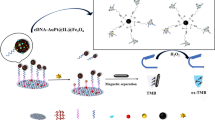
Abstract
The authors describe a rapid enzyme-free colorimetric assay for food- and water-borne pathogens by using the multi-functional MnO2-doped magnetic ferrite nanoparticles (MCDs-MnO2 NPs). These nanoparticles not only can recognize, absorb, and separate the analyte from the matrix efficiently but also can directly catalyze the oxidation of TMB into a blue colored product without H2O2. In the presence of target, the nanoparticles preferentially bind to the target surface and the interaction between nanoparticles and TMB can be blocked, providing turn-off sensing of bacteria. By combining the superior capture efficiency of magnetic beads with the high catalytic activity of the enzyme mimic, the turn-off colorimetric assay can detect the gram-positive bacterium and the gram-negative bacterium by the naked eye at a low detection limit (102 cfu mL−1) with a wide line arrange from 10 to 106 cfu mL−1. With the advantage of simplicity, sensitivity, and specificity, this proposed approach showed promise in rapid instrumental and on-site visual detection of bacteria for determination of water contamination.

Graphical abstract




Similar content being viewed by others
References
Law JW, Ab Mutalib NS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol. 2015;5:770–88.
Cho IH, Ku S. Current technical approaches for the early detection of foodborne pathogens: challenges and opportunities. Int J Mol Sci. 2017;18(10):2078–100.
Díaz-Amaya S, Zhao M, Lin LK, Ostos C, Allebach JP, Chiu GT. Inkjet printed nanopatterned aptamer-based sensors for improved optical detection of foodborne pathogens. Small. 2019;15(24):1805342.
Borgohain R, Baruah S. Development and testing of ZnO nanorods based biosensor on model Gram-positive and gram-negative bacteria. IEEE Sensors J. 2017;17(9):2649–53.
Florentin A, Lizon J, Asensio E, Forin J, Rivier A. Water and surface microbiologic quality of point-of-use water filters: a comparative study. Am J Infect Control. 2016;44(9):1061–2.
Adkins JA, Boehle K, Friend C, Chamberlain B, Bisha B, Henry CS. Colorimetric and electrochemical bacteria detection using printed paper-and transparency-based analytic devices. Anal Chem. 2017;89(6):3613–21.
Altintas Z, Akgun M, Kokturk G, Uludag Y. A fully automated microfluidic-based electrochemical sensor for real-time bacteria detection. Biosens Bioelectron. 2018;100:541–8.
Lazcka O, Del Campo FJ, Muñoz FX. Pathogen detection: a perspective of traditional methods and biosensors. Biosens Bioelectron. 2007;22(7):1205–17.
Wang H, Zhou Y, Jiang X, Sun B, Zhu Y, Wang H. Simultaneous capture, detection, and inactivation of bacteria as enabled by a surface-enhanced Raman scattering multifunctional chip. Angew Chem Int Ed Engl. 2015;54(17):5132–6.
Wang W, Liu L, Song S, Tang L, Kuang H, Xu C. A highly sensitive ELISA and immunochromatographic strip for the detection of Salmonella typhimurium in milk samples. Sensors (Basel). 2015;15(3):5281–92.
Postollec F, Falentin H, Pavan S, Combrisson J, Sohier D. Recent advances in quantitative PCR (qPCR) applications in food microbiology. Food Microbiol. 2011;28(5):848–61.
Liu P, Han L, Wang F, Li X, Petrenko VA, Liu A. Sensitive colorimetric immunoassay of Vibrio parahaemolyticus based on specific nonapeptide probe screening from a phage display library conjugated with MnO2 nanosheets with peroxidase-like activity. Nanoscale. 2018;10(6):2825–33.
Zhang X, Mao X, Li S, Dong W, Huang Y. Tuning the oxidase mimics activity of manganese oxides via control of their growth conditions for highly sensitive detection of glutathione. Sensor Actuators B Chem. 2018;258:80–7.
Liu J, Meng L, Fei Z, Dyson PJ, Zhang L. On the origin of the synergy between the Pt nanoparticles and MnO2 nanosheets in Wonton-like 3D nanozyme oxidase mimics. Biosens Bioelectron. 2018;121:159–65.
Ouyang H, Lu Q, Wang W, Song Y, Tu X, Zhu C. Dual-readout immunochromatographic assay by utilizing MnO2 nanoflowers as the unique colorimetric/chemiluminescent probe. Anal Chem. 2018;90(8):5147–52.
Gong J, Han X, Zhu X, Guan Z. Layer-by-layer assembled multilayer films of exfoliated layered double hydroxide and carboxymethyl-β-cyclodextrin for selective capacitive sensing of acephatemet. Biosens Bioelectron. 2014;61:379–85.
Stobiecka M, Chalupa A. Modulation of plasmon-enhanced resonance energy transfer to gold nanoparticles by protein survivin channeled-shell gating. J Phys Chem B. 2015;119(41):13227–35.
Bhaisare ML, Gedda G, Khan MS, Wu HF. Fluorimetric detection of pathogenic bacteria using magnetic carbon dots. Anal Chim Acta. 2016;920:63–71.
Xiong Y, Chen S, Ye F, Su L, Zhang C, Shen S. Preparation of magnetic core–shell nanoflower Fe3O4@MnO2 as reusable oxidase mimetics for colorimetric detection of phenol. Anal Methods. 2015;7(4):1300–6.
Wang Q, Pang H, Dong Y, Chi Y, Fu F. Colorimetric determination of glutathione by using a nanohybrid composed of manganese dioxide and carbon dots. Mikrochim Acta. 2018;185(6):291.
Abdelhamid HN, Wu HF. Probing the interactions of chitosan capped CdS quantum dots with pathogenic bacteria and their biosensing application. J Mater Chem B. 2013;1(44):6094–106.
Funding
This study received financial support from the National Natural Science Foundation of China (Grant Nos. 81602895, 81602894, and 81872668), the Education Department of Jilin Province (JJKH20180240KJ), Health Commission of Jilin Province (2018Q033), and Norman Bethune Health Science Center of Jilin University (2018A05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 864 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Zhao, C., Zhao, W. et al. Multi-functional MnO2-doped Fe3O4 nanoparticles as an artificial enzyme for the colorimetric detection of bacteria. Anal Bioanal Chem 412, 3135–3140 (2020). https://doi.org/10.1007/s00216-020-02563-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02563-2