Abstract
Accumulating evidence indicates that chronic circadian rhythm disruption is associated with the development of neurodegenerative diseases induced by exposure to neurotoxic chemicals. Herein, we examined the relationship between cellular circadian rhythm disruption and cytotoxicity in neural cells. Moreover, we evaluated the potential application of an in vitro cellular circadian rhythm assay in determining circadian rhythm disruption as a sensitive and early marker of neurotoxicant-induced adverse effects. To explore these objectives, we established an in vitro cellular circadian rhythm assay using human glioblastoma (U87 MG) cells stably transfected with a circadian reporter vector (PER2-dLuc) and determined the lowest-observed-adverse-effect levels (LOAELs) of several common neurotoxicants. Additionally, we determined the LOAEL of each compound on multiple cytotoxicity endpoints (nuclear size [NC], mitochondrial membrane potential [MMP], calcium ions, or lipid peroxidation) using a multiparametric high-content screening (HCS) assay using transfected U87 MG cells treated with the same neurotoxicants for 24 and 72 h. Based on our findings, the LOAEL for cellular circadian rhythm disruption for most chemicals was slightly higher than that for most cytotoxicity indicators detected using HCS, and the LOAEL for MMP in the first 24 h was the closest to that for cellular circadian rhythm disruption. Dietary antioxidants (methylselenocysteine and N-acetyl-l-cysteine) prevented or restored neurotoxicant-induced cellular circadian rhythm disruption. Our results suggest that cellular circadian rhythm disruption is as sensitive as cytotoxicity indicators and occurs early as much as cytotoxic events during disease development. Moreover, the in vitro cellular circadian rhythm assay warrants further evaluation as an early screening tool for neurotoxicants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases have been associated with the disruption of daily rhythms (Carter et al. 2021; Logan and McClung 2019; Nassan and Videnovic 2022). According to human and rodent studies, circadian rhythm disruption can be attributed to shift work, light exposure at night, pinealectomy, or genetic defects (e.g., lack of circadian genes) (Antoch et al. 2008; Fu and Lee 2003; Kondratov et al. 2006; Lee et al. 2010; National Toxicology 2021; Salgado-Delgado et al. 2011). Numerous clinical studies have revealed a direct correlation between abnormal clock function and the severity of neurodegeneration and sleep disturbances (Hastings and Goedert 2013; Leng et al. 2019; Shen et al. 2023). Furthermore, animal models of Alzheimer’s disease (Sterniczuk et al. 2010), Parkinson’s disease (Kudo et al. 2011), and Huntington’s disease (Oakeshott et al. 2011) have confirmed the adverse effects of circadian clock disruption on neurodegeneration.
Investigations exploring neurological diseases using laboratory animal models are costly, time-consuming, and unsuitable for screening large numbers of chemicals. Moreover, in vivo studies lack adequate sensitivity to predict human neurotoxicity and often fail to generate sufficient information for regulatory decision-making (Bal-Price et al. 2008). Therefore, it is imperative to establish and validate cell-based in vitro assays to monitor changes in cell-autonomous rhythms using initial screening methods for neurotoxic chemicals, given that neurotoxicity prediction is an important feature of toxicological profiling of chemical compounds and environmental toxicants. Furthermore, chemicals responsible for various diseases have been shown to alter cellular circadian rhythm (Audira et al. 2019; Boughattas et al. 1989; Chen et al. 2012; Fifel and Videnovic 2020; Hwang et al. 2014; Wilsbacher et al. 2002; Wong and Cortopassi 2002); Neurotoxicity induced by external factors, such as chemicals, can result in disease, with major toxicity mechanisms including excess nitrogen oxide production, reactive oxygen species (ROS)-induced oxidative stress, mitochondrial dysfunction (Amo et al. 2011; Zerin et al. 2015), calcium channel dysfunctions (Baev et al. 2022), and DNA damage (Zhang 2018); these factors have been found to modulate cellular circadian rhythm (Cavieres-Lepe and Ewer 2021; Fagiani et al. 2022; Fanjul-Moles and López-Riquelme 2016). Therefore, the impact of cellular circadian rhythm disruption on toxicological effects is crucial for assessing the neurotoxicity of chemical compounds and environmental toxicants.
