Abstract
Histamine exerts cAMP-dependent positive inotropic effects (PIE) and positive chronotropic effects (PCE) on isolated left and right atria, respectively, of transgenic mice which overexpress the human H2-receptor in the heart (=H2-TG). To determine whether these effects are antagonized by phosphodiesterases (PDEs), contractile studies were done in isolated left and right atrial preparations of H2-TG. The contractile effects of histamine were tested in the additional presence of the PDE-inhibitorserythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA, 1 μM, PDE2-inhibitor) or cilostamide (1 μM, PDE3-inhibitor), rolipram (10 μM, a PDE4-inhibitor), and their combinations. Cilostamide (1 μM) and EHNA (1 μM), rolipram (1 μM), and EHNA (1 μM) and the combination of rolipram (0.1 μM) and cilostamide (1 μM) each increased the potency of histamine to elevate the force of contraction (FOC) in H2-TG. Cilostamide (1 μM) and rolipram (10 μM) alone increased and EHNA (1 μM) decreased alone, and their combination increased the potency of histamine to increase the FOC in H2-TG indicating that PDE3 and PDE4 regulate the inotropic effects of histamine in H2-TG. The PDE inhibitors (EHNA, cilostamide, rolipram) alone did not alter the potency of histamine to increase the heart beat in H2-TG whereas a combination of rolipram, cilostamide, and EHNA, or of rolipram and EHNA increased the potency of histamine to act on the beating rate. In summary, the data suggest that the PCE of histamine in H2-TG atrium involves PDE 2 and 4 activities, whereas the PIE of histamine are diminished by activity of PDE 3 and 4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effects of histamine are mediated by receptors known as H1, H2, H3, and H4-histamine receptors (Jutel et al., 2009). The cardiac H2-histamine-receptors mediate the PIE of histamine in isolated human cardiac preparations (Levi et al., 1981). These PIE in the human heart were accompanied by and hence probably mediated by an increase in cAMP (atrial preparations: Sanders et al., 1996). We failed to notice a PIE or PCE of histamine in WT atrium of mice (Gergs et al., 2019b, 2020) and therefore, we generated mice overexpressing human H2 receptors and in those mice detected an increase in force of contraction and the beating rate by histamine (Gergs et al., 2019b, 2020, this manuscript).
Histamine acts in many ways similar to serotonin in the heart. Indeed, in the human failing ventricle, treatment of trabeculae carneae with 3-isobutyl-1-methylxanthine (IBMX), an unspecific phosphodiesterase (PDE) inhibitor, uncovered a hidden effect: under these conditions, 5-HT elicited a PIE via 5-HT4-receptor receptors (Brattelid et al., 2004). The same group reiterated some of these effects in a rat model. Rat ventricular preparations express both 5-HT2A and 5-HT4 receptors on the mRNA level but signal only through 5-HT2A(Brattelid et al., 2012, Läer et al., 1998) under normal conditions. However, with aortic banding to induce hypertrophy in rats, the authors noticed that a PIE of 5-HT via 5-HT4 receptors could be detected.
The degradation of cAMP is solely brought about by cAMP-specific PDEs. PDEs degrade cAMP to inactive 5´-AMP and modulate cAMP-dependent signaling. Thus, PDEs in the heart will diminish or even terminate the responses to receptors, such as β-adrenoceptors, 5-HT4 receptors, or H2-receptors, that lead to cAMP production.
Among these PDEs are PDE1, a Ca2+/calmodulin-activated PDE; PDE2, a cGMP-activated PDE; PDE3, a cGMP-inhibited PDE; and PDE4, a cGMP-independent, cAMP-specific PDE. PDEs can be classified as cAMP selective (PDE4, 7, 8) or cGMP selective (PDE5, 6, 9), or hydrolyzing both cAMP and cGMP (PDE1, 2, 3, 10, 11) (Bobin et al., 2016, Conti and Beavo, 2007). PDE2, PDE3, and PDE4 provide the major PDE activity for cAMP in the heart. To dissect the role of PDEs for inotropy and chronotropy in the heart, drugs that are specific inhibitors have been used in many previous studies. This study used the same inhibitors as previous investigators at the same concentrations to facilitate interpretation of the present work. Specifically, this study used erythro-9-(2-Hydroxy-3-nonyl)adenine hydrochloride (EHNA), as a PDE2-inhibitor; cilostamide, as a PDE3-inhibitor; and rolipram, as a PDE4-inhibitor(for specificity, see Maurice et al., 2014, Table 1 in Gergs et al., 2019b).
The present study was started to determine whether the inotropic and chronotropic effects of histamine in our mouse model, that mimics the cardiac effects of histamine on PIE and PCE by H2-histamine receptors (H2-TG), are sensitive to typical PDE inhibitors in the atrium of transgenic mice engineered to express a functional H2-receptor on atrial and ventricular cardiomyocytes (Gergs et al., 2019a). This study tested whether or not PDE 2, 3, or 4 alone or in concert are important for H2-receptor-mediated effects in the contracting left atrium (electrically driven) and in the sinus node of the spontaneously beating right atrium of H2-TG.
Materials and methods
Transgenic mice
Transgenic mice (H2-TG) with cardiac myocyte-specific overexpression of the human H2- histamine receptor and their littermate control mice (WT) were generated as described by Gergs et al. (2019a). Heart-specific expression was achieved via the α-myosin heavy-chain promoter. The animals were in average about 150 days of age (75 female and 65 male animals). Contraction experiments were performed on left and right atrial preparations as previously described by Gergs et al. (2013). All mice were housed under conditions of optimum light, temperature, and humidity with food and water provided ad libitum. Animals were handled and maintained according to approved protocols of the animal welfare committee of the University of Halle-Wittenberg, Halle, Germany, (approval reference number 42502-02-691 MLU).
Contractile studies in mice
In brief, right or left atrial preparations were isolated and mounted in organ baths as described by Gergs et al. (2013, 2017, 2019b) and Neumann et al. (2003). The organ baths’ bathing solution contained 119.8 mM NaCl 5.4 mM KCl, 1.8 mM CaCl2, 1.05 mM MgCl2, 0.42 mM NaH2PO4, 22.6 mM NaHCO3, 0.05 mM Na2EDTA, 0.28 mM ascorbic acid, and 5.05 mM glucose. It was continuously gassed with 95% O2 and 5% CO2 and maintained at 37 °C and pH 7.4, as described by Neumann et al. (2003) and Kirchhefer et al. (2004). Preparations were attached to a bipolar stimulating electrode and suspended individually in 10-ml glass tissue chambers for recording isometric contractions. Force of contraction (FOC) was measured with inductive force transducers connected to a chart recorder. Time parameters (time to peak tension =t1 or time of tension relaxation =t2 in milli seconds, ms) and minimum and maximum of the first derivate versus time of force of contraction with respect to time of single contractions (dF/dTmin and dF/dTmax in milli Newton per milli second, mN/ms) were evaluated from digitized recordings. Each muscle was stretched to the length of maximal FOC. The left atrial preparations from mice were electrically stimulated at 1 Hz with rectangular pulses of 5 ms duration; the voltage was ~ 10–20% greater than the threshold of initiation of contraction. Spontaneously beating right atrial preparations from mice were used to study any chronotropic effects.
