Abstract
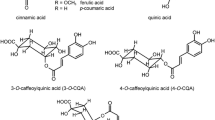
Antioxidant (AO) capacity of instant, espresso, filter and Turkish/Greek coffee brews, coffee substitutes (roasted chicory root, barley, pea, chickpea, carob and dried fig) and individual compounds (phenolic acids, flavonoids, methylxanthines, N-methyl pyridinium and HMW melanoidins) was assessed using DC polarographic assay based on decrease of anodic current originating from hydroxo-perhydroxo mercury complex formed in alkaline solutions of H2O2 at potential of mercury dissolution, as well as three spectrophotometric assays (DPPH, ABTS and FRAP). A large difference between applied assays ability to recognize various types of individual AOs was noticed. Only according to DC polarographic assay significant AO activity was ascribed to methylxanthines and N-methyl pyridinum. The total content of phenolics (TPC) present in complex samples was determined by FC assay. The highest TPC was ascribed to instant coffees and coffee substitutes while the lowest to decaffeinated filter coffee. Complex samples were grouped based on principal components analysis, phenolics AO coefficient, calculated as the ratio between AO capacity and TPC, and relative AO capacity index (RACI), calculated by assigning equal weight to all applied assays including FC. The highest values of RACI were ascribed to instant coffee brews, followed by substitutes while the lowest to the decaffeinated espresso coffee.


Similar content being viewed by others
References
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. LWT 28:25–30
Carlsen MH, Halvorsen BL, Holte K, Bohn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I, Berhe N, Willett WC, Phillips KM, Jacobs DR, Blomhoff R (2010) The total antioxidant content of more than 3100 foods beverages spices herbs and supplements used worldwide. Nutr J 9:3
Carlsson ACC, Grafenstein J, Budnjo A, Laurila JL, Bergquist J, Karim A, Kleinmaier R, Brath U, Erdelyi M (2012) Symmetric halogen bonding is preferred in solution. J Am Chem Soc 134:5706–5715
Custodio L, Fernandes E, Escapa AL, Fajardo A, Aligue R, Albericio F, Neng NR, Nogueira JMF, Romano A (2011) Antioxidant and cytotoxic activities of carob tree fruit pulps are strongly influenced by gender and cultivar. J Agric Food Chem 59:7005–7012
El Qouatli SE, Rosie NT, Valery HG, Oubenamar H, Hissou H, Najih R, Chtaini A (2011) Electrochemical analysis of the antioxidant capacity of coffee. Bulletin of the Catalysis Society of India 10(3):15–20
Fukushima Y, Ohie T, Yonekawa Y, Yonemoto K, Aizawa H, Mori Y, Watanabe M, Takeuchi M, Hasegawa M, Taguchi C, Kondo K (2009) Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J Agric Food Chem 57:1253–1259
Hečimović I, Belščak-Cvitanović A, Horžić D, Komes D (2011) Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem 129:991–1000
Jurgonski A, Milala J, Juskiewicz J, Zdunczyk Z, Krol B (2011) Composition of chicory root peel seed and leaf ethanol extracts and biological properties of their non-inulin fractions. Food Technol Biotech 49:40–47
Ludwig IA, Sanchez L, Caemmerer B, Kroh LW, Paz de Pena M, Cid C (2012) Extraction of coffee antioxidants: impact of brewing time and method. Food Res Int 48:57–64
Niseteo T, Komes D, Belščak-Cvitanović A, Horžić D, Budeč M (2012) Bioactive composition and antioxidant potential of different commonly consumed coffee brews affected by their preparation technique and milk addition. Food Chem 134:1870–1877
Oliveira-Neto JR, Garcia Rezende S, de Fatima Reis C, Benjamin SR, Rocha MV, de Souza Gil E (2016) Electrochemical behavior and determination of major phenolic antioxidants in selected coffee samples. Food Chem 190:506–512
Omwamba M, Hu Q (2010) Antioxidant activity in barley (Hordeum Vulgare L) grains roasted in a microwave oven under conditions optimized using response surface methodology. J Food Sci 75:C66–C73
Papetti A, Daglia M, Aceti C, Quaglia M, Gregotti C, Gazzani G (2006) Isolation of an in vitro and ex vivo antiradical melanoidin from roasted barley. J Agric Food Chem 54:1209–1216
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Bio Med 26:1231–1237
Sahin H, Topuz A, Pischetsrieder M, Ozdemir F (2009) Effect of roasting process on phenolic antioxidant and browning properties of carob powder. Eur Food Res Technol 230:155–161
Segev A, Badani H, Galili L, Hovav R, Kapulnik Y, Shomer I, Galili S (2012) Effects of baking roasting and frying on total polyphenols and antioxidant activity in colored chickpea seeds. Food Nutr Sci 3:369–376
Vinson JA, Zubik L, Bose P, Samman N, Proch J (2005) Dried fruits: excellent in vitro and in vivo antioxidants. J Am Coll Nutr 24:44–50
Wang Y, Ho CT (2009) Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem 57:8109–8114
Yardim Y (2012) Electrochemical behavior of chlorogenic acid at a boron-doped diamond electrode and estimation of the antioxidant capacity in the coffee samples based on its oxidation peak. J Food Sci 77:C408–C413
Ziyatdinova G, Aytuganova I, Nizamova A, Budnikov H (2013) Differential pulse voltammetric assay of coffee antioxidant capacity with MWNT-modified electrode food. Anal Method 6:1629–1638
Acknowledgements
Authors sincerely express appreciation to Branko J. Drakulić for constructive suggestions as well assynthesis and characterization of N-methyl pyridinum.
Funding
This work was supported by the Ministry of Education and Science of Republic of Serbia, Grants 43010 and 31093.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Gorjanović, S., Komes, D., Laličić-Petronijević, J. et al. Antioxidant efficiency of polyphenols from coffee and coffee substitutes-electrochemical versus spectrophotometric approach. J Food Sci Technol 54, 2324–2331 (2017). https://doi.org/10.1007/s13197-017-2672-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2672-y