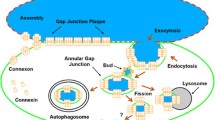
Abstract
Connexin 43 (Cx43), the most widely expressed and abundant vertebrate gap junction protein, is phosphorylated at multiple different serine residues during its life cycle. Cx43 is phosphorylated soon after synthesis and phosphorylation changes as it traffics through the endoplasmic reticulum and Golgi to the plasma membrane, ultimately forming a gap junction structure. The electrophoretic mobility of Cx43 changes as the protein proceeds through its life cycle, with prominent bands often labeled P0, P1 and P2. Many reports have indicated changes in “phosphorylation” based on these mobility shifts and others that occur in response to growth factors or other biological effectors. Here, we indicate how phosphospecific and epitope-specific antibodies can be utilized to show when and where certain phosphorylation events occur during the Cx43 life cycle. These reagents show that phosphorylation at S364 and/or S365 is involved in forming the P1 isoform, an event that apparently regulates trafficking to or within the plasma membrane. Phosphorylation at S325, S328 and/or S330 is necessary to form a P2 isoform; and this phosphorylation event is present only in gap junctions. Treatment with protein kinase C activators led to phosphorylation at S368, S279/S282 and S262 with a shift in mobility in CHO, but not MDCK, cells. The shift was dependent on mitogen-activated protein kinase activity but not phosphorylation at S279/S282. However, phosphorylation at S262 could explain the shift. By defining these phosphorylation events, we have begun to sort out the critical signaling pathways that regulate gap junction function.




Similar content being viewed by others
References
Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE (2000) Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 87:656–662
Cooper CD, Lampe PD (2002) Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem 277:44962–44968
Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF (1990) Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol 10:1754–1763
Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E (2004) Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J Cell Sci 117:507–514
Goodenough DA, Paul DL (2003) Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol 4:285–294
Lampe PD, Cooper CD, King TJ, Burt JM (2006) Analysis of connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci 119:3435–3442
Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF (2000) Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 126:1503–1512
Loewenstein WR, Azarnia R (1988) Regulation of intercellular communication and growth by the cellular src gene. Ann N Y Acad Sci 551:337–346
Musil LS, Cunningham BA, Edelman GM, Goodenough DA (1990) Differential phosphorylation of gap junction protein connexin43 in junctional communication-competent and deficient cell lines. J Cell Biol 111:2077–2088
Musil LS, Goodenough DA (1991) Biochemical analysis of connexin43 intracellular transport, phosphorylation and assembly into gap junctional plaques. J Cell Biol 115:1357–1374
Rivedal E, Opsahl H (2001) Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis 22:1543–1550
Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83:1359–1400
Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G (2003) Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J 17:1355–1357
Simon AM, Goodenough DA, Paul DL (1998) Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol 8:295–298
Sohl G, Willecke K (2004) Gap junctions and the connexin protein family. Cardiovasc Res 62:228–232
Solan JL, Fry MD, TenBroek EM, Lampe PD (2003) Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci 116:2203–2211
Sosinsky GE, Solan JL, Gaietta GM, Ngan L, Mackey M, Lampe PD (2007) The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structurally specific antibodies. (in press)
Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF (1998) Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem 273:9188–9196
Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF (1996) Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J Biol Chem 271:3779–3786
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383:725–737
Acknowlegement
These studies were supported by grants from the National Institutes of Health (GM055632 to P. D. L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solan, J.L., Lampe, P.D. Key Connexin 43 Phosphorylation Events Regulate the Gap Junction Life Cycle. J Membrane Biol 217, 35–41 (2007). https://doi.org/10.1007/s00232-007-9035-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-007-9035-y