The characteristics of the cellular circadian rhythm have been investigated in the suprachiasmatic nucleus of various laboratory animal species (Brown et al. 2019; Rivkees 2007). Importantly, the existence of the circadian rhythm has been confirmed in peripheral tissues and cells (Balsalobre et al. 1998), such as fibroblasts (Izumo et al. 2003), and changes in cellular circadian rhythm following chemical exposure have been documented and quantitatively analyzed (Hirota and Kay 2009). Cell experiments have shown that circadian rhythm disruption is associated with shortened or prolonged circadian periods, increased or decreased amplitude, and/or advanced or delayed rhythmic expression of circadian rhythm-related genes (Chen et al. 2012; Izumo et al. 2006). Subsequently, in vitro tests were developed to analyze the circadian rhythm of the liver (Guenthner et al. 2014; Saini et al. 2013; Yang et al. 2010), brain (Slat et al. 2017), and mammary gland (Fang et al. 2017) cells.
Alterations in the circadian rhythm in response to environmental chemicals have been studied in both in vivo and in vitro models (Grill and Maganti 2011; Richardson et al. 2019). For example, an in vitro investigation using liver cells to examine changes in the cellular metabolic rhythms of environmental substances has been reported, along with a review paper exploring cellular rhythm in zebrafish in relation to environmental pollutants (Ndikung et al. 2020; Zheng et al. 2021). However, properties that induce cellular circadian rhythm disturbance remain poorly established for several of these substances. The molecular mechanisms underlying changes in the cellular circadian rhythm have been identified through animal experiments and in vitro tests (Fang et al. 2015; Partch et al. 2014).
In the present study, we aimed to explore the relationship between disruption of the cellular circadian rhythm and cytotoxicity in neural cells. Accordingly, we combined in vitro circadian rhythm reporter assays and multiparametric high-content screening (HCS), which provides a mechanistic basis for effortless neurotoxicity screening based on different cellular functions without intending to study the detailed molecular biological mechanisms. We selected neurotoxicants known to cause oxidative stress (e.g., chlorpyrifos [CPF] and 1-methyl-4-phenylpyridinium [MPP +]) (Li et al. 2021; Singh et al. 2018), mitochondrial damage/dysfunction (e.g., CPF) (Yamada et al. 2017), and calcium channel inhibition (e.g., 4,4′-dichlorodiphenyltrichloroethane [DDT]) (Costa et al. 2008), as well as antibiotics capable of inducing neurotoxic effects (e.g., ciprofloxacin [CPX]; polymyxin B [PMX]) (Grill and Maganti 2011; Xiao et al. 2019), and heavy metals (e.g., methylmercury (II) chloride [MHg]) (Davidson et al. 2004; Yuan and Atchison 2016) as environmental contaminants, and tested these compounds in an in vitro assay using U87 MG cells. Comparing the chemical concentrations that induce cellular circadian rhythm changes with those that alter different mechanistic endpoints of cytotoxicity can provide valuable insights into the association between cellular rhythm disruption and general neurotoxicity.
Materials and methods
Materials
MHg was purchased from Sigma-Aldrich (442534). Additionally, we procured the following pesticides: CPF (45395; Sigma-Aldrich); MPP + iodide (N048; Sigma-Aldrich); DDT (N10876; Sigma-Aldrich). We also purchased the following antibiotics: PMX sulfate salt (Sigma P4932; Sigma-Aldrich) and CPX (17850; Sigma-Aldrich). Depending on the solvent employed for chemical dissolution, a vehicle control group was established: vehicle control: 0.1% dimethyl sulfoxide (DMSO [D12345; Invitrogen])-treated media or naïve media. For example, MPP + , PMX, and CPX were dissolved in distilled water or 0.1 N HCl and stored in aliquots at concentrations of 30 mM or 20 mM; MHg, DDT, and CPF were dissolved in DMSO and stored in aliquots with concentrations of 5 mM or 10 mM, and then diluted in a medium for experimental use.
