Abstract
The focus of the present research was to study inhibition of lipoxygenase activity by rapeseed native polyphenols and the interactions between those compounds and the enzyme. The enzyme and polyphenolic compounds (polyphenols, phenolic acids) were extracted from rapeseed (Brassica napus) varieties Aviso and PR45DO3. The total phenolic compounds concentration in tested rapeseed was 1,485–1,691 mg/100 g d.m. (dry matter) and the free phenolic acids content in both rapeseed varieties was about 76 μg/100 g d.m. The isolated proteins showed lipoxygenase activity. Prooxidant properties of phenolic compounds in the presence of lipoxygenase and linoleic acid were observed rather in the case of extracts containing a relatively high concentration of miscellaneous polyphenols. Antioxidant properties were recorded in the case of phenolic acid extracts which contain only 1.4–1.9% of phenolics present in raw phenolic extracts. We propose that the prooxidant effect of phenolic compounds comes from quinone and oxidized polyphenols formation. The observed antioxidant activity of phenolic acid extracts is probably due to their ability to scavenge free radicals formed from linoleic acid. However, reduction of lipoxygenase ferric to ferrous ions, which prevent the activation of the enzyme and inhibited its activity, was also observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last five years, the culture of rapeseed in Poland has been constantly increasing, and also the forecast for the period up to the end of 2011 shows further increase—this concerns the crop as well as the area under cultivation. Rapeseed, cultivated not only as a raw material for the oil industry, should be characterized by high quality and also considerable durability. The seeds are a biological material in which various biochemical processes occur during post-harvest processing (purifying, drying, and storing). Many of the processes mentioned above are catalyzed by enzymes like lipoxygenase. Lipoxygenase (EC 1.13.11.12) is an enzyme of the oxidoreductases class, commonly found in all plants and animals. Lipoxygenase belongs to a subclass of dioxygenases containing iron and catalyzes oxidation of free or esterified polyunsaturated fatty acids to hydroperoxides. This enzyme catalyzes the oxidation of fatty acids which contain a cis,cis-1,4-pentadiene moiety [1]. Plant lipoxygenase consists of a single chain with a molecular mass of about 75–100 kDa and an activity over a wide range of optimum pH varying from 6.0 to 9.0 [2]. The formation of free radicals by lipoxygenase activity on polyunsaturated fatty acids is one of the major reasons for interest in this enzyme by food technologists due to the fact that the products obtained might react with other food ingredients like vitamins, pigments, proteins and polyphenols, reducing the quality of raw materials and foodstuffs. Lipoxygenase like lipase has a negative influence during the storage of oilseeds. During oxidation of polyunsaturated fatty acid acylglycerol hydroperoxides are formed [3]. In the case of rapeseed it causes deterioration of quality parameters of oil and post-extraction meal produced from the seeds [4, 5]. Extent of these transformations is conditioned, first of all, by the presence of water, and also by the content of natural antioxidants. Natural antioxidants determine lipid stability in stored seeds and account for an adequate nutritive value of produced oils [6]. Rapeseed is characterized by high content of phenolic compounds, their content is tenfold comparing to other oilseeds [7]. The most common phenolic compound is sinapic acid and its derivatives, especially sinapin which constitutes 80% phenolic compounds in rapeseed [8–11]. Sinapic acid exists also as glucopyranosyl sinapate [12]. Only a small part of sinapic acid, less than 16%, is present as the free sinapic acid [8].
