activities of green and roasted coffee. J Agric Food Chem 48:1449–1454
Lemanska K, Szymusiak H, Tyrakowska B, Zielinski R, Soffers AE, Rietjens IM (2001) The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic Biol Med 31:869–881
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol 299:152–178
Box JD (1983) Investigation of the Folin–Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res 17:511–525
Sacchetti G, Di Mattia C, Pittia P, Mastrocola D (2009) Effect of roasting degree, equivalent thermal effect and coffee type on the radical scavenging activity of coffee brews and their phenolic fraction. J Food Eng 90:74–80
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S
Casal S, Mendes E, Oliveira MBPP, Ferreira MA (2005) Roast effects on coffee amino acid enantiomers. Food Chem 89:333–340
Borrelli RC, Visconti A, Mennella C, Anese M, Fogliano V (2002) Chemical characterization and antioxidant properties of coffee melanoidins. J Agric Food Chem 50:6527–6533
Rufián-Henares JA, Morales FJ (2007) Effect of in vitro enzymatic digestion on antioxidant activity of coffee melanoidins and fractions. J Agric Food Chem 55:10016–10021
Gornas P (2015) Unique variability of tocopherol composition in various seed oils recovered from by-products of apple industry: rapid and simple determination of all four homologues (alpha, beta, gamma and delta) by RP-HPLC/FLD. Food Chem 172:129–134
Delgado-Andrade C, Rufian-Henares JA, Morales FJ (2005) Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J Agric Food Chem 53:7832–7836
Gómez-Ruiz JÁ, Ames JM, Leake DS (2008) Antioxidant activity and protective effects of green and dark coffee components against human low density lipoprotein oxidation. Eur Food Res Technol 227:1017–1024
Yilmaz Y, Toledo R (2005) Antioxidant activity of water-soluble Maillard reaction products. Food Chem 93:273–278
Layrisse M, García-Casal MN, Solano L, Barón MA, Arguello F, Llovera D, Ramírez J, Leets I, Tropper E (2000) Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J Nutr 130:2195–2199
Chen X, Ahn DU (1998) Antioxidant activities of six natural phenolics against lipid oxidation induced by Fe2+ or ultraviolet light. J Am Oil Chem Soc 75:1717–1721
Wen X, Enokizo A, Hattori H, Kobayashi S, Murata M, Homma S (2005) Effect of roasting on properties of the zinc-chelating substance in coffee brews. J Agric Food Chem 53:2684–2689
Yoshimura Y, Iijima T, Watanabe T, Nakazawa H (1997) Antioxidative effect of Maillard reaction products using glucose–glycine model system. J Agric Food Chem 45:4106–4109
Lopez-Galilea I, Andriot I, de Pena MP, Cid C, Guichard E (2008) How does roasting process influence the retention of coffee aroma compounds by lyophilized coffee extract? J Food Sci 73:S165–S171
Acknowledgments
This study was partially supported by Grant 508/82-4 from Poznan University of Life Sciences, Poznan, Poland, and by Grant N312 1410 33 from the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Compliance with ethical requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Górnaś, P., Dwiecki, K., Siger, A. et al. Contribution of phenolic acids isolated from green and roasted boiled-type coffee brews to total coffee antioxidant capacity. Eur Food Res Technol 242, 641–653 (2016). https://doi.org/10.1007/s00217-015-2572-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2572-1