Establishment of an in vitro cellular bioluminescence assay using U87 MG neural cells
U-87 MG (ATCC® HTB-14™) is a well-recognized human glioblastoma cell line widely used in the field of neuroscience. U87 MG cells have a regular and strong cellular circadian rhythm after transfection with a circadian gene (i.e., BMAL1) reporter vector (Jung et al. 2013; Slat et al. 2017). As described in our previous report (Fang et al. 2015, 2017), U87 MG cells were transfected with a circadian reporter vector pGL [PER2P (Luc2P/Neo)] expressing destabilized firefly luciferase driven by the human PER2 promoter using FuGene HD™ transfection reagent (LightSwitch Genomics), and stably transfected cells were selected and cultured with 1000 μg/mL G418 (Invitrogen). The stably transfected cells were used to examine the cellular circadian rhythm using an in vitro cellular bioluminescence assay. U87 MG/PER2P-dLuc cells were cultured in Eagle’s minimum essential medium (EMEM) (11430030; Gibco) supplemented with 5% fetal bovine serum (FBS) (26140079; Gibco) and 1% penicillin/streptomycin (15140122; Gibco) at 37 °C in an atmosphere of 95% humidity and 5% CO2.
Cell viability assay
U87 MG/PER2-dLuc cells were subjected to the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) (M2128; Sigma-Aldrich) assay. To determine whether PER2P influences cell toxicity and establish working concentrations of the chemicals used in the current study (concentrations: MHg [1–9 µM]; CPF [20–180 µM]; MPP + [1 mM–1000 mM]; DDT [100–1000 µM]; PMX [0.1–1.0 mM]; CPX [0.76–100 µM]), we performed MTT cell viability assays and calculated the IC50/IC15 inhibitory concentrations of transfected cells. Briefly, U87 MG/PER2-dLuc cells were seeded in 96-well plates at a density of 105 cells/mL, incubated for 48 h, and treated with relevant chemicals at various concentrations for 72 h. Subsequently, the media with chemicals were decanted and 20μl of MTT reagent solution (5 mg/ml) with 80μl of the media was added to all wells the plates and were incubated for 2 h until the formation of formazan crystals. The reagent solution was decanted, and 100μl of DMSO (D8418; Sigma-Aldrich) was added. Absorbance was measured at 570 nm using a spectrophotometer (Molecular Devices). The values of triplicate same-treatment wells were averaged. For each compound, concentrations resulting in 15% (IC15) and 50% (IC50) growth inhibition were determined using GraphPad Prism 9.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Assessment of the effects of neurotoxicants on cellular circadian rhythm
Briefly, cultured U87 MG/PER2 -dLuc cells were seeded in 35-mm dishes at 3 × 105 cells/dish and incubated until 70–80% confluency was reached. The cells were FBS-starved for three days at 70% confluency and then synchronized with 50% horse serum (16050122; Gibco) (Balsalobre et al. 1998). After decanting and washing, recording medium (EMEM with 20% of growing medium containing 5% FBS, 6.5 mM sodium bicarbonate, 10 mM HEPES buffer [pH 7.2], 0.1 mM luciferin [E1602; Promega], and 50 U/mL penicillin and 50 µg/mL streptomycin) was added. Subsequently, the cells were treated with each compound. The 35-mm dishes were sealed with a sterile glass cover slide using silicon grease and subjected to continuous monitoring with a LumiCycle 32™ (Actimetrics) for 5 days. The analytical method was optimized according to the instruction manual of the LumiCycle Analysis Software (Actimetrics). Using this software, data were detrended (running average), and best fits to a sine wave were estimated using the Levenberg–Marquardt algorithm for period, phase, amplitude, and damping rate measurements, as previously reported (Chen et al. 2012; Fang et al. 2015, 2017).