Phenolic compounds occurring in plants (flavonoids, phenolic acid) may modify the activity of lipoxygenase. According to Zadernowski et al. [13] rapeseed phenols inhibit the action of this enzyme. Some flavonoids (isoflavones), present in soybean, inhibit lipoxygenase by reduction of the iron present in the active site of the enzyme from ferric to the resting ferrous state [14]. Polyphenols may also undergo oxidation to semi-quinones or quinones, which bind to sulfhydryl or amino groups of the enzyme causing inhibition [15]. Phenolic compounds inhibit peroxidation of linoleic acid also by free radical scavenging. The scavenging-radical activity of rapeseed polyphenols is well established [3, 16]. On the other hand plant polyphenols may act as a prooxidants. It may come from autoxidation yielding phenoxyl radicals or quinone formation in the presence of lipoxygenase [15, 17]. That is why the enzymatic activity of lipoxygenase and antioxidant/prooxidant potential of polyphenols in the presence of this enzyme are determined by many factors. The nature and mechanism of those processes is quite complex and not ultimately explained. The focus of the present investigation is to study inhibition of lipoxygenase activity by rapeseed native polyphenols and the interaction between those compounds and enzyme.
Materials and Methods
The source of lipoxygenase and phenolic compounds was rapeseed (Brassica napus) varieties Aviso and PR45DO3. The experimental material was obtained from the fields in the middle region of Wielkopolska (Poland), crop 2011. Folin-Ciocalteu reagent, 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH), linoleic acid and all standard of phenolic acids (protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, o-coumaric acid and sinapic acid) were purchased from Sigma (St. Louis, MO). Acetonitrile and orthophosphoric acid (HPLC-grade both) were obtained from Merck (Darmstadt, Germany). All other solvents and chemicals used in this study were of analytical grade. Deionized water was used in the resin-based column chromatography, while ultrapure water purified via the Milli-Q system (Millipore, Bedford, USA) was used during HPLC analysis.
Lipoxygenase Extraction and Purification
Lipoxygenase was partially purified using the procedure described below. The protein was extracted from ground defatted seed by using a modification of the procedure described by Khalyfa et al. [18]. Briefly, the defatted ground seeds were suspended in 20 mM Tris-HCl buffer at pH 7.5 in the proportion 1:6 (w/v) with mechanical stirring for 16 h at 4 °C. The resulting suspension was centrifuged for 30 min at 20,000 g, the pellet was discard and the supernatant was subjected to second additional centrifugation steps (30 min at 20,000 g). Obtained supernatant was treated with solid ammonium sulfate in proportion 1:2 (w/v) and mechanical stirring for 30 min at 4 °C. The precipitate was obtained by centrifugation at 20,000 g for 30 min. The supernatant discarded and the precipitate resuspended in minimal amount of 20 mM Tris–HCl buffer at pH 7.5. The protein extract obtained was desalted by applying to HiTrap Desalting column (Pharmacia, Uppsala, Sweden) equilibrated with 20 mM Tris–HCl buffer at pH 7.5. Desalted proteins were collected in 2-ml fractions. The absorbance of the fractions was measured at 280 nm. The desalt step was carried out by using an automatic FPLC system (Pharmacia, Upssala, Sweden). Analysis of the oxidation of linoleic acid by lipoxygenase was performed immediately after isolation of the enzyme from the rapeseeds.
Protein Concentration Measurements
Protein content was assayed by the colorimetric Bradford method [19] using BSA as a standard. Analyses were carried out at wavelength λ = 595 nm (UV–Vis spectrophotometer SP 8001, Metertech Inc. Taipei, Taiwan). To determine protein concentration, the standard curve based on BSA was made (y = 0.6192x; R 2 = 0.9966).
Methanol Extracts of Phenolic Compounds
All samples were defatted using an automatic Soxhlet Büchi Extraction System B-811 (Büchi Labortechnik AG, Flawil, Switzerland). The extraction with n-hexane was carried out for two hours. To obtain rapeseed phenols, each sample was extracted with 80% methanol. The samples were mixed with the solvent (1:15 w/v), shaken for 30 min. After centrifugation (10 min at 5,000×g) (model 6K15, Sigma, Osterode am Harz, Germany), the precipitate was re-extracted twice more by following the same steps. The three supernatants were combined and evaporated under reduced pressure using an R-215 Rotorvapor (Büchi Labortechnik AG, Flawil, Switzerland) to a volume of 25 ml.
