Abstract
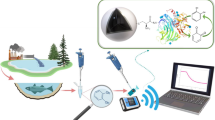
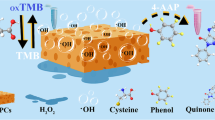
A stainless steel mesh (SSM) with the feature of flexibility was employed as the colorimetric biosensor substrate, and aptamer was bond onto the surface of the SSM. Through the cross-linking of ionic liquids (ILs), AuPt nanoparticles were deposited onto the surface of Fe3O4 material to obtain a magnetic nanozyme with high peroxidase catalytic activity and rapid color change. Through the competing interaction of OTA and cDNA with aptamer, AuPt@IL@Fe3O4 signal probe was separated to catalyze the 3,3′,5,5′-tetramethylbenzidine/hydrogen peroxide (TMB/H2O2) system to observe the color by bare eye and record the absorbance at 652 nm using a UV-spectrophotometer. Through the study of the catalytic properties on the basis of the Michaelis equation, AuPt@IL@Fe3O4 nanozyme presented a Vmax of 3.85 × 10−8 M s−1 and Km of 0.01 mM. Under the optimized conditions, the linear range of the colorimetric biosensor towards OTA was 5–100 ng mL−1, and the detection limit was 0.078 ng mL–1. This biosensor was applied to beer and corn samples with recoveries of 70.4–102.6% and 93.3–104.7%, respectively. Results showed that this sensor is a portable, rapid, economical, sensitive visual sensing platform towards mycotoxin in real samples.
Graphical abstract








Similar content being viewed by others
References
Chen X, Gao D, Sun F, Li Z, Wang Y, Qiu C, He K, Wang J (2022) Nanomaterial-based aptamer biosensors for ochratoxin A detection: a review. Anal Bioanal Chem 414(9):2953–2969. https://doi.org/10.1007/s00216-022-03960-5
Zhu W, Li L, Zhou Z, Yang X, Hao N, Guo Y, Wang K (2020) A colorimetric biosensor for simultaneous ochratoxin A and aflatoxins B1 detection in agricultural products. Food Chem 319:126544. https://doi.org/10.1016/j.foodchem.2020.126544
Suo Z, Liang X, Jin H, He B, Wei M (2021) A signal-enhancement fluorescent aptasensor based on the stable dual cross DNA nanostructure for simultaneous detection of OTA and AFB1. Anal Bioanal Chem 413(30):7587–7595. https://doi.org/10.1007/s00216-021-03723-8
Huang R, Xiong LL, Chai HH, Fu JJ, Lu Z, Yu L (2019) Sensitive colorimetric detection of ochratoxin A by a dual-functional Au/Fe3O4 nanohybrid-based aptasensor. RSC Adv 9(66):38590–38596. https://doi.org/10.1039/c9ra07899a
Lin X, Li C, He C, Zhou Y, Wang Z, Duan N, Wu S (2021) Upconversion nanoparticles assembled with gold nanourchins as luminescence and surface-enhanced raman scattering dual-mode aptasensors for detection of Ochratoxin A. ACS Appl Nano Mater 4(8):8231–8240. https://doi.org/10.1021/acsanm.1c01421
Jiang L, Han Y, Li Y, Li Z, Zhang S, Zhu X, Liu Z, Chen Y, Fernandez-Garcia S, Tang Y, Chen X (2022) Split-type assay for wide-range sensitive sensing of ochratoxin A with praseodymia nanorods. Colloids Surf A Physicochem Eng Asp 652:129804. https://doi.org/10.1016/j.colsurfa.2022.129804
Zhang ZH, Zhu M, Wang F, Meng H, Feng L (2021) Electrochemical/visual dual-readout aptasensor for ochratoxin A detection integrated into a miniaturized paper-based analytical device. Biosens Bioelectron 180:113146. https://doi.org/10.1016/j.bios.2021.113146
Tian F, Zhou J, Jiao B, He Y (2019) A nanozyme-based cascade colorimetric aptasensor for amplified detection of ochratoxin A. Nanoscale 11(19):9547–9555. https://doi.org/10.1039/c9nr02872b
Setlem K, Mondal B, Shylaja R, Parida M (2020) Dual aptamer-DNAzyme based colorimetric assay for the detection of AFB1 from food and environmental samples. Anal Biochem 608:113874. https://doi.org/10.1016/j.ab.2020.113874
Lv X, Frahat Foda M, He J, Zhou J, Cai J (2023) Robust and facile label-free colorimetric aptasensor for ochratoxin A detection using aptamer-enhanced oxidase-like activity of MnO2 nanoflowers. Food Chem 401:134144. https://doi.org/10.1016/j.foodchem.2022.134144
