Abstract
Background and purpose
Uncoupling of cardiac gap junction channels is an important arrhythmogenic mechanism in ischemia/reperfusion. Antiarrhythmic peptide AAP10 (H-Gly-Ala-Gly-Hyp-Pro-Tyr-CONH2) has been shown to prevent acidosis-induced uncoupling and ischemia-related increase in dispersion. Previous structure–effect investigations and subsequent computer modeling studies indicated that the tricyclic antidepressant desipramine may exert similar effects as AAP10.
Methods
We assessed the binding of 14C-AAP10 to membranes of rabbit cardiac ventricles and its displacement with desipramine in a classical radioligand binding and competition study. Gap junction currents were measured between isolated pairs of human atrial cardiomyocytes under normal and acidotic (pH 6.3) conditions with or without 1 μmol/l desipramine using dual whole-cell voltage clamp. The effect of 1 μmol/l desipramine was assessed in isolated rabbit hearts (Langendorff technique) undergoing local ischemia by coronary occlusion with 256-channel electrophysiological mapping and subsequent analysis of connexin43 (Cx43) expression, phosphorylation (Western blot), and subcellular localization (immunohistology).
Results
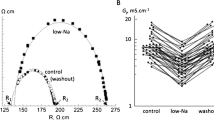
We found saturable 14C-AAP10 binding to cardiac membranes (K D, 0.29 ± 0.11 nmol/l; B max, 42.5 ± 7.2 pmol/mg) which could be displaced by desipramine with a K D.High = 0.14 μmol/l and a K D.Low = 22 μmol/l. Acidosis reduced the gap junction conductance in human cardiomyocyte pairs from 24.1 ± 4.7 to 11.5 ± 2.5 nS, which could be significantly reversed by desipramine (26.6 ± 4.8 nS). In isolated hearts, ischemia resulted in significantly increased dispersion of activation–recovery intervals, loss of membrane Cx43, and dephosphorylation of Cx43, which all could be prevented by desipramine.
Conclusion
Desipramine seems to prevent the uncoupling of cardiac gap junctions and ischemia-related increase in dispersion.






Similar content being viewed by others
Abbreviations
- AAP10:
-
Synthetic antiarrhythmic peptide 10
- AAPnat:
-
Natural antiarrhythmic peptide
- ARI:
-
Activation–recovery interval
- BCL:
-
Basic cycle length
- BSA:
-
Bovine serum albumin
- CF:
-
Coronary flow
- Cx:
-
Connexin
- Cx43:
-
Connexin43
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- LVP:
-
Left ventricular pressure
- ML:
-
Membrane length
- QRS:
-
Ventricular activation complex in the electrocardiogram
- RV:
-
Right ventricle
References
Baldessarini RJ (1996) Drugs and the treatment of psychiatric disorders. In: Hardman JG, Limbird LE (eds) Goodman & Gilman’s the pharmacological basis of therapeutics, 9th edn. McGraw-Hill, New York, pp 431–459
Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kléber AG, Schuessler RB, Saffitz JE (2000) Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 87:656–662
Bril A, Rochette L (1988) Antiarrhythmic properties of antidepressant drugs after coronary artery occlusion and reperfusion in rats. Pharmacology 36:16–26
Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF (1990) Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol 10:1754–1763
DaTorre SD, Creer MH, Pogwizd SM, Corr PB (1991) Amphipathic lipid metabolites and their relation to arrhythmogenesis in the ischemic heart. J Mol Cell Cardiol 23(suppl1):11–22
Daugherty A, Frayn KN, Redfern WS, Woodward B (1986) The role of catecholamines in the production of ischemia-induced ventricular arrhythmias in the rat in vivo and in vitro. Br J Pharmacol 87:265–277
De Groot JR, Wilms-Schopman FJ, Opthof T, Remme CA, Coronel R (2001) Late ventricular arrhythmias during acute regional ischemia in the isolated blood perfused pig heart. Role of electrical cellular coupling. Cardiovasc Res 50:362–372
De Maziére MGL, Scheuermann DW (1990) Structural changes in cardiac gap junctions after hypoxia and reoxygenation: a quantitative freeze fracture analysis. Cell Tissue Res 261:183–194
Dekker LRC, Fiolet JWT, VanBavel E, Coronel R, Opthof T, Spaan JAE, Janse MJ (1996) Intracellular Ca2+, intercellular electrical coupling and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ Res 79:237–246
Delpón E, Valenzuela C, Pérez O, Tamargo J (1993) Electrophysiological effects of the combination of imipramine and desipramine in guinea pig papillary muscles. J Cardiovasc Pharmacol 21:13–20
Dhein S, Müller A, Gerwin R, Klaus W (1993) Comparative study on the proarrhythmic effects of some class I antiarrhythmic agents. Circulation 87:617–631