Total Phenolic Contents
The content of total phenolic compounds in methanol extracts was determined by the Folin–Ciocalteu method. An aliquot (0.025 ml) of the methanolic extract was placed in a volumetric flask (10 ml). Water (5 ml) and Folin–Ciocalteu reagent (0.5 ml) were added. After 3 min, saturated sodium carbonate (1 ml) was added. The flask was filled with water up to 10 ml. After 1 h, the solution absorbance was measured at λmax = 725 nm against a reagent blank using a UV–Vis spectrophotometer SP 8001 (Metertech Inc., Taipei, Taiwan). The total phenolic content was determined after preparation of a standard curve and on that basis, the total phenolic compounds were measured as sinapic acid equivalents (SAE).
Isolation of Phenolic Acid
In order to isolate the fraction of phenolic acids the Chromabond® System (Macherey–Nagle, Germany) was applied together with the SPE Bakerbond spe™ columns filled with a quaternary amine (500 mg). The process comprised 4 stages: (1) conditioning of the columns (10 ml methanol, 10 ml distilled water and 10 ml 0.15% solution NaHCO3), (2) placing of the sample (3 ml), (3) washing of the column (15 ml 0.15% solution NaHCO3), (4) free phenolic acids elution with a mixture of 0.2 M H3PO4 and methanol (2:1 v/v) (10 ml). The pH value of the eluate obtained was adjusted to about 3 using 1 M NaOH [20]. After purification by SPE, recovery of the phenolic acids standards amounted to 96.7% for the vanillin and ferulic acids, 98.2% for the chlorogenic acid and 99.7% for the remaining phenolic acids. The recovery of phenolic acids from the SPE cartridge was determined by passing standard solutions containing 0.05 mg/mL of standard compounds (protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, o-coumaric acid and sinapic acid) through the SPE cartridges under the same conditions as stated above for isolation of phenolic acids from rapeseed extracts. The amount of the individual phenolic acids before and after SPE was determined using HPLC.
Determination of the Phenolic Acids Composition
Identification and quantification of free phenolic acids was achieved using analytical reversed-phase high performance liquid chromatography (HPLC 600 Pump and Controller—Waters, Milford, MA, USA) using a XBridgeTM C18 column (4.6 × 100 mm; 3.5μm) (Waters, Milford, MA, USA). A gradient program was used with the mobile phase, combining solvent A (acetonitrile: water 50:50 v/v) and solvent B (water adjusted to pH 2.7 with orthophosphoric acid) as follows: 0–50% A (60 min), 50–0% A (9 min). The flow rate was 1.0 ml/min. The injection volume was 10 μl while the column temperature was maintained at 30 °C. The signal was monitored at 200–600 nm with the diode array detector (PDA detector 2998 Waters, Milford, MA, USA). Peak identification was performed by comparing retention times and diode array spectral characteristics with the standards. The presence of the sinapic acid derivative was noted based on similarities in spectra obtained from the diode array detector (PDA detector 2998 Waters, Milford, MA, USA). Because the standards of sinapic acid derivatives are not commercially available for quantitative determinations, sinapic acid was used as an equivalent.
Antioxidant Activity Determination
The method consisted of spectrophotometric measurement of the intensity of the color change in solution depending on the amount of DPPH•. The reaction was initiated by mixing polyphenol methanolic extract with 3 ml methanol and then adding 1 ml of DPPH• (0.012 g/100 ml). Absorbance at λmax = 517 nm (UV–Vis spectrophotometer SP 8001, Metertech Inc. Taipei, Taiwan) was checked after 30 min of incubation in the dark. The activity of the extract in scavenging DPPH• was expressed as ARP (antiradical power), according to the formula:
Where, EC50 is the concentration of antioxidant required to cause a 50% reduction in the original concentration of DPPH.