Banding
Aortic constriction of mice was performed as described previously (Müller et al., 2009).
Data analysis
Data shown are means ± standard error of the mean. Statistical significance was estimated by analysis of variance followed by Bonferroni´s t test. A p value of less than 0.05 was considered significant. Experimental data for agonist-induced positive inotropic and chronotropic effects were analyzed by fitting sigmoidal curves to the experimental data with GraphPad Prism 5.0. All other statistical analyses were performed as indicated in the figures and tables. Statistical evaluation was conducted with GraphPad Prism 5.0 (GraphPad Software, San Diego, California, USA).
Drugs and materials
(-)-Isoprenaline (+)-bitartrate, serotonin (5-HT) hydrochloride, and histamine were purchased from Sigma-Aldrich (Deisenhofen, Germany). Rolipram, EHNA, and cilostamide were obtained from Tocris (Wiesbaden, Germany). All other chemicals were of the highest purity grade commercially available. Deionized water was used throughout the experiments. Stock solutions were freshly prepared daily.
Results
To facilitate comparisons, all EC-50 values have been put together in Table 3.
Effects without PDE inhibitors
As seen in the original recording (Fig. 1c, top) and summarized in Fig. 2 a (open circles), histamine exerted a PIE in isolated electrically stimulated (1 Hz) left atrial preparations of H2-TG that was concentration dependent (-log EC50 = − 7.07 ± 0.04 M (n = 63), EC50: effective concentration in M for 50 % effect) but histamine was completely lacking a PIE in WT (original tracing in Fig. 1c and summarized in Fig. 2a (open squares) in agreement with our published data (Gergs et al. 2019b). At the same time, t2 (=time of relaxation) amounted to 33.6 ± 0.74 ms and (n = 65) in WT and 32.3 ± 0.81 ms (n = 67) in H2-TG under basal conditions, and by 1 μM histamine remained unaltered at 33.39 ± 0.74 ms in WT and shortened to 27.71 ± 0.37 ms (n = 67) in H2-TG (compare Table 1, bottom). In a similar way, 1 μM histamine shortened t1 (time to peak tension) in H2-TG, but not in WT (see Table 1, top)
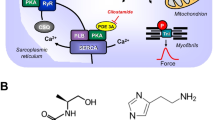
a Scheme of the putative subcellular localization of phosphodiesterase (PDEs) 2-4. Their inhibitors used in this study are also depicted. Ca2+ enters the mammalian heart cell via the L-type Ca2+ channel (LTCC). This process can be enhanced by histamine via a cascade starting with the H2-receptor, the occupation of which by histamine elevates the activity of adenylyl cyclase (AC) in the sarcolemma via stimulatory G-proteins (Gs), elevates subsequent production of cAMP and, thereby, activates cAMP-dependent protein kinase (PKA). PKA increases cardiac force generation and relaxation by increasing the phosphorylation state (P) of LTCC, phospholamban (PLB), and other regulatory proteins. Trigger Ca2+ initiates release of Ca2+ from the sarcoplasmic reticulum via ryanodine receptors (RYR) into the cytosol, where Ca2+ activates myofilaments and leads to increased inotropy. In diastole, Ca2+ is taken up into the sarcoplasmic reticulum via a sarcoplasmic reticulum Ca2+ ATPase (SERCA), whose activity is higher when the phosphorylation state of PLB is elevated by PKA. PDE2-4 have been localized to the LTCC, PDE3 and PDE4 to PLB, PDE4 to G-protein coupled receptors, RYR, and the myofilaments, and PDE2 to the cytosol. Not shown here: PDE3 and PDE4 are also localized to the nucleus. EHNA preferentially inhibits PDE2, cilostamide PDE3, rolipram PDE4. b Schematic description of the order of drug application to isolated atria, including incubation times and concentrations of drugs. c Original recordings of the force of contraction (FOC) in left atria from transgenic mice that overexpress the H2-receptor (H2-TG) (top), in the presence of 0.1 μM rolipram,cilostamide and EHNA (H2-TG, middle tracing) and littermate control (WT) (bottom). Concentration response curves for histamine are shown. Note the fast inset of action and the concentration dependence of the positive inotropic effect (PIE) in H2-TG (top), the leftward shift of the curve (middle) and the lack of any PIE in WT (bottom). d Original recordings of the FOC in right atria from H2-TG (top) and WT (bottom). High temporal resolution is shown (see time bar) to make two single contractions visible. Note that the time between beats is shortened (positive chronotropic effect = PCE) of 1 μM histamine in H2-TG and the lack of a PIE in WT
a Effect of histamine alone on the FOC of isolated electrically driven (1 Hz) left atrium of H2-TG (closed circles) or WT (squares). FOC before histamine is indicated as Ctr (control). First effect in the figure means that all left atrial contraction experiments with the initial concentration response curve to histamine (please compare Fig. 1b for the experimental design) in all groups #1-#8 are summarized here. Numbers in brackets mean number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr or WT. b Effects of histamine alone in isolated spontaneously beating right atrium of H2-TG (closed circles) or WT (squares). FOC before histamine addition is indicated as Ctr. Beating rate in beats per minute (bpm) is depicted on the ordinate. First effect in the figure means that all right atrial contraction experiments with the initial concentration response curve to histamine (please compare Fig. 1b for the experimental design) in all groups #1-#8 are summarized here. Numbers in brackets indicate number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr or WT, x indicates a significantly higher (p < 0.05) basal beating rate (in the absence of external histamine) vs WT. c Effect of histamine alone (open squares) or in the additional presence of 1 μM erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA) on the FOC of isolated electrically driven (1 Hz) left atrium of H2-TG (closed circles) or WT (squares). FOC before application of EHNA (=Ctr 1) and after EHNA (=Ctr 2) are indicated. d Effect of histamine alone (open squares) alone or in the additional presence of 1 μM EHNA in isolated spontaneously beating right atrium of H2-TG (closed circles) or WT (squares). FOC before (Ctr 1) and after EHNA (Ctr 2) are indicated. Beating rate in beats per minute (bpm) is depicted on the ordinate. Abscissae indicate negative decadic logarithm of increasing histamine concentrations. Numbers in brackets indicate number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr 2 or WT
Likewise, dF/dTmax (=maximum rate of tension development) amounted to 302.6 ± 14.31 mN/ms (n = 67) in WT and 271.9 ± 11.80 mN/ms (n = 68) in H2-TG under basal conditions, and were augmented in H2-TG but not WT by 1 μM histamine namely amounted to 267.6 ± 12.82 mN/ms (n = 67) in WT and 647.9 ± 18.96 mN/ms (n = 68) in H2-TG(compare also Table 2 top). Similarly, 1 μM histamine shortened dF/dTmin (=minimum rate of tension development= rate of relaxation) in H2-TG but not WT (see Table 2, bottom).