Rotariu L, Lagarde F, Jaffrezic-Renault N, Bala C (2016) Electrochemical biosensors for fast detection of food contaminants – trends and perspective. TrAC Trends Anal Chem 79:80–87. https://doi.org/10.1016/j.trac.2015.12.017
Chen Y, Jiao L, Yan H, Xu W, Wu Y, Wang H, Gu W, Zhu C (2020) Hierarchically porous S/N codoped carbon nanozymes with enhanced peroxidase-like activity for total antioxidant capacity biosensing. Anal Chem 92(19):13518–13524. https://doi.org/10.1021/acs.analchem.0c02982
Fang Z, Zhou X, Wang X, Shi X (2023) Development of a 3-plex droplet digital PCR for identification and absolute quantification of salmonella and its two important serovars in various food samples. Food Control 145:109465. https://doi.org/10.1016/j.foodcont.2022.109465
Long L, Liu J, Lu K, Zhang T, Xie Y, Ji Y, Wu X (2018) Highly sensitive and robust peroxidase-like activity of Au-Pt core/shell nanorod-antigen conjugates for measles virus diagnosis. J Nanobiotechnology 16(1):46. https://doi.org/10.1186/s12951-018-0371-0
Yuan P, Deng Z, Qiu P, Yin Z, Bai Y, Su Z, He J (2023) Bimetallic metal−organic framework nanorods with peroxidase mimicking activity for selective colorimetric detection of salmonella typhimurium in food. Food Control 144:109357. https://doi.org/10.1016/j.foodcont.2022.109357
Zhu D, Liu B, Wei G (2021) Two-dimensional material-based colorimetric biosensors: a review. Biosensors 11(8):259. https://doi.org/10.3390/bios11080259
Wang X, Dong S, Gai P, Duan R, Li F (2016) Highly sensitive homogeneous electrochemical aptasensor for antibiotic residues detection based on dual recycling amplification strategy. Biosens Bioelectron 82:49–54. https://doi.org/10.1016/j.bios.2016.03.055
Hosseini M, Khabbaz H, Dadmehr M, Ganjali MR, Mohamadnejad J (2015) Aptamer-based colorimetric and chemiluminescence detection of Aflatoxin B1 in foods samples. Acta Chim Slov 62:721–728. https://doi.org/10.17344/acsi.2015.1358
Tian F, Zhou J, Fu R, Cui Y, Zhao Q, Jiao B, He Y (2020) Multicolor colorimetric detection of ochratoxin A via structure-switching aptamer and enzyme-induced metallization of gold nanorods. Food Chem 320:126607. https://doi.org/10.1016/j.foodchem.2020.126607
Ali MH, Elsherbiny ME, Emara M (2019) Updates on aptamer research. Int J Mol Sci 20(10):2511. https://doi.org/10.3390/ijms20102511
Xu X, Wang L, Zou X, Wu S, Pan J, Li X, Niu X (2019) Highly sensitive colorimetric detection of arsenite based on reassembly-induced oxidase-mimicking activity inhibition of dithiothreitol-capped Pd nanozyme. Sensors Actuators B Chem 298:126876. https://doi.org/10.1016/j.snb.2019.126876
Gai P, Pu L, Wang C, Zhu D, Li F (2023) CeO2@NC nanozyme with robust dephosphorylation ability of phosphotriester: a simple colorimetric assay for rapid and selective detection of paraoxon. Biosens Bioelectron 220:114841. https://doi.org/10.1016/j.bios.2022.114841
Wan Y, Zhao J, Deng X, Chen J, Xi F, Wang X (2021) Colorimetric and fluorescent dual-modality sensing platform based on fluorescent nanozyme. Front Chem 9:774486. https://doi.org/10.3389/fchem.2021.774486
Wu L, Zhou S, Wang G, Yun Y, Liu G, Zhang W (2021) Nanozyme applications: a glimpse of insight in food safety. Front Bioeng Biotechnol 9:727886. https://doi.org/10.3389/fbioe.2021.727886
Chang J, Li H, Hou T, Duan W, Li F (2018) Paper-based fluorescent sensor via aggregation induced emission fluorogen for facile and sensitive visual detection of hydrogen peroxide and glucose. Biosens Bioelectron 104:152–157. https://doi.org/10.1016/j.bios.2018.01.007
Xu Y, Zhang Y, Song X, Liu H (2019) Facile hydrothermal synthesis of Fe3O4 nanoparticle and effect of crystallinity on performances for supercapacitor. Funct Mater Lett 12(02):1950019. https://doi.org/10.1142/s179360471950019x