Linoleic Acid Peroxidation in the Presence of Rapeseed Lipoxygenase and Phenolic Extracts
For linoleic acid peroxidation experiments, a solution containing 73 μg/ml of lipoxygenase from rapeseed varieties Aviso (LOXA) and PR45DO3 (LOXP) and 98 μM of linoleic acid in 20 mM Tris–HCl buffer (pH 7.5) was prepared. In each sample to volume 2.03 ml of protein–linoleic acid mixture 20–100 μl of phenolic extract from Aviso (PEA) and from PR45DO3 (PEP) or 66–330 μl of phenolic acid extract from Aviso (PAA) and from PR45DO3 (PAP) was added. Each sample was completed with buffer to a final volume of 2.36 ml. As a result of the extraction procedures, the range of volumes 20–100 μl (phenolic extract) and 66–330 μl (phenolic acid extract) containing phenolic compounds were obtained from the equal amounts of plant material. The samples were incubated in the dark at room temperature (22 °C). The peroxidation was stopped by adding 2 ml of phosphoric acid solution (pH 2.7).
HPLC Analysis of Linoleic Acid Hydroperoxides (FAOOHs)
The procedures of Banni et al. [21] were adopted for HPLC analysis of the linolenic acid hydroperoxides. Identification and quantification of the hydroperoxides was achieved using analytical reversed-phase high performance liquid chromatography (HPLC—Waters, Milford, MA, USA) using a LiChrosorb RP-18 column (250 × 4.6, 5 μm) (Merck, Germany). An isocratic program was used with the mobile phase, combining acetonitrile, water and acetic acid (60:40:0.12 v/v). The flow rate was 1.5 ml/min. The signal was monitored at 200–300 nm with the diode array detector (PDA detector 2998 Waters, Milford, MA, USA). The content of individual fatty acid hydroperoxides (FAOOHs) in all samples was calculated on the basis of calibration curves made for pure 13-hydroperoxy-octadecadienoic acid (13-HPODE) and 13-hydroperoxy-octadecatrienoic acid (13-HPOTE) standards which were synthesized as described by Nogala-Kalucka et al. [22]
Spectroscopic Studies
The absorption spectra were measured in the range 250–500 nm with a UV–Vis SP 8001 spectrophotometer (Metertech Inc., Taipei, Taiwan) in a 1 cm × 1 cm quartz cuvette. The concentration of rapeseed lipoxygenase dissolved in 20 mM Tris–HCl buffer (pH 7.5) was 73 μg/ml and linoleic acid, 98 μM. To each sample, a volume of 2.03 ml of lipoxygenase–linoleic acid mixture, 10 μl of phenolic extract or 33 μl of phenolic acid extract was added.
Statistical Analysis
Results are presented as means ± standard deviation from three replicates of each experiment. A p value <0.05 was used to denote significant differences between mean values determined by the analysis of variance (ANOVA) with the assistance of Statistica 7.0 (StatSoft, Inc., Tulsa, OK) software.