Similarly, histamine exerted a concentration-dependent positive chronotropic effect (=PCE, -log EC50 = 7.13 ± 0.06, n = 43) in right atrial preparations of H2-TG (original recording, Fig. 1d, top, summarized in Fig. 2b, (open circles)) but histamine was devoid of any PCE in WT (original tracing: Fig. 1d bottom, Fig. 2b (squares)). In Fig. 2 a and Fig. 2 b, the contractile initial (=first) effects of histamine in all subgroups (Fig. 1b: #1-#8) of studied atria were summarized, which explains the larger number of experiments and small error bars in these figures.
Effects of PDE inhibitors alone
EHNA (1 μM), a PDE2 inhibitor, alone, was ineffective to increase the FOC in left atrial preparations (Ctr 2 = contractile value after complete stabilization of the effect of a PDE-inhibitor vs. Ctr 1 = pre-drug contractile value) of WT (squares) and H2-TG (closed circles) in Fig. 2 c. The effects in the absence of any PDE inhibitor are additionally plotted (open circles, Fig. 2c). Likewise, 1 μM EHNA alone did not increase the beating rate in right atrial preparations from WT or H2-TG (Ctr 2 vs. Ctr 1) in Fig. 2 d. EHNA shifted the PIE of histamine in H2-TG slightly, but significantly, to the right (circles, -log EC50 Ctr = 7.11 ± 0.11; -log EC50 EHNA = 7.01 ± 0.14; p < 0.05).
Cilostamide (1 μM), a PDE 3 inhibitor, alone was ineffective to increase the FOC in left atrial preparations from WT (squares) or H2-TG (closed circles) (Ctr 2 vs. Ctr 1 in Fig. 3a). Similarly, cilostamide did not increase the beating rate in right atrial preparations from WT or H2-TG (Ctr 2 vs. Ctr 1), as seen in Fig. 3 b. However, cilostamide shifted the PIE of histamine to lower concentrations of histamine in H2-TG (-log EC50 Ctr = 7.03 ± 0.08; -logEC50 cilostamide: 7.55 ± 0.06; p < 0.05) (Fig. 3a). In contrast, cilostamide failed to alter the EC50 of histamine with respect to the PCE (-log EC50 Ctr = 7.49 ± 0.11; versus - logEC50 cilostamide: 7.88 ± 0.09).
a Effect of histamine alone (open circles) or in the additional presence of 1 μM cilostamide on the force of contraction (FOC) of isolated electrically driven (1 Hz) left atrium of H2-receptor overexpressing mice (H2-TG, closed circles) or littermate mice (WT, squares). FOC before (Ctr 1) and after cilostamide (Ctr 2) are indicated. FOC is given on the ordinate in milli Newton (mN). b Effect of histamine alone (open circles) or in the additional presence of 1 μM cilostamide in isolated spontaneously beating right atrium of H2-TG (closed circles) or WT (squares). Beating rate in beats per minute (bpm) is depicted on the ordinate. Beating rates before (Ctr 1) and after cilostamide (Ctr 2) are indicated. Abscissae indicate negative decadic logarithm of increasing histamine concentrations Numbers in brackets indicate number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr or WT
Rolipram (10 μM), a PDE 4 inhibitor, increased the FOC in left atrial preparations in WT and in H2-TG (Ctr 2 vs. Ctr 1 in Fig. 4a). At the same time T2 and dF/dTmax amounted to 27.13 ± 1.43 ms and 195.3 ± 74.2 mN/ms in WT and 30.66 ± 2.51 ms and 201.7 ± 23.3 in H2-TG under basal, and amounted in the presence of 10 μM rolipram to 26.12 ± 0.89 ms and 250.8 ± 63.5 mN/ms in WT and 28.94 ± 2.67 ms and 258.4 ± 35.2 mN/ms in H2-TG (Tables 1 and 2). Rolipram (10 μM) shifted the potency of histamine to increase the FOC (Fig. 4a) (FOC: (-log EC50 Ctr = 6.97 ± 0.09; - logEC50 rolipram: 7.57 ± 0.06) in H2TG and in the presence of 1 μM histamine T2 amounted to 26.10 ± 0.74 ms and dF/dTmax was measured as 707.4 ± 26.8 mN/ms. Rolipram did not alter the beating rate in WT and H2-TG (Fig. 4b). Rolipram did not alter the PCE of histamine in H2-TG, but it also did not reveal a PIE of histamine in WT (Fig. 4) or a PCE of histamine in WT (Fig. 4a, b).
a Effect of histamine alone (open circles) or in the additional presence of 10 μM rolipram on the force of contraction (FOC) of isolated electrically driven (1 Hz) left atrium of histamine2-receptor overexpressing mice (H2-TG, closed circles) or littermate mice (WT, squares). FOC before (Ctr 1) and after rolipram (Ctr 2) is indicated. FOC is given on the ordinate in milli Newton from pre-drug values (mN). b Effect of histamine alone (open circles) or in the additional presence of 10 μM rolipram in isolated spontaneously beating right atrium of H2-TG atrium (closed circles) or WT (squares). Beating rates before (Ctr 1) and after cilostamide (Ctr 2) are indicated. Abscissae indicate negative decadic logarithm of increasing histamine concentrations Numbers in brackets indicate number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr or WT
Effects of combinations of PDE inhibitors alone
The combination of rolipram (0.1 μM) and cilostamide (1 μM) elevated the FOC (Fig. 5) in H2-TG (closed circles) and in WT (closed squares, Ctr 2 vs. Ctr 1, Fig. 5a). At the same time T2 and dF/dTmax amounted to 25.81 ± 1.94 ms and 209.8 ± 38.8 mN/ms in WT and 23.29 ± 1.05 ms and 195.4 ± 27.3 mN/ms in H2-TG under basal conditions, and remained unchanged in the presence of 0.1 μM rolipram and 1 μM cilostamide at 25.81 ± 1.40 ms and 513.6 ± 120.9 mN/ms in WT and 23.62 ± 0.92 ms or 445.1 ± 91.2 mN/ms in TG (compare Tables 1 and 2). The combination of 0.1 μM rolipram and 1 μM cilostamide increased the beating rate (Fig. 5b) in H2-TG (open circles) and WT (closed squares). Rolipram (0.1 μM) and cilostamide (1 μM) shifted the concentration response curve of histamine on FOC in H2-TG to lower concentrations (-log EC50 Ctr = 7.31 ± 0.06; - logEC50 rolipram+cilostamide: 9.06 ± 0.13 (closed circles, Fig. 5a) and 1 μM histamine prolonged t2 to 26.06 ± 0.61 ms and elevated dF/dTmax to 704.6 ± 47.7 mN/ms.