Dhein S, Manicone N, Müller A, Gerwin R, Ziskoven U, Irankhahi A, Minke C, Klaus W (1994) A new synthetic antiarrhythmic peptide reduces dispersion of epicardial activation recovery interval and enhances cellular communication. Antiarrhythmic properties in regional ischemia. Naunyn-Schmiedeberg's Arch Pharmacol 350:174–184
Dhein S, Weng S, Grover R, Tudyka T, Gottwald M, Schaefer T, Polontchouk L (2001) Protein kinase Cα mediates the effect of antiarrhythmic peptide on gap junction conductance. Cell Adhesion Commun 8:257–264
Dhein S, Hagen A, Jozwiak J, Dietze A, Garbade J, Barten M, Kostelka M, Mohr FW (2010) Improving cardiac gap junction communication as a new antiarrhythmic mechanism: the action of antiarrhythmic peptides. Naunyn Schmiedebergs Arch Pharmacol 381:221–234
Du XJ, Woodcock EA, Little PJ, Esler MD, Dart AM (1998) Protection of neuronal uptake-1 inhibitors in ischemic and anoxic hearts by norepinephrine-dependent and -independent mechanisms. J Cardiovasc Pharmacol 32:621–628
Durrer D, Van der Tweel LH (1954) Spread of activation in the left ventricular wall of the dog. Activation conditions at the epicardial surface. Am Heart J 47:192–203
Eloff BC, Gilat E, Wan X, Rosenbaum DS (2003) Pharmacological modulation of cardiac gap junction to enhance cardiac conduction. Evidence supporting a novel target for antiarrhythmic therapy. Circulation 108:3157–3163
Giardina EG, Bigger JT, Glassman AH, Perel JM, Kantor SJ (1979) The electrocardiographic and antiarrhythmic effects of imipramine hydrochloride at therapeutic plasma concentrations. Circulation 60:1045–1052
Gottwald E, Gottwald M, Dhein S (1998) Enhanced dispersion of epicardial activation-recovery intervals at sites of histologic inhomogeneity during regional cardiac ischaemia and reperfusion. Heart 79:474–480
Grover R (2000) Molecular Modeling und Rezeptorentwicklung des antiarrhythmischen Peptides AAP10. Thesis, Faculty for Mathematics and Natural Sciences, University of Cologne, Cologne
Grover R, Dhein S (1998) Spatial structure determination of antiarrhythmic peptide using nuclear magnetic resonance spectroscopy. Peptides 19:1725–1729
Grover R, Dhein S (2001) Structure–activity relationships of novel peptides related to the antiarrhythmic peptide AAP10 which reduce the dispersion of epicardial action potential duration. Peptides 22:1011–1021
Gu H, Ek-Vitorin JF, Taffet SM, Delmar M (2000) Coexpression of connexins 40 and 43 enhances the pH sensitivity of gap junctions. A model for synergistic interactions among connexins. Circ Res 86:e98–e103
Hagen A, Dietze A, Dhein S (2009) Human cardiac gap junction coupling: effects of antiarrhythmic peptide AAP10. Cardiovasc Res 83:405–415
Hatanaka K, Kawata K, Toyofuku T, Yoshida K (2004) Down-regulation of connexin43 in early myocardial ischemia and protective effect by ischemic preconditioning in rat hearts in vivo. Jpn Heart J 45:1007–1019
Hong HK, Park MH, Lee BH, Jo SH (2010) Block of human ether-a-go-go-related gene (hERG) K + channel by the antidepressant desipramine. Biochem Biophys Res Commun 394:536–541
Imamura M, Lander HM, Levi R (1996) Activation of histamine H3-receptors inhibits carrier-mediated norepinephrine release during protracted myocardial ischemia. Comparison with adenosine A1-receptors and alpha2-adrenoceptors. Circ Res 78:475–481
Jain SK, Schuessler RB, Saffitz JE (2003) Mechanisms of delayed electrical uncoupling induced ischemic preconditioning. Circ Res 92:1138–1144
Jozwiak J, Dhein S (2008) Local effects and mechanisms of antiarrhythmic peptide AAP10 in acute regional myocardial ischemia: electrophysiological and molecular findings. Naunyn Schmiedebergs Arch Pharmacol 378:459–470
Kjolbye AL, Diksheteyn M, Eloff BC, Deschenes I, Rosenbaum DS (2007) Maintenance of intercellular coupling by the antiarrhythmic peptide rotigaptide suppresses arrhythmogenic discordant alternans. Am J Physiol Heart Circ Physiol 294:H41–H49
Kléber AG, Riegger CB, Janse MJ (1987) Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res 61:271–279
Kuo CS, Munakata K, Reddy CP, Surawicz B (1983) Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential duration. Circulation 67:1356–1367
Kurz T, Offner B, Schreieck J, Richardt G, Tölg R, Schömig A (1995) Nonexocytotic noradrenaline release and ventricular fibrillation in ischemic rat hearts. Naunyn Schmiedeberg’s Arch Pharmacol 352:491–496
Lampe PD, Cooper CD, King TJ, Burt JM (2006) Analysis of connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci 119(Pt 16):3435–3442
Lesh MD, Pring M, Spear JF (1989) Cellular uncoupling can unmask dispersion of action potential duration in ventricular myocardium. Circ Res 65:1426–1440