Assessment of the effects of neurotoxicants on cellular circadian rhythm in the presence of antioxidants
To determine the effects of antioxidants, U87 MG/PER2-dLuc cells were treated as previously described with neurotoxicants (MHg, CPF, MPP + , DDT, PMX, or CPX) at respective lowest-observed-adverse-effect levels (LOAELs), along with one of the following antioxidants: 12.5 or 25 µM methylselenocysteine (MSC) (M6680; Sigma-Alrich) or 10 or 20 µM N-acetyl-l-cysteine (NAC) (A7250; Sigma-Alrich) added in the recording medium, as previously described. The cellular circadian rhythm was recorded as described above.
HCS assay
A multiparametric analysis of compound toxicity at the level of individual cells was conducted using the HCS method established by Tolosa et al. (2012) to assess the liver toxicity of chemicals and drugs. Briefly, U87 MG/PER2-dLuc cells were seeded in 96-well plates specific for HCS at a density of 104 cells/well. The cells were incubated with the chemicals at various concentrations (concentrations: MHg [0.03–8 µM]; CPF [0.7–180 µM]; MPP + [0.6 µM–160 µM]; DDT [0.5–120 µM]; PMX [4–1000 µM]; CPX [0.9–240 µM]) for 24 or 72 h. Following these treatments, cells were treated with a mixture of Hoechst 33342 (Hoechst) (B2261; Sigma-Alrich) at 1.5 µg/mL to assess the nuclear size (NC), 75 ng/mL tetramethyl rhodamine methyl ester (TMRM) (T-668; Molecular Probes) as a mitochondrial membrane potential (MMP) sensor, 0.24 µM fluo-4 acetoxymethyl ester (Fluo-4 AM) (F14217; Molecular Probes) to detect intracellular calcium ions (Ca2+), and 1.5 µg/mL of (E,E)-3,5-bis-(4-phenyl-1,3-butadienyl)-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY 665/676) (b-3932; Molecular Probes) to evaluate lipid peroxidation. After incubation for 1 h, the cells were imaged using an IN Cell Analyzer 6000 (GE Healthcare Life Sciences) and analyzed using IN Cell Miner HCM. The data were analyzed with IN Cell Analyzer 6000 Analysis Software. Nuclear morphological alterations were assessed based on the Hoechst staining. The nucleus was defined as the main object detected using an edge detection algorithm. To separate individual cells, segmentation was applied. All measurements were restricted to live cells. Lipid peroxidation was detected based on BODIPY fluorescence intensity in the cytoplasm. The cellular MMP was defined as the TMRM fluorescence intensity in punctate cytosolic regions surrounding the nucleus. Fluo-4 intensity was used to measure changes in the cytosolic-free Ca2+ concentration. An intensity algorithm with a fixed threshold was used to measure TMRM, Fluo-4, and BODIPY fluorescence. Each measurement was performed in individual cells. The values of triplicate same-treatment wells were averaged and normalized to the average value of vehicle control (0.1% DMSO-treated or naïve cells).
Statistical analysis
The values of triplicate same-treatment wells were averaged and normalized to the average value of the negative control group, and differences in parameters between concentrations were analyzed using one-way ANOVA followed by Duncan’s tests. A p-value < 0.05 was considered significant.
Results
Toxicity of chemicals in U87 MG/PER2-dLuc cells
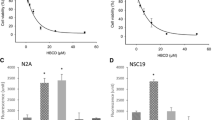
To determine the concentration range of each chemical to be employed in the in vitro cellular bioluminescence assay and HCS, we measured the viability of U87 MG/PER2-dLuc cells treated with individual compounds using the MTT assay. Cell viability as a function of concentration is presented in Fig. 1. Among the examined compounds, MHg exhibited the highest toxicity toward U87 MG/PER2-dLuc cells, with an IC50 of 3.97 µM, whereas CPX exerted the lowest toxicity (IC50 = 98.3 µM). The order of toxicity was as follows: MHg > DDT > MPP + > CPF > PMX > CPX. For each examined compound, concentrations covering the IC50–IC15 range were used in the in vitro cellular bioluminescence assay.