a Effect of histamine alone (open circles) in the additional presence of rolipram (0.1 μM) and 1 μM cilostamide on the force of contraction (FOC) of isolated electrically driven (1 Hz) left atrium of histamine2-receptor overexpressing mice (H2-TG, circles) or littermate mice (WT, squares). FOC before (Ctr 1) and after rolipram (left tick) and cilostamide (right tick, Ctr 2) are indicated. FOC is given on the ordinate in milli Newton (mN). b Effect of histamine alone (open circles) in the presence of 0.1 μM rolipram and 1 μM cilostamide in isolated spontaneously beating right atrium of H2-TG (circles) or WT (squares). Beating rate in beats per minute (bpm) is depicted before (Ctr 1) and after rolipram and cilostamide (Ctr 2) are indicated on the ordinate. c Effect of histamine alone (open circles) or in the presence of 1 μM rolipram and 1 μM cilostamide on the FOC of isolated electrically driven (1 Hz) left atrium of H2-TG (circles) or WT (squares). FOC before (Ctr 1) and after rolipram and cilostamide (Ctr 2) are indicated. FOC is given on the ordinate in milli Newton (mN). d Effect of histamine alone (open circles) or in the presence of 1 μM rolipram and 1 μM cilostamide in isolated spontaneously beating right atrium of H2-TG or WT. Beating rate before (Ctr 1) and after rolipram and cilostamide (Ctr 2) are indicated. Abscissae indicate negative decadic logarithm of increasing histamine concentrations. Numbers in brackets indicate number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr or WT. e Effect of histamine alone (open circles) or in the presence of 10 μM rolipram and 1 μM cilostamide on the FOC of isolated electrically driven (1 Hz) left atrium of H2-TG (circles) or WT (squares). FOC before (Ctr 1) and after rolipram and cilostamide (Ctr 2) are indicated. FOC is given on the ordinate in milli Newton (mN). f Effect of histamine alone (open circles) or in the presence of 10 μM rolipram and 1 μM cilostamide in isolated spontaneously beating right atrium of H2-TG or WT. Beating rate before (Ctr 1) and after rolipram and cilostamide (Ctr 2) are indicated. Abscissae indicate negative decadic logarithm of increasing histamine concentrations. Numbers in brackets indicate number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr or WT
In WT, the FOC was not further increased by histamine under these conditions (squares, Fig. 5a). In the right atrium, histamine was ineffective to increase further the already augmented beating rate in H2-TG (closed circles) or WT (closed squares, Fig. 5b). Using higher concentrations of rolipram, 1 μM rolipram or 10 μM rolipram increased FOC to such high levels that additionally applied histamine was ineffective to raise FOC further in WT as well as H2-TG (Fig. 5c, e). At the same time, dF/dTmax (but not t2) were changed in the presence of 1 μM rolipram or 10 μM rolipram and 1 μM cilostamide (see Tables 1 and 2). Likewise, 1 μM rolipram or 10 μM rolipram in the presence of cilostamide elevated beating rate in WT and H2-TG (Fig. 5d, f). Additionally, applied histamine failed to increase beating rate further (Fig. 5d, f).
The combination of cilostamide (1 μM) and EHNA (1 μM) did not elevate the FOC (Ctr2 vs. Ctr1 in Fig. 6a) but the beating rate in H2-TG (Ctr2 vs. Ctr1 in Fig. 6b). In WT, the FOC and beating rate remained unaltered (Fig. 6a). In the right atrium, under these conditions, histamine did not increase the beating rate in H2-TG at lower concentrations (log EC50 Ctr = 6.98 ± 0.27; - logEC50 cilostamide+EHNA: 7.4 ± 0.24 (closed circles, Fig. 6b).
a Effect of histamine alone (open circles) or in the additional presence of 1 μM cilostamide and 1 μM EHNA on the force of contraction (FOC) of isolated electrically driven (1 Hz) left atrium of histamine-receptor overexpressing mice (H2-TG, closed circles) or littermate mice (WT, squares). FOC before (Ctr 1) and after cilostamide (left tick in Ctr 2) and after EHNA (right tick in Ctr 2) are indicated. FOC is given on the ordinate in milli Newton (mN). b Effect of histamine alone (open circles) or in the presence of 1 μM cilostamide and 1 μM EHNA in isolated spontaneously beating right atrium of H2-TG (H2-TG, closed circles) or WT (squares). Beating rate in beats per minute (bpm) is depicted before (Ctr1) and after cilostamide and EHNA (Ctr2) is indicated on the ordinate
Effects of combinations of PDE in the presence of histamine
The combination of rolipram and EHNA elevated the FOC (Ctr 2 vs. Ctr 1 in Fig. 7a) in WT but hardly in H2-TG (Ctr2 vs. Ctr1). At the same time, T2 and dF/dTmax amounted to 29.10±2.28 ms and 185.0 ± 38.3 mN/ms in WT and 21.47 ± 1.09ms and 115.6 ± 11.6 mN/ms in H2-TG under basal, and in the presence of 1 μM rolipram and 1 μM EHNA amounted to 27.06 ± 2.30 ms and 252.9 ± 42.2 mN/ms in WT and 20.87 ± 1.02 ms or 147.5 ± 28.3 mN/ms in H2-TG (compare Tables 1 and 2). The combination increased the beating rate in H2-TG and WT (Fig. 7b). Under these conditions, the potency of histamine to increase the FOC in H2-TG was elevated (log EC50 Ctr = 6.89 ± 0.21; - logEC50 rolipram+EHNA: 7.43 ± 0.18). Also, the potency of histamine to increase the PCE in H2TG was elevated (log EC50 Ctr = 7.09 ± 0.22; - logEC50 rolipram+EHNA: 7.51 ± 0.31).