Limbird LE (1996) Cell surface receptors: a short course on theory and methods, 2nd edn. Klüwer Academic, Dordrecht pp 1–232
Millar CK, Kralios FA, Lux RL (1985) Correlation between refractory periods and activation recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation 72:1372–1379
Mollerup S, Hofgaard JP, Braunstein TH, Kjenseth LE, Rivedal E, Holstein-Rathlou NH, Nielsen MS (2011) Norepinephrine inhibits intercellular coupling in rat cardiomyocytes by ubiquitination of connexin43 gap junctions. Cell Commun Adhes 18:57–65
Müller A, Dhein S (1993) Sodium channel blockade enhances dispersion of the cardiac action potential duration. A computer simulation study. Basic Res Cardiol 88:11–22
Müller A, Schaefer T, Linke W, Tudyka T, Gottwald M, Klaus W, Dhein S (1997) Actions of the antiarrhythmic peptide AAP10 on cellular coupling. Naunyn Schmiedeberg's Arch Pharmacol 356:76–82
Müller A, Lauven M, Berkels R, Dhein S, Polder HR, Klaus W (1999) Switched single electrode voltage clamp amplifiers allow precise measurement of gap junction conductance. Am J Physiol 276:C980–C987
Musil LS, Cunningham BA, Edelman GM, Goodenough DA (1990) Differential phosphorylation of gap junction protein connexin43 in junctional communication-competent and deficient cell lines. J Cell Biol 111:2077–2088
Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, Kuhn-Reignier F, DeVivie ER, Dhein S (2001) Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol 38:883–891
Schömig A, Richard G (1990) Cardiac sympathetic activity in myocardial ischemia: release and effects of noradrenaline. Basic Res Cardiol 85(suppl1):9–30
Shekher A, Sharma PL, Pandhi P, Singh M (1996) Role of noradrenaline uptake inhibition and alpha-adrenoceptors in reperfusion ventricular arrhythmias in vivo and in-vitro. Indian J Exp Biol 34:1085–1090
Solan JL, Lampe PD (2007) Key connexin43 phosphorylation events regulate the gap junction life cycle. J Membr Biol 217:35–41
Staudacher I, Wang L, Wan X, Obers S, Wenzel W, Tristam F, Koschny R, Staudacher K, Kisselbach J, Koelsch P, Schweizer PA, Katus HA, Ficker E, Thomas D (2011) hERG K + channel-associated cardiac effects of the antidepressant drug desipramine. Naunyn Schmiedeberg’s Arch Pharmacol 383:119–139
Tansey EE, Kwaku KF, Hammer PE, Cowan DB, Federman M, Levitsky S, McCully JD (2006) Reduction and redistribution of gap and adherens junction proteins after ischemia and reperfusion. Ann Thorac Surg 82:1472–1479
Verrecchia F, Duthe F, Duval S, Duchatelle I, Sarrouilhe D, Hervé JC (1999) ATP counteracts the rundown of gap junctional channels of rat ventricular myocytes by promoting protein phosphorylation. J Physiol (London) 516:447–459
Vetterlein F, Mühlfeld CH, Cetegen C, Volkmann R, Scgrader CH, Hellige G (2006) Redistribution of connexin43 in regional acute ischemic myocardium: influence of ischemic preconditioning. Am J Physiol Heart Circ Physiol 291:H813–H819
Weingart R, Maurer P (1988) Action potential transfer in cell pairs isolated from adult rat and guinea pig ventricles. Circ Res 63:72–80
Weng S, Lauven M, Schaefer T, Polontchouk L, Grover R, Dhein S (2002) Pharmacological modulation of gap junction coupling by an antiarrhythmic peptide via protein kinase C activation. FASEB J 16:1114–1116
Wu J, McHowat J, Saffitz JE, Yamada KA, Corr PB (1993) Inhibition of gap junctional conductance by long chain acylcarnitines and their preferential accumulation in junctional sarcolemma during hypoxia. Circ Res 72:879–889
Xing D, Kjølbye AL, Nielsen MS, Petersen JS, Harlow KW, Holstein-Rathlou NH, Martins JB (2003) ZP123 increases gap junctional conductance and prevents reentrant ventricular tachycardia during myocardial ischemia in open chest dogs. J Cardiovasc Electrophysiol 14:510–520
Zahradník I, Minarovic I, Zahradníková A (2008) Inhibition of the cardiac L-type calcium channel current by antidepressant drugs. J Pharmacol Exp Ther 324:977–984
Acknowledgments
This paper was generously supported by grants to Stefan Dhein given by DFG (Bonn, Germany) and ProCordis (Leipzig, Germany).
Conflict of interest
There is no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Joanna Jozwiak and Anna Dietze contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 230 kb)
Rights and permissions
About this article
Cite this article
Jozwiak, J., Dietze, A., Grover, R. et al. Desipramine prevents cardiac gap junction uncoupling. Naunyn-Schmiedeberg's Arch Pharmacol 385, 1063–1075 (2012). https://doi.org/10.1007/s00210-012-0795-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-012-0795-2