Cell viability of U87 MG/PER2-dLuc cells post-treatment. U87 MG/PER2-dLuc cells were treated with each compound at the indicated doses, and cell viability was examined using the MTT assay. The IC50–IC15 concentration range of each compound was estimated using GraphPad Prism 9.0 (GraphPad). CPF, chlorpyrifos; CPX, ciprofloxacin; DDT, 4,4′-dichlorodiphenyltrichloroethane; MHg, methylmercury; MPP + ,1 -methyl-4-phenylpyridinium; PMX, polymyxin B
Cellular circadian rhythm of U87 MG cells transfected with a circadian reporter vector and toxicity of chemicals on transfected U87 MG/PER2-dLuc cells
The cellular circadian rhythm was evaluated based on circadian parameters, including amplitude, period, and phase, as described previously (Fang et al. 2015, 2017). U87 MG cells stably transfected with the circadian reporter vector (U87 MG/PER2-dLuc) exhibited a regular and strong cellular circadian rhythm for at least 7 days (Fig. 2), comparable with that observed in human mammary epithelial cells (Fang et al. 2015, 2017). This finding indicated that our circadian luciferase reporter vector and in vitro cellular bioluminescence assay could be applied to U87 MG human neural cells.
Disruption of the circadian rhythm in the in vitro cellular bioluminescence assay using U87 MG/PER2-dLuc neural cells
According to bioluminescence assay results, 2.0 µM MHg (Fig. 3A) and 1.5 µM CPF (Fig. 3B) induced circadian rhythm disruption of U87 MG/PER2-dLuc neural cells. Additionally, MPP + disrupted the cellular circadian rhythm at concentrations ≥ 30 µM, with an advanced phase and decreased amplitude, although no disruptive effect was observed at a lower concentration (10 µM) (Fig. 3C). DDT appeared to be well tolerated except at the highest concentration (10 µM), at which the robust rhythm was gradually abolished (Fig. 3D). PMX disrupted the cellular circadian rhythm at concentrations ≥ 30 µM, with an advanced phase and decreased amplitude, but showed no disruptive effect at a lower concentration (15 µM) (Fig. 3E). CPX increased the circadian rhythm at concentrations ≥ 40 µM. After treatment with ≥ 40 µM CPX, reporter activities were enhanced during the first circadian cycle and persisted even in subsequent cycles (Fig. 3F). Table 1 summarizes the effects of all examined compounds on cellular circadian rhythm.
In vitro cellular circadian rhythms of U87 MG/PER2-dLuc cells disrupted by neurotoxicants. Bioluminescence assays were performed for each treatment using U87 MG/PER2-dLuc reporter cells after synchronization with 50% horse serum. X-axis, time (hours) post-chemical treatment; Y-axis, bioluminescence (count/min). (A) MHg, (B) CPF, (C) MPP + , (D) DDT, (E) PMX, and (F) CPX. CPF, chlorpyrifos; CPX, ciprofloxacin; DDT, 4,4′-dichlorodiphenyltrichloroethane; MHg, methylmercury; MPP + ,1 -methyl-4-phenylpyridinium; PMX, polymyxin B
Antioxidants restored the chemical-induced circadian rhythm disruption
The dietary antioxidant agents MSC and NAC are known to reduce oxidative stress and prevent the loss of or restore the normal pattern of cellular circadian rhythm (Fang et al. 2015). To determine whether disrupted cellular circadian rhythms are associated with oxidative stress, we simultaneously administered NAC (10 or 20 μM) or MSC (12.5 or 25 μM) at the minimum disrupted concentration for each chemical, as previously reported (Fang et al. 2017).