a Effect of histamine alone (open circles) or in the additional presence 1 μM rolipram and 1 μM EHNA on the FOC of isolated electrically driven (1 Hz) left atrium of H2-TG (closed circles) or WT (squares). FOC before (Ctr1) and after rolipram (left tick), EHNA, (right hand tick in Ctr2) are indicated. FOC is given on the ordinate in milli Newton (mN). b Effect of histamine alone (open circles) in the presence of 1 μM rolipram and 1 μM EHNA in isolated spontaneously beating right atrium of H2-TG (closed circles) or WT (squares). Beating rate before (Ctr 1) and after rolipram (left tick) and EHNA (right tick, Ctr 2) is indicated on the ordinate. Abscissae indicate negative decadic logarithm of increasing histamine concentrations. Numbers in brackets indicate number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr or WT
Finally, to inhibit PDE2, 3, and 4 together, we applied a combination of rolipram (0.1 μM), cilostamide (1 μM), and EHNA (1 μM). Under these conditions, the FOC (Fig. 8a) was greatly augmented in WT (Ctr2 vs. Ctr1) and H2-TG). At the same time, T2 and dF/dTmax amounted to 24.78 ± 2.02 ms and 169.3 ± 24.0 mN/ms in WT and 23.70 ± 1.50 ms and 127.5 ± 36.2 mN/ms in H2-TG under basal, and were changed in the presence of 0.1 μM rolipram, 1 μM EHNA, and 1 μM cilostamide to 22.66 ± 1.79 ms and 391.5 ± 106.0 mN/ms in WT and 21.66 ± 1.03 ms and 212.4 ± 64.7 mN/ms(compare Tables 1 and 2). Additionally, applied histamine at 1 μM prolonged T2 to 24.81 ± 1.06 ms elevated dF/dTmax 602.5 ± 52.1 mN/ms(Fig. 8a, Tables 1 and 2).
a Effect of histamine alone (open circles) or in the additional presence of 0.1 μM rolipram, 1 μM cilostamide and 1 μM EHNA on the FOC of isolated electrically driven (1 Hz) left atrium of H2-TG (closed circles) or WT (squares). FOC before (Ctr 1) and after cilostamide (middle tick), EHNA (right tick), and rolipram, (left hand tick in Ctr 2) are indicated. FOC is given on the ordinate in milli Newton (mN). b Effect of histamine alone (open circles) or in the additional presence of 0.1 μM rolipram 1 μM cilostamide and 1 μM EHNA in isolated spontaneously beating right atrium of H2-TG (closed circles) or WT (squares). Beating rate before (Ctr 1) and after rolipram, cilostamide and EHNA (Ctr 2) is indicated on the ordinate. Abscissae indicate negative decadic logarithm of increasing histamine concentrations. Numbers in brackets indicate number of experiments. ★, +indicate first significant difference (p < 0.05) vs. Ctr or WT
Moreover, the beating rate was increased by triple inhibition in H2-TG (Ctr2 vs. Ctr1 in Fig. 8b) and in WT (Fig. 8b). The response to histamine on FOC and beating rate under triple PDE inhibitions were shifted to the left in H2-TG (Fig. 8a, b) (FOC: -log EC50 Ctr = 6.89 ± 0.13; - logEC50 rolipram+cilostamide+EHNA: 8.36 ± 0.17) (beating rate: -log EC50 Ctr = 6.72 ± 0.15; - logEC50 rolipram+cilostamide+EHNA: 7.49 ± 0.11, Table 3). No hidden PIE or hidden PCE to histamine was unveiled under these conditions in WT (Fig. 8a, b).
Moreover, we wanted to know whether a hidden PIE to H2 could be detected under pathological conditions in WT animals. Hence, we studied the effect of histamine on FOC in left atria from mice with aortic banding and sham operated animals. In mice with aortic banding, the relative hearts weight was higher than hearts from sham operated animals (data not shown). However, histamine (or serotonin studied for comparison) failed to increase FOC (Fig. 9). However, in the same atria, the PIE of the β-adrenoceptor agonist isoprenaline was blunted in atria from banded animals compared to sham operated animals (Fig. 9).
Data on banded mouse hearts Comparison of effects in atrium of H2-TG and WT. Relative heart weight was increased upon banding (TAC vs Sham, bar diagram, numbers in bars indicated numbers of mice studied). Histamine failed to increase force of contraction in electrically driven left atrial preparations from Sham- and TAC- mice (upper right inset, ordinate gives developed for in milli Newton (mN). Hypertrophy led to attenuated positive inotropic effects of the β-adrenoceptor agonist isoprenaline. Ordinates in mN (lower left inset) or in percent of pre-drug value (lower right inset). * indicated p < 0.05 versus pre-drug values (Ctr). Numbers in brackets indicate the numbers of mice studied
Discussion
To facilitate the discussion of the present results, we have summarized the main findings in Table 4.
We studied an interaction of PDE inhibition on the PIE and PCE of histamine in H2-TG mice, because they mimic the PIE of histamine in the human heart by using the same human H2-histamine receptor (Fig. 10). In the future, we would like to extend the present study by investigating the role of PDE isoenzymes for the PIE of histamine in electrically stimulated human atrial strips. We had worked before on the effect of serotonin in the human and mouse heart and noticed similarities to histamine. For instance, we noticed that in WT mice heart, serotonin (5-HT) did not have any effect on force of contraction or beating rate (Läer et al., 1998). This prompted us to generate transgenic mice that overexpress human 5-HT4 receptor (which is the receptor subtype responsible for inotropic effect in human atrium and ventricle (e.g., Gergs et al., 2009): we detected an increase in force of contraction by serotonin in 5-HT4-TG but no effect in WT (Gergs et al., 2010). For comparison, we started to study histamine, because like serotonin, it can exert an increase in force of contraction in human atrium (via H2-histamine receptors) and like serotonin acts in the human heart via an increase in cAMP concentrations and phospholamban phosphorylation. Surprisingly, and like for serotonin, we failed to notice a PIE of histamine in WT atrium (Gergs et al., 2019b, 2020) and therefore, as for serotonin, we generated mice overexpressing human H2 receptors and in those mice detected an increase in force of contraction by histamine (Gergs et al., 2019b, 2020, this manuscript). We had reported before that serotonin exerts a positive inotropic effect in rats by action on 5-HT2 receptors (Läer et al., 1998). Colleagues in Norway confirmed this work and extended it by showing that in failing rat hearts (due to aortic banding or experimentally induced myocardial infarction) in addition to a 5-HT2-receptor mediated PIE also a 5-HT4-receptor mediated PIE of serotonin occurred accompanied by increased expression of the mRNA for the 5-HT4-receptor (reviewed in: Levy et al., 2008). The positive inotropic effect of 5-HT4 receptor stimulation by serotonin in failing rat hearts was potentiated by the PDE inhibitors we used in the current study (Afzal et al., 2008). Based on these rat data, the Oslo group also studied human samples (Afzal et al., 2008) and found that in electrically stimulated human left ventricular trabeculae (from explanted failing heart), PDE 3 and 4 are most relevant for the PIE of serotonin. We used their choice of PDE inhibitors in the past to address the question of the role of specific PDEs in mouse hearts overexpressing 5-HT4 receptors (Neumann et al., 2019) and noticed that for the serotonin induced PIE in 5-HT4-TG, mainly PDE4 is involved (Neumann et al., 2019). Here, the question arose: can we use the H2-TG mouse to find whether and which PDEs decrease the PIE and PCE of histamine in the present mouse model of the human H2 receptor? Is the histamine action on force and frequency in H2-TG reduced by same endogenous PDEs as that of serotonin in 5-HT4 mice or man? Does the effect of histamine on the sinus node of H2-TG involve PDE (serotonin acts in sinus node of 5-HT4 TG and probably also in living humans without participation of PDEs: Neumann et al., 2019). Using a similar approach, others have studied which PDEs attenuate the PCE of noradrenaline in WT mouse atrium (Galindo-Tovar et al., 2016).