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583. https://doi.org/10.1038/nnano.2007.260
Vojoudi H, Ghasemi JB, Hajihosseinloo A, Bastan B, Badiei A (2021) One-pot synthesis of hematite-alumina hollow sphere composite by ultrasonic spray pyrolysis technique with high adsorption capacity toward PAHs. Adv Powder Technol 32(4):1060–1069. https://doi.org/10.1016/j.apt.2021.02.015
Wang Z, Ma H, Wang F, Li M, Zhang L, Xu X (2016) Controllable synthesis and magnetic properties of monodisperse Fe3O4 nanoparticles. Chin Phys Lett 33(10):107501. https://doi.org/10.1088/0256-307x/33/10/107501
Feng X, Fu H, Bai Z, Li P, Song X, Hu X (2022) Colorimetric detection of glucose by a hybrid nanomaterial based on amplified peroxidase-like activity of ferrosoferric oxide modified with gold–platinum heterodimer. New J Chem 46(1):239–249. https://doi.org/10.1039/d1nj04491e
Xu C, Yang Z, Yan H, Li J, Yu H, Zhang L, Shu J (2022) Synergistic dual conversion reactions assisting Pb-S electrochemistry for energy storage. Proc Natl Acad Sci U S A 119(12):e2118675119. https://doi.org/10.1073/pnas.2118675119
Yu L, Chang J, Zhuang X, Li H, Hou T, Li F (2022) Two-dimensional cobalt-doped Ti3C2 MXene nanozyme-mediated homogeneous electrochemical strategy for pesticides assay based on in situ generation of electroactive substances. Anal Chem 94(8):3669–3676. https://doi.org/10.1021/acs.analchem.1c05300
Guo D, Huang Q, Zhao R, Guo W, Fan K, Han Z, Zhao Z, Nie D (2023) MIL-101(Cr)@Fe3O4 nanocomposites as magnetic solid-phase extraction adsorbent for the determination of multiple mycotoxins in agricultural products by ultra-high-performance liquid chromatography tandem mass spectrometry. Food Control 146:109540. https://doi.org/10.1016/j.foodcont.2022.109540
Luo S, Liu Y, Rao H, Wang Y, Wang X (2017) Fluorescence and magnetic nanocomposite Fe3O4@SiO2@Au MNPs as peroxidase mimetics for glucose detection. Anal Biochem 538:26–33. https://doi.org/10.1016/j.ab.2017.09.006
Vojoudi H, Bastan B, Ghasemi JB, Badiei A (2019) An ultrasensitive fluorescence sensor for determination of trace levels of copper in blood samples. Anal Bioanal Chem 411(21):5593–5603. https://doi.org/10.1007/s00216-019-01940-w
Modheji M, Emadi H, Vojoudi H (2020) Efficient pre-concentration of As(III) in food samples using guanidine-modified magnetic mesoporous silica. J Porous Mater 27(4):971–978. https://doi.org/10.1007/s10934-020-00873-5
Zhu Q, Maeno S, Sasaki M, Miyamoto T, Fukushima M (2015) Monopersulfate oxidation of 2,4,6-tribromophenol using an iron(III)-tetrakis(p-sulfonatephenyl)porphyrin catalyst supported on an ionic liquid functionalized Fe3O4 coated with silica. Appl Catal B Environ 163:459–466. https://doi.org/10.1016/j.apcatb.2014.08.035
Funding
This work was supported by the National Natural Science Foundation of China (No. 31901766), financial support from the Talents of High-Level Scientific Research Foundation, Qingdao Agricultural University (No.1119014), postgraduate innovation gram of Qingdao Agricultural University (No. QNYCX22096), Rural Revitalization Science and Technology Innovation Plan Project of Shandong Province (2022TZXD0031), and Science & Technology Specific Projects in Agricultural High-tech Industrial Demonstration Area of the Yellow River Delta (2022SZX26).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 652 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Q., Xin, S., Tan, X. et al. Ionic liquids functionalized Fe3O4-based colorimetric biosensor for rapid determination of ochratoxin A. Microchim Acta 190, 364 (2023). https://doi.org/10.1007/s00604-023-05943-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05943-4