Although treatment with 1.5 µM CPF abolished the cellular circadian rhythm, as indicated by the disappearance of the second luminescence peak in the reporter activity assay, both 20 µM NAC and 12.5 µM (or 25 µM) MSC restored the circadian rhythm in CPF-treated cells and helped maintain a normal cellular rhythm (Fig. 4A). Similarly, co-treatment with NAC or MSC restored the cellular rhythm disrupted by 10 µM DDT to a normal rhythm (Fig. 4B). Co-treatment with NAC and MSC reduced the first high-altitude peak induced by 2.0 µM MHg (Fig. 4C). These antioxidant agents could restore the MPP + -disrupted rhythmicity; however, the restored rhythm was not similar to that of control cells (Fig. 4D). Conversely, co-treatment with PMX and CPF and antioxidants failed to consistently restore disrupted rhythms (data not shown).
Antioxidants restore cellular circadian rhythms disrupted by chemicals in U87 MG/PER2-dLuc cells in vitro. A MSC and NAC restore the cellular circadian rhythm disrupted by CPF in U87 MG/PER2-dLuc cells. In cells treated with B DDT, C MHg, or D MPP + , MSC and NAC restore the cellular circadian rhythm to a certain extent. CPF chlorpyrifos, CPX ciprofloxacin, DDT,4,4′-dichlorodiphenyltrichloroethane, MHg methylmercury, MPP + 1 -methyl-4-phenylpyridinium, MSC methylselenocysteine, NAC N-acetyl-l-cysteine, PMX polymyxin B
Changes in nuclear size induced after chemical treatment for 24 and 72 h
To examine the effects of the chemicals on the nuclear size of U87 MG/PER2-dLuc cells, Hoechst 33342 was used as a molecular probe to detect the area of nuclear fluorescence in treated cells when compared with that in non-treated control cells. Relative changes in the nuclear size indicated adverse effects associated with cytotoxicity. MHg had dual effects, with a slight increase followed by a significant decrease in nuclear size at 4–8 µM of MHg (above the IC50) after 24 h of treatment (p < 0.01). Moreover, MHg exerted substantial hormesis, with moderate biphasic changes in nuclear size observed at 2–8 µM of MHg (around the IC50) after 72 h of treatment (Fig. 5A). After 72 h of treatment, MPP + could sequentially reduce nuclear size at concentrations of 80–120 µM (around the IC50) (Fig. 5C). After 24 h of treatment, DDT significantly reduced the nuclear size at concentrations at 120 µM (above the IC50) (p < 0.01) (Fig. 5D).
Relative changes in cytotoxic parameters in HCS in U87 MG/PER2-dLuc cells treated with the neurotoxicants for 24 and 72 h. U87 MG/PER2-dLuc cells were treated with A MHg, B CPF, C MPP + , D DDT, E PMX, or F CPX for 24 or 72 h, and then subjected to HCS assay as described in Materials and Methods. In the legend, the indicators (Y-axis) measured for each administered substance (A–E) are explained from left to right: nuclear size, MMP, lipid peroxidation, and Ca2+ levels as the ratio of the test substance to the vehicle control. Values are the mean ± standard deviation (SD) of triplicates. “a” and “aa” indicate p < 0.05 and p < 0.01, respectively, vs. 0 µM for 24 h. “b” and “bb” indicate p < 0.05 and p < 0.01, respectively, vs. 0 µM for 72 h. CPF, chlorpyrifos; CPX, ciprofloxacin; DDT, 4,4′-dichlorodiphenyltrichloroethane; HCS, high-content-screening; MHg, methylmercury; MMP, mitochondrial membrane potential; MPP + ,1 -methyl-4-phenylpyridinium; PMX, polymyxin B
Neurotoxicant treatment for 24 h reduced MMP levels
To examine the effects of selected chemicals on the MMP of cells, TMRM was used as a molecular probe to detect the MMP, and the fluorescence intensity of treated cells was compared with that of non-treated control cells. A relative reduction in TMRM fluorescence greater than that of negative controls indicates a decrease in the MMP, a well-known adverse effect associated with cytotoxicity. Following 24 h of treatment, we observed that all examined chemicals could significantly reduce MMP at concentrations substantially lower than the IC50 value (p < 0.01 or p < 0.05) (Fig. 5A–F). Cytotoxicity, as indicated by a decrease in the MMP, was observed at the lowest concentrations, in ascending order: 0.5 µM for MHg, 0.94 µM for CPX, 1.41 µM for CPF, 3.91 µM for PMX, 15 µM for DDT, and 40 µM for MPP + (Fig. 5). Conversely, MMP levels were not significantly altered by most chemicals following 72 h of treatment; one exception was CPX, which could significantly decrease MMP levels at concentrations ≥ 0.94 µM even after 72-h treatment (p < 0.01). Interestingly, we observed that certain capable of reducing MMP levels showed a tendency for biphasic reactions, decreasing and then increasing MMP with increasing concentrations; however, this observation was considered to be hormesis (O'Brien et al. 2006).