Results and discussion
Phenolic Content and DPPH Radicals Scavenging Ability
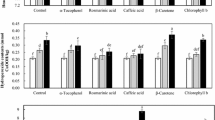
Total phenolic contents in rapeseed and rapeseed extracts and the DPPH radicals scavenging ability are presented in Table 1. The total phenolic compounds in tested methanol extracts was determined by the Folin–Ciocalteu method. The total phenolics contents in PR45DO3 and Aviso varied statistically and ranged from 1,485 mg/100 g (variety PR45DO3) to 1,691 mg/100 g (cv. Aviso). The content of reducing compounds was higher in Aviso and it correlated with antioxidant activity (ARP = 20.00). Free phenolic acids were isolated from crude methanol extracts by a solid phase extraction technique. Compounds that are able to reduce Folin–Ciocialteu reagent were determined again in the resulting purified extract containing the free phenolic acids. In relation to the total content of phenolic compounds, there was 1.42% of free phenolic acids in the extract from Aviso seeds and 1.92% of free phenolic acids in the extract from PR45DO3 seeds (statistically relevant variation was observed). Shahidi and Naczk [29] presented data where the total content of phenolic compounds in rapeseed meal was 1,080.2–1,807.0 mg/100 g. According to Cai and Arntfield [28] the content of total phenolic compounds in rapeseed meal averaged from 17.2 to 22.9 mg/g, depending on temperature, time of extraction or concentration of the solvent used. Amarowicz et al. [23] were studying the antioxidant properties of tannin extract from the husk of rapeseed and determined the content of total phenolic compounds to be from 128 to 296 mg/g in of extract in terms of sinapic acid. The assay used for the total phenols content determination has several disadvantages including a low specificity for phenols, a lack of relevance to biological oxidative processes and interferences with other compounds. It measures the reducing capacity of phenols or other reducing agents present in samples that can react with the Folin-Ciocalteu reagent (e.g., nitric compounds, saccharides) [24].
The main phenolic acids found in Aviso and PR45DO3 were sinapic acid and its low molecular derivatives (Table 2). Total free phenolic acid content in both rapeseed varieties was about 76 μg/100 g d.m. Those results are compatible with numerous literature data [25–27]. The content of sinapic acid in Aviso and PR45DO3 was 23.5 and 24.3 μg/100 g d.m., respectively. Cai and Arntfield [28] examined the content of sinapic acid in rapeseed meal using extraction with 70–100% methanol. The level of sinapic acid found was in the range from 0.34 mg/g d.m. (100% methanol, 75 °C, 20 min) to 0.40 mg/g d. m. (70% methanol, 75 °C, 20 min). The results mentioned above are in accordance with findings of Kozłowska et al. [25, 26] (41.3–51.6 mg/100 g d.m.). Shahidi and Naczk [29] report the content of sinapic acid in rapeseed in the range 27.6–67.7 mg/100 g d.m. The concentration of sinapic acid derivatives in the Aviso and PR45DO3 was 44.57 and 43.81 mg/100 g d.m., respectively and it was twice as high as the concentration of sinapic acid (Table 2).
Linoleic Acid Peroxidation in the Presence of Rapeseed Lipoxygenase and Phenolic Extracts
In order to check out lipoxygenase activity of purified proteins, linoleic acid hydroperoxides concentration measurement were carried out. In the presence of lipoxygenase from Aviso (LOXA) and PR45DO3 (LOXP), linoleic acid undergoes peroxidation and linoleic acid hydroperoxides are formed. After 3 min of incubation, the concentration of total hydroperoxides in the sample reached 16.2% and 15.0% of their initial value in the case of LOXA and LOXP, respectively (Figs. 1 and 2). The main hydroperoxide produced in the presence both LOXA and LOXP was 13-hydroperoxy-octadecadienoic acid (13-HPODE t–t) (Fig. 3). LOXA and LOXP were enzymatically active in pH 7.5, what is characteristic for canola lipoxygenases [18]. 13-positional isomers are products typical for many plant lipoxygenases [30], however soybean lipoxygenases, which generates mainly 13-derivatives possess optimal activity in an alkaline environment (pH 9.0), [31, 32]. When Aviso phenolic extract (PEA), (20–100 μl) was added to the samples containing linoleic acid and LOXA a prooxidant effect was observed (Fig. 1). After 3 min of incubation no significant differences between samples with and without PEA were observed. In the case of free PAA, a distinct antioxidant effect was observed when 330 μl of the PAA was added. In the third minute of the experiment the concentration of the hydroperoxides in the above mentioned sample amounted to 75% of the value obtained for a blank sample (Fig. 1). In the system containing LOXP a weak prooxidant effect was observed when 20 μl of PR45DO3 rapeseed phenolics extract (PEP) was added to the sample. A clear antioxidant effect was seen at 330 μl additive of the PAP and the concentration of total hydroperoxides yielded 51% of the concentration observed for the control sample. As we can see prooxidant properties were observed rather in those extracts containing a relatively high concentration of miscellaneous phenolic compounds and characterized by high ability to scavenge DPPH free radicals (see Table 1). Antioxidant properties were recorded in those phenolic acid extracts which contained only 1.42–1.92% of phenolics present in the raw phenolic extracts and their free radicals scavenging activity was more than 10 times weaker than those observed for total phenolics (Table 1). It suggests that besides free radical scavenging other mechanisms are also involved in the antioxidant/prooxidant activity of those phenolic compounds.