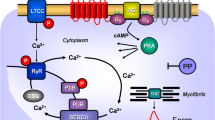
Scheme. Hypothetical simplified explanation which PDE might be mainly involved in the basal (absence of histamine) contractile state in mouse left atrium (Force) or mouse right atrium (beating rate) in H2-TG or WT. Likewise the situation if one constructs a concentration response curves to histamine (Histamine CRC) is depicted in the lower half of the figure. This indicated that force under basal conditions can be elevated either by inhibition of PDE IV alone or the joint inhibition of PDEIII and PDE IV In contrast, in the presence of histamine the effect of histamine is potentiated (shifted sinistrally) by either inhibition of PDE III or PDE IV. The interpretation for beating rate is different: only joint inhibition of PDEs will potentiate the chronotropic effect of histamine. In contrast, inhibition of PDE II reduces the potency of histamine to increase FOC in H2-TG, conceivably by elevating cAMP and activating PKA in a compartment that can where PDE III can be phosphorylated and activated by PKA
Right atria, role of PDE isoenzymes in basal conditions
How the heartbeat is generated, maintained, decreased, and increased in the mammalian heart is controversial. Clearly PDEs play a role (for review: Vinogradova and Lakatta, 2009, Kaumann, 2011). In new born pigs, PDE3 and PDE4 controlled the heartbeat while in adult pigs, the PDE4 was mainly important (Galindo-Tovar et al., 2009). Another study detected no PDE2-inhibitor effect on the beating rate in isolated right atrial preparations from WT mice with the compound 100 nM Bay 60-7550 but 1 μM Bay 60-7550 increased the beating rate (Galindo-Tovar et al., 2016). Like the current study, Galindo-Tovar et al. (2016) noted that 10 μM EHNA alone did not increase FOC in isolated WT mouse right atrial preparations. Cilostamide and rolipram, administered together, caused marked increases in sinoatrial rate in isolated right atrium from WT mouse (Galindo-Tovar and Kaumann, 2008).
In our previous work (Gergs et al., 2019b), and in the present work, an increase in the beating rate in WT or H2-TG right atria by cilostamide was not detected. Rolipram (1 μM) tended to transiently increase sinoatrial rate in isolated mouse right atrium (Galindo-Tovar and Kaumann, 2008). Inhibition by rolipram and cilostamide together has exerted a substantial PCE (mouse: Galindo-Tovar and Kaumann, 2008, Galindo-Tovar et al., 2009). This is in line with the present findings (Fig. 5b, d, and f).
The addition of cilostamide to a combination of EHNA and rolipram increased the beating rate probably maximally. In healthy humans, the infusion of milrinone, a clinically approved PDE3 inhibitor, led to a sustained increase in the heart rate, suggesting that at least PDE3 is involved in the regulation of the heartbeat in man (Chiu et al., 1999). A new PDE4 inhibitor, namely roflumilast, was reported to have no effect on heart rate in humans (Rabe et al., 2005).
Right atria, role of PDE isoenzymes with histamine
As in the present study and our earlier study (Gergs et al., 2019b), others noted that the combination of 300 nM cilostamide with 1 μM rolipram increased the beating rate in right atrial preparations of WT mice to a maximum, making it impossible to stimulate the beating rate further with isoprenaline (Galindo-Tovar and Kaumann, 2008).
Galindo-Tovar et al. (2009) argued that the lack of potentiation of the chronotropic effects of rolipram, cilostamide, and concurrent rolipram and cilostamide means that the cAMP pool governing H2-receptor-mediated sinoatrial tachycardia is protected from PDE3 and PDE4 and represents a compartment distinct from the cAMP compartment in which both PDE3 and PDE4 reduce basal sinoatrial beating. They speculated that increases in maximum PCE of serotonin in the presence of rolipram and concurrent rolipram plus cilostamide could be due to additivity of the tachycardia caused by 5-HT and the PDE inhibitors (Galindo-Tovar et al., 2009).
Left atria, role of PDE isoenzymes under basal conditions
Rolipram but not cilostamide alone increased the FOC in isolated left atrial preparations from adult pigs. However, the combination of rolipram and cilostamide increased the FOC significantly (Galindo-Tovar et al., 2009). Cilostamide (300 nM) did not significantly increase left atrial contractility in WT mice (Galindo-Tovar and Kaumann 2008), which agrees with our previous (Gergs et al., 2019b) and the present study’s observations.
Left atria role of PDE isoenzymes with histamine
In ventricular preparations from pig, rat, and man, serotonin usually fails to induce a PIE. However, this PIE can be disclosed by preincubation with cilostamide alone (in rat and man) and is even higher in the combined presence of cilostamide and rolipram (rat and man: Afzal et al., 2008, pig: Galindo-Tovar et al., 2009). It is noteworthy that in WT mice, neither cilostamide, nor rolipram, nor EHNA, nor their combinations, unveiled a PIE to 5-HT(Gergs et al., 2019b) or to histamine (this study).
Limitations of the study
One micromolar of EHNA is likely insufficient to inhibit mouse cardiac PDE2 completely. The IC50 value in human heart for PDE 2 inhibition was reported by 0.8 μM (Podzuweit et al., 1995). Looking at Fig. 2 in Podzuweit et al. 1995, one can estimate that 1 μM EHNA can inhibit about 60 % of total PDE2. An advantage is that, at 1 μM, EHNA does not yet inhibit the activity of human cardiac PDE 3 and/or PDE 4 (Podzuweit et al., 1995). In constrast, Méry et al. (1995) found that 10 μM EHNA is needed to inhibit the PDE 2 in the heart sufficiently. However, Méry et al. (1995) studied PDE 2 activity from frog heart and frogs are not a mammalian species, in contrast to mice. Hence, we think that PDE inhibitor EHNA (namely 1 μM) was reasonable choice for the mouse heart in the present work. In subsequent studies, one might use the newer PDE 2 inhibitor Bay 60-7750 instead of EHNA. However, we wanted to facilitate direct comparison to our earlier work on 5-HT4 receptors stimulation and PDE2 where we also used 1 μM EHNA (Neumann et al., 2019).