Treatment with neurotoxicants for 24 and 72 h reduced calcium ion levels
Next, the Fluo-4 AM probe was used to evaluate the effects of selected chemicals on the endoplasmic reticulum. An increase in green fluorescence indicates an increase in the intracellular free Ca2+ concentration. At concentrations ranging from 0.7 to 45 µM, CPF increased the intracellular Ca2+ level at both the 24- and 72-h timepoints (Fig. 5B). Treatment with MPP + at concentrations of 40 and 0.63 µM increased intracellular Ca2+ levels at 24- and 72-h timepoints, respectively (Fig. 5C). However, treatment with the other chemicals did not significantly enhance intracellular Ca2+ levels after 24 h of treatment (Fig. 5A, E and F). Following treatment for 72 h, the other chemicals (i.e., other than MPP + and CPF) could enhance intracellular Ca2+ levels at concentrations lower than the IC15 value (Fig. 5A, D–F). At 72 h, intracellular Ca2+ levels were elevated at concentrations above 0.03 µM for MHg, 0.47 µM for DDT, and 0.94 µM for CPX (Fig. 5A, D and E).
Treatment with chemicals for increased lipid peroxidation primarily after 72 h
BODIPY 665/676 was used as a molecular probe to examine the oxidative degradation of cell membranes. Increased red fluorescence indicates enhanced lipid peroxidation induced by oxidative stress (Choi et al. 2007). As shown in Fig. 5, cytotoxicity, as indicated by an increase in lipid peroxidation, was observed in ascending order at serial low concentrations. Treatment with most chemicals for 24-h did not significantly alter the lipid peroxidation levels. However, MHg significantly reduced lipid peroxidation levels at concentrations above the IC50 value, 8.0 µM, 24 h after treatment.
Treatment with certain chemicals could significantly increase lipid peroxidation levels 72 h after treatment. Specifically, chemically treated cells showed increased lipid peroxidation at concentrations above 0.031 μM for MHg, 160 µM for MPP + , and 0.94 µM for CPX after 72 h of treatment (Fig. 5).
Association between disruption of cellular circadian rhythm and toxic indicators of cellular function
Table 1 summarizes the effects of all examined compounds on cellular circadian rhythm and individual toxic indicators of cellular function. The LOAELs of all compounds for the cellular circadian rhythm were below or approximated the IC15 value, except that of CPX (40 µM) for circadian disruption, which was in the range of LC15–LC50 (20–98 µM). These results revealed that the nuclear size was relatively stable, especially at low doses of each chemical. However, nuclear size was significantly altered (increased or decreased) by most chemicals at high doses, especially MHg, DDT, and PMX, after both 24 or 72 h of treatment (p < 0.01 or p < 0.05). For examined chemicals, the LOAEL for circadian rhythm disruption was slightly higher than that of most cytotoxicity indices detected using HCS. The LOAEL for MMP reduction in the first 24 h was slightly lower than that for circadian disruption and the closest to it. For CPF and DDT, the LOAEL for calcium ions closely approximated the LOAEL for circadian disruption at 24 h. Considering MHg, MPP + , and CPX, the LOAELs for lipid peroxidation were increased at 72 h.