Lipoxygenase–Phenolic Compounds Interactions—Spectroscopic Studies
In order to check interactions between enzymatically active proteins and phenolic compounds UV–Vis spectra were obtained. The spectra of PEA and PEP extracts in the presence of LOXA and LOXP are presented in Figs. 4 and 5. As a result of incubation of polyphenols extracts with proteins, quinones formation (absorption band with a maximum at 323 nm) were observed. Enzymatic formation of quinoid products from polyphenols have been observed by other authors. According to Chedea et al. [15] polyphenol oxidation products with absorption maxima at 324.5 nm occur as a result of incubation of polyphenols from grape seeds with lipoxygenase [15]. Formation of o-quinones from catechins (absorption at 335 nm) and from quercetin in the presence of lipoxygenase and hydrogen peroxide (335 nm) have been observed by other authors [33, 34]. In the case of PEA and PEP extracts an increase in absorbance at 283 nm was also noticed (Figs. 4 and 5). This band can be attributed to oxidized polyphenols, what is in accordance with the conclusion of Chedea et al. [15] who observed the formation of oxidized polyphenolic compounds after incubation of catechins and polyphenols from grape in the presence of lipoxygenase in a primary leukocyte culture. The discovered products of rapeseed polyphenol oxidation may be the reason of the prooxidant effect of PEA and PEP. This conclusion seems to be confirmed by a stronger prooxidant effect of PEA (see Figs. 1 and 2) what is in accordance with a higher increase in polyphenol oxidation products concentration in the case of PEA in comparison to PEP (see Fig. 4 and 5). The spectra of phenolic acid extracts (PAA and PAP) with proteins A and P are presented in Figs. 6 and 7. As we can see in Fig. 7, incubation of PAP with LOXP resulted in a decrease in the absorption band with a maximum of about 340 nm. Appearance of this band comes from the oxidation of Fe2+ to Fe3+ in the lipoxygenase molecule in the presence of a substrate (linoleic acid), resulting in enzyme activation [14]. Decreasing of this band indicates the reduction of ferric to ferrous ions, what prevents the activation of lipoxygenase and inhibits its enzymatic activity. Such phenomena were not observed when a LOXA and PAA mixture was investigated (Fig. 6). The comparison of the highest hydroperoxide formation inhibition level of PAA (about 25% after 3 min of incubation; Fig 1) and PAP (about 49%, Fig. 2) points that inhibition of rapeseed proteins lipoxygenase activity by phenolic acids plays an important role in the antioxidant activity of those compounds. The composition of phenolic acids and the sum of all phenolic acids were similar in rapeseed Aviso and PR45DO3 (see Table 2). It was noted that slight differences between individual phenolic acids occur, but the sum of phenolic acids which are p-hydroxybenzoic acid derivatives as well as the sum of phenolic acids which are cinnamic acid derivatives in the investigated rapeseeds were not statistically different. Therefore we can assume that different influences of PAA and PAP on the LOXA and LOXP activity is due to differences in proteins structure. This assumption may be supported by the fact that a slight reduction of ferric to ferrous ions in the LOXP was observed also in the presence of PEP (see Fig. 5). The decrease of absorbance value at about 280 nm in the mixture of LOXP and PAP was also observed. Such a decrease in absorbance was observed only in the samples in which reduction of lypoxygenase ferric to ferrous ions was observed. That is why we assume that it come from changing of the phenolic compounds absorption coefficient as a result of protein–phenolic acid interactions. In the samples containing LOXA and PAA no spectral evidence for inhibition of lipoxygenase activity by phenolic acids was noticed. However after 75 min of incubation we observed the formation of oxidized phenolic acids (282 nm) and quinone/phenolic acid dimmers (shoulder 325–450 nm). Absorption band in this range coming from phenolic acid quinone and phenolics dimmers were confirmed by other authors [15, 35].