Interestingly, PDE2 inhibition by EHNA can reduce the potency of histamine to increase FOC in left atrium of H2-TG (Fig. 3c). This might come about in an indirect way: PDE 3 also hydrolyses cGMP. Hence, if PDE 3 is inhibited, cGMP levels in the heart will rise (Maurice et al., 2014). The generated cGMP can allosterically stimulate PDE 2 activity (Martins et al., 1982; cartoon in Fig. 8b in Neumann et al., 2019). On the other hand, an increase in cAMP would also activate cAMP-dependent protein kinase. Activated cAMP-dependent protein kinase would phosphorylate and activate PDE 3 and PDE 4 (Smith et al., 1991; MacKenzie et al., 2002). Hence, one might envision the following steps: the PDE 2 inhibitor EHNA can increase cellular cAMP (in a certain subcellular compartment relevant for inotropy), this would activate cAMP-dependent protein kinase and subsequently PDE 3 and 4 are activated and might reduce cAMP levels, at least in that compartment that would normally increase force of contraction after H2-receptor stimulation in H2-TG. In this way, EHNA might reduce the potency of histamine. Moreover, we find that PDE4 is involved in several processes (Table 4). This might be due to the expression of different isoforms of PDE 4 in the heart. For instance, PDE 4 isoforms have been reported to have different subcellular locations in the heart or other tissue (review: Maurice et al., 2014, cartoon: Fig. 1, this work). On the one hand, PDE 4 activity can be increased by activation of cAMP-dependent protein kinases (Maurice et al., 2014). On the other hand, inhibition of PDE 4 would presumably increase cAMP levels. Moreover, an increase in cAMP leading to an increase in the activity of cAMP dependent protein kinase would phosphorylate and activate PDE 3. Thus, rolipram might also increase PDE 3 activity in this indirect way (Maurice et al., 2014). Hence, indirect effects of rolipram might contribute to its contractile effect in the present study, not only its direct effects on PDE 4. Another caveat is in order: even though some of the experiments for beating rate (Table 3, bottom) show no significant effects of PDE inhibitors on EC50-values, the number of experiments is quite low in some experimental groups and with a higher number of experiments significant effects of PDE inhibitors might become visible.
In summary, we show for the first time that tachycardia induced by histamine on the human H2 receptors is modulated by PDEs only in concert while PDE 3 or 4 alone are sufficient to attenuate the histamine-induced PIE in atrium from H2-TG, providing another example for functional compartments of PDE in the mammalian heart.
References
Afzal F, Andressen KW, Mørk HK, Aronsen JM, Sjaastad I, Dahl CP, Skomedal T, Levy FO, Osnes JB, Qvigstad E (2008)5-HT4-elicited positive inotropic response is mediated by cAMP and regulated by PDE3 in failing rat and human cardiac ventricles. Br J Pharmacol 155(7):1005–1014. https://doi.org/10.1038/bjp.2008.339
Bobin P, Belacel-Ouari M, Bedioune I, Zhang L, Leroy J, Leblais V, Fischmeister R, Vandecasteele G (2016) Cyclic nucleotide phosphodiesterases in heart and vessels: a therapeutic perspective. Arch Cardiovasc Dis 109(6-7):431–443. https://doi.org/10.1016/j.acvd.2016.02.004
Brattelid T, Kvingedal AM, Krobert KA, Andressen KW, Bach T, Hystad ME, Kaumann AJ, Levy FO (2004) Cloning, pharmacological characterization and tissue distribution of a novel 5-HT4 receptor splice variant, 5-HT4(i). Naunyn Schmiedeberg's Arch Pharmacol 369(6):616–628. https://doi.org/10.1007/s00210-004-0919-4
Brattelid T, Qvigstad E, Moltzau LR, Bekkevold SV, Sandnes DL, Birkeland JA, Skomedal T, Osnes JB, Sjaastad I, Levy FO (2012) The cardiac ventricular 5-HT4 receptor is functional in late foetal development and is reactivated in heart failure. PLoS One 7(9):e45489. https://doi.org/10.1371/journal.pone.0045489
Chiu YJ, Hu SH, Reid IA (1999) Inhibition of phosphodiesterase III with milrinone increases renin secretion in human subjects. J Pharmacol Exp Ther 290(1):16–19
Conti M, Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem.76:481–511. doi: https://doi.org/10.1146/annurev.biochem.76.060305.150444
Galindo-Tovar A, Kaumann AJ (2008)Phosphodiesterase-4 blunts inotropism and arrhythmias but not sinoatrial tachycardia of (-)-adrenaline mediated through mouse cardiac beta(1)-adrenoceptors. Br J Pharmacol 153(4):710–720. https://doi.org/10.1038/sj.bjp.0707631
Galindo-Tovar A, Vargas ML, Escudero E, Kaumann AJ (2009) Ontogenic changes of the control by phosphodiesterase-3 and -4 of 5-HT responses in porcine heart and relevance to human atrial 5-HT(4) receptors. Br J Pharmacol 156(2):237–249. https://doi.org/10.1111/j.1476-5381.2008.00023.x
Galindo-Tovar A, Vargas ML, Kaumann AJ (2016) Inhibitors of phosphodiesterases PDE2, PDE3, and PDE4 do not increase the sinoatrial tachycardia of noradrenaline and prostaglandin PGE1 in mice. Naunyn Schmiedeberg's Arch Pharmacol 389(2):177–186. https://doi.org/10.1007/s00210-015-1178-2
Gergs U, Neumann J, Simm A, Silber RE, Remmers FO, Läer S (2009) Phosphorylation of phospholamban and troponin I through 5-HT4-receptors in the isolated human atrium. Naunyn-Schmiedeberg´s Arch Pharmacol 379:349-359 doi: https://doi.org/10.1007/s00210-008-0371-y
Gergs U, Baumann M, Böckler A, Buchwalow IB, Ebelt H, Fabritz L, Günther S, Hauptmann S, Kirchhof P, Klöckner U, Pönicke K, Rückschloß U, Schmitz W, Schulz N, Werner F, Neumann J (2010) Cardiac overexpression of the human 5-HT4-receptor in mice. Am J Physiol Heart Circ Physiol 299:H788–H798. https://doi.org/10.1152/ajpheart.00691.2009
Gergs U, Böckler A, Ebelt H, Hauptmann S, Keller N, Otto V, Pönicke K, Schmitz W, Neumann J (2013) Human 5-HT4-receptor stimulation in atria of transgenic mice. Naunyn Schmiedeberg's Arch Pharmacol 386(5):357–367. https://doi.org/10.1007/s00210-013-0831-x