Conclusion
The proteins isolated from rapeseed Aviso and PR45DO3 showed lipoxygenase activity. Prooxidant properties of phenolic compounds in the presence of lipoxygenase and linoleic acid were observed rather in the case of extracts containing relatively high concentration of miscellaneous polyphenols. Antioxidant properties were recorded in the case of phenolic acid extracts, which contained only 1.4–1.9% of phenolics present in raw phenolic extracts. Results shown suggest that the prooxidant effect of phenolic compounds comes from quinone and oxidized polyphenols formation. The observed antioxidant activity of phenolic acids extracts is probably due to their ability to scavenge free radicals formed from linoleic acid. However, the reduction of lipoxygenase ferric to ferrous ions, which prevents the activation of enzyme and inhibits its activity, was also observed.
References
Axelrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenase from soybeans. Methods Enzymol 91:441–451
Galliar T, Phillips DR (1971) Lipoxygenase from potato tubers: partial purification and properties of an enzyme that specifically oxygenates the 9-position of linoleic acid. Biochem J 124:431–438
Shahidi F, Naczk M (2004) Phenolics in food and nutraceuticals. CRC Press, Boca Raton
Tys J, Piekarski W, Jackowska I, Kaczor A, Zając G, Starobrat P (2003) Technological and economic conditions of production of biofuel from rapeseed. Acta Agroph 99:35–85
Tys J, Sujak A, Bogdan A (2002) Changes to the composition of colorants caused by the temperature of drying rapeseed. Intern Agroph 16:307–312
Hofius D, Sonnewald U (2003) Vitamin E biosynthesis: biochemistry meets cell biology. Trends Plant Sci 8:6–8
Zadernowski R, Kozlowska H (1983) Phenolic acids in soybean and rapeseed flours. Lebens-Wissen Technol 16:110–114
Kozłowska H, Naczk M, Shahidi F, Zadernowski R (1990) Phenolic acids and tannins in rapeseed and canola. In: Shahidi F (ed) Canola and rapeseed production, chemistry, nutrition and processing technology. Van Nostrand Reinhold, USA, pp 193–210
Lacki K, Duvnjak Z (1998) Decrease of phenolic content in Canola meal using a polyphenol oxidase preparation from Trametes versicolor: effect of meal saccharification. Biotechnol Tech 12:31–34
Zukalova H, Vaak J (1999) New horizons for an old crop. www. regional.org.au/au/gcird
Thiyam U, Kuhlmann A, Stöckmann H, Schwarz K (2004) Prospects of rapeseed oil by-products with respect to antioxidative potential. C R Chimie 7:611–616
Amarowicz R, Shahidi F (1994) Chromatographic separation of glucopyranosyl sinapate from canola meal. JAOCS 71:551–552
Zadernowski R, Nowak-Polakowska H, Amarowicz R (1998) Phenolic compounds of rapeseed as factors protecting lipids against hydrolytic and oxidative changes. Oilseed Crops 19:563–572
Mahesha HG, Singh SA, Appu Rao AG (2007) Inhibition of lipoxygenase by soy isoflavones: Evidence of isoflavones as redox inhibitors. Arch Biochem Bioph 461:176–185
Chedea VA, Braicu C, Socaciu C (2010) Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem 121:132–139
Siger A, Nogala-Kałucka M, Lampart-Szczapa E (2008) The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J Food Lipids 15:137–149
Galati G, Sabzevari O, Wilson JX, O’Brien PJ (2002) Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicol 177:91–104
Khalyfa A, Kermasha S, Alli I (1990) Partial purification and characterization of lypoxygenase of canola seed (Brassica napus var. Westar). J Agric Food Chem 11:2003–2008
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Lampart-Szczapa E, Siger A, Trojanowska K, Nogala-Kałucka M, Małecka M, Pachołek B (2003) Chemical composition and antibacterial activities of lupin seeds extracts. Nahrung/Food 47:286–290
Banni S, Day BD, Evans RW, Corongiu FP, Lombardi B (1995) Detection of conjugated diene isomers of linoleic acid in liver lipids of rats fed a choline-devoid diet indicates that the diet does not cause lipoperoxidation. Nutrit Biochem 6:281–289
Nogala-Kałucka M, Kupczyk B, Polewski K, Siger A, Dwiecki K (2007) Influence of native antioxidants on the formation of fatty acid hydroperoxides in model systems. Eur J Lipids Sci Technol 109:1028–1037
Amarowicz R, Naczk M, Shahidi F (2000) Antioxidant activity of crude tannins of canola and rapeseed hulls. JAOCS 77:957–961
Moore J, Yu L (2008) Methods for antioxidant capacity estimation of wheat and wheat-based food products. In: Yu L (ed) Wheat antioxidant. Wiley, Hoboken, pp 118–172
Kozłowska H, Rotkiewicz DA, Zadernowski R (1983) Phenolic acids in rapeseed and mustard. JAOCS 60:1119–1123
Kozłowska H, Zadernowski R, Sosulski FW (1983) Phenolic acids in oilseed flours. Nahrung/Food 27:449–453
Siger A, Nogala-Kałucka M, Lampart-Szczapa E, Hoffmann A (2004) Phenolic compound contents in new rape varieties. Oilseeds Plant 25:263–274
Cai R, Arntfield SD (2001) A rapid high-performance liquid chromatographic method for the determination of sinapine and sinapic acid in canola seed and meal. JAOCS 78:903–910
Shahidi F, Naczk M (1992) An overview of the phenolics of canola and rapeseed: chemical, sensory and nutritional significance. JAOCS 69:917–924
Allen CL, Lancaster JE, Robinson DS (1999) Lipoxygenase activity in seeds from New Zealand native plants. New Zealand J Bot 37:737–745
Márczy JS, Simon ML, Mózsik L, Szajáni B (1995) Comparative study on the lipoxygenase activities of soybean cultivars. J Agric Food Chem 43:313–315
Kumar V, Rani A, Tindwani C, Jain M (2003) Lipoxygenase isozymes and trypsin inhibitor activities in soybean influenced by growing location. Food Chem 83:79–83
Dangles O, Fargeix G, Dufour C (2000) Antioxidant properties of anthocyans and tannins, a mechanistic investigation with catechin and the 3′,4′,7-trihydroxyflavylium ion. J Chem Soc–Perkin Tran 2:1653–1663
Pinto MC, Macias P (2005) Oxidation of dietary polyphenolics by hydroperoxidase activity of lipoxygenase. J Agric Food Chem 53:9225–9230
Munoz J, Garcia-Molina F, Varon R, Rodriguez-Lopez JN, Garcia-Ruiz PA, Garcia-Canovas F, Tudela J (2007) Kinetic characterization of the oxidation of chlorogenic acid by polyphenol oxidase and peroxidase. Characteristics of the o-quinone. J Agric Food Chem 55:920–928
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dwiecki, K., Siger, A., Czubiński, J. et al. The Interactions Between Rapeseed Lipoxygenase and Native Polyphenolic Compounds in a Model System. J Am Oil Chem Soc 89, 379–387 (2012). https://doi.org/10.1007/s11746-011-1923-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1923-8