Gergs U, Fritsche J, Fabian S, Christ J, Neumann J (2017) Desensitization of the human 5-HT4 receptor in isolated atria of transgenic mice. Naunyn Schmiedeberg's Arch Pharmacol 390(10):987–996. https://doi.org/10.1007/s00210-017-1403-2
Gergs U, Trapp T, Bushnaq H, Simm A, Silber RE, Neumann J (2019a)Age-dependent protein expression of serine-/threonine-phosphatases and their inhibitors in the human cardiac atrium. Advances in Medicine, Volume 2019, Article ID 2675972, 9 pages, 2019. https://doi.org/10.1155/2019/2675972
Gergs U, Bernhardt G, Buchwalow IB, Edler H, Fröba J, Keller M, Kirchhefer U, Köhler F, Mißlinger N, Wache H, Neumann J (2019b) Initial characterization of transgenic mice overexpressing human histamine H2 receptors. J Pharmacol Exp Ther 369:129–141. https://doi.org/10.1124/jpet.118.255711
Gergs U, Kirchhefer U, Bergmann F, Künstler B, Mißlinger N, Au B, Mahnkopf M, Wache H, Neumann J (2020) Characterization of stressed transgenic mice overexpressing H2-histamine-receptors. J Pharmacol Exp Ther 374:479–488. https://doi.org/10.1124/jpet.120.000063
Jutel M, Akdis M, Akdis CA (2009) Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy 39(12):1786–1800. https://doi.org/10.1111/j.1365-2222.2009.03374.x
Kaumann AJ (2011) Phosphodiesterases reduce spontaneous sinoatrial beating but not the ‘fight or flight’ tachycardia elicited by agonists through Gs-protein-coupled receptors. Trends Pharmacol Sci 32(7):377–383. https://doi.org/10.1016/j.tips.2011.03.003
Kirchhefer U, Baba HA, Hanske G, Jones LR, Kirchhof P, Schmitz W, Neumann J (2004)Age-dependent biochemical and contractile properties in atrium of transgenic mice overexpressing junctin. Am J Physiol Heart Circ Physiol 287(5):H2216–H2225. https://doi.org/10.1152/ajpheart.00137.2004
Läer S, Remmers F, Stein B, Scholz H, Müller FU, Neumann J (1998) Receptor mechanisms involved in 5-HT induced inotropic action in the rat isolated atrium. Br J Pharmacol 123(6):1182–1188. https://doi.org/10.1038/sj.bjp.0701702
Levi R, Malm JR, Bowman FO, Rosen MR (1981) The arrhythmogenic actions of histamine on human atrial fibers. Circ Res 49(2):545–550. https://doi.org/10.1161/01.res.49.2.545
Levy FO, Qvigstad E, Krobert KA, Skomedal T, Osnes JB (2008) Effects of serotonin in failing cardiac ventricle: signalling mechanisms and potential therapeutic implications. Neuropharmacology 55(6):1066–1071. https://doi.org/10.1016/j.neuropharm.2008.07.010
Martins TJ, Mumby MC, Beavo JA (1982) Purification and characterization of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase from bovine tissues. J Biol Chem 257(4):1973-9)
Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC (2014) Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov 13(4):290–314. https://doi.org/10.1038/nrd4228
MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, Millen J, Beard MB, van Heeke G, Houslay MD (2002) Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1). Br J Pharmacol 136(3):421–433. https://doi.org/10.1038/sj.bjp.0704743
Méry PF, Pavoine C, Pecker F, Fischmeister R (1995) Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic GMP-stimulated phosphodiesterase in isolated cardiac myocytes. Mol Pharmacol 48(1):121–130
Müller P, Kazakov A, Jagoda P, Semenov A, Böhm M, Laufs U (2009) ACE inhibition promotes upregulation of endothelial progenitor cells and neoangiogenesis in cardiac pressure overload. Cardiovasc Res 83(1):106–114. https://doi.org/10.1093/cvr/cvp123
Neumann J, Boknik P, Matherne GP, Lankford A, Schmitz W (2003) Pertussis toxin sensitive and insensitive effects of adenosine and carbachol in murine atria overexpressing A1–adenosine receptors. Br J Pharmacol 138(1):209–217. https://doi.org/10.1038/sj.bjp.070501
Neumann J, Käufler B, Gergs U (2019) Which phosphodiesterase can decrease cardiac effects of 5-HT4 receptor activation in transgenic mice? Naunyn Schmiedeberg's Arch Pharmacol 392(8):991–1004. https://doi.org/10.1007/s00210-019-01653-y
Podzuweit T, Nennstiel P, Müller A (1995) Isozyme selective inhibition of cGMP-stimulated cyclic nucleotide phosphodiesterases by erythro-9-(2-hydroxy-3-nonyl) adenine. Cell Signal 7(7):733–738. https://doi.org/10.1016/0898-6568(95)00042-n
Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbröker D, Bethke TD (2005)Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 366(9485):563–571. https://doi.org/10.1016/S0140-6736(05)67100-0
Sanders L, Lynham JA, Kaumann AJ (1996) Chronic beta 1-adrenoceptor blockade sensitises the H1 and H2 receptor systems in human atrium: rôle of cyclic nucleotides. Naunyn Schmiedeberg's Arch Pharmacol 353(6):661–670. https://doi.org/10.1007/BF00167185
Smith CJ, Vasta V, Degerman E, Belfrage P, Manganiello VC (1991)Hormone-sensitive cyclic GMP-inhibited cyclic AMP phosphodiesterase in rat adipocytes. Regulation of insulin- and cAMP-dependent activation by phosphorylation. J Biol Chem 266(20):13385–13390
Vinogradova TM, Lakatta EG (2009) Regulation of basal and reserve cardiac pacemaker function by interactions of cAMP-mediated PKA-dependent Ca2+ cycling with surface membrane channels. J Mol Cell Cardiol 47(4):456–474. https://doi.org/10.1016/j.yjmcc.2009.06.014
Acknowledgements
The work contains parts of the medical doctoral thesis of Rafaela Voss. The technical assistance of S. Reber and P. Willmy is gratefully acknowledged. The data were generated in-house and do not come from a paper mill.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.N. designed the research; R.V., U.G., C.W., and U.L. performed or contributed to the research by providing animals or reagents; R.V. and U.G. analyzed the data; U.G., R.V., and J.N. wrote and revised the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neumann, J., Voss, R., Laufs, U. et al. Phosphodiesterases 2, 3 and 4 can decrease cardiac effects of H2-histamine-receptor activation in isolated atria of transgenic mice. Naunyn-Schmiedeberg's Arch Pharmacol 394, 1215–1229 (2021). https://doi.org/10.1007/s00210-021-02052-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02052-y