Discussion
Herein, we evaluated the applicability of an in vitro circadian reporter assay using human glioblastoma cells (U87 MG/hPER2-dLuc) to assess the potential toxicity of environmental chemicals. The combined application of the multiparametric HCS method and the in vitro circadian reporter system can provide a mechanistic basis for effortless neurotoxicity screening based on different cellular functions. Additionally, we evaluated whether a cellular bioluminescence assay could be employed as an early and sensitive biomarker for evaluating the toxicity of neurotoxicants. Comparing concentrations that induced changes in cellular circadian rhythm with those that altered different mechanistic endpoints, we gained insight into the association between cellular rhythm dysfunction and cytotoxicity in neural cells. We found that chemicals well-known to induce neurotoxicity, including a heavy metal (MHg), pesticides (CPF, MPP + , and DDT), and antibiotic drugs (CPX and PMX), disrupted circadian rhythm by altering the amplitude and/or advancing the phase with continued high amplitude. Moreover, our findings revealed that a multiparametric cellular image-based HCS method can be utilized to assess the cytotoxic effects of environmental toxicants based on indicators of cellular functions in U87 MG/hPER2-dLuc. Assessing cytotoxic endpoints between the cellular circadian rhythm and indicators of cellular functions suggests that the circadian rhythm disruption assay correlates with the mechanism of cytotoxicity. Therefore, the assay sensitivity to potential cytotoxic effects can be evaluated and compared.
We demonstrated that chemicals could disrupt the circadian rhythm of PER2 expression and parameters of HCS, such as NC, MMP, CA, and LP. This finding suggests that the in vitro circadian reporter cell system is associated with the molecular endpoint of cytotoxicity and possesses the characteristics of conventional in vitro cell-based toxicity assays. Thus, the circadian rhythm of PER2 expression may be a useful biomarker. Heavy metals and pesticides are more cytotoxic than antibiotics, even at lower concentrations, severely disrupting normal, synchronized circadian rhythms. When the minimum concentration for a disturbed circadian rhythm of PER2 was compared with that in the HCS assay at 24 or 72 h, the minimal disruptive concentration of tested chemicals was similar to the concentration that significantly altered MMP at 24 h, indicating that disruption of the circadian expression of PER2 is related to the molecular indicator of cytotoxicity. Notably, we observed that co-treatment with antioxidant chemicals could prevent or restore cellular circadian rhythm disruption induced by MHg, CPF, MPP + , and DDT, indicating that oxidative stress-mediated MMP reduction could be a highly relevant factor associated with circadian rhythm disruption. However, circadian rhythm disruption mediated by PMX and CPX was not closely or consistently associated with cytotoxicity endpoints. Moreover, co-treatment with PMX or CPX and antioxidants afforded inconsistent or unreproducible results. Accordingly, further studies are warranted to confirm the effects of antioxidants on the actions of PMX and CPX. In addition, in vitro assessments of U87 MG/PER2-dLuc cells in the presence of neurotoxins and antioxidants could be valuable in identifying the defense mechanism of antioxidants.
We tested the values of cytotoxicity parameters for HCS. The chronological, biological neurotoxicity following different durations of chemical exposure revealed a distinct trend at 24 and 72 h. A reduction in the MMP level during the early phase (24 h) of the circadian period is assumed to be the earliest and most sensitive indicator of cellular circadian rhythm disruption, whereas elevated intracellular Ca2+ levels and lipid peroxidation appear to be secondary markers of the early/late or the late phase (24/72 h or 72 h), respectively. These results are similar to the liver cell model, which initially showed a reduction in NC and MMP, followed by a gradual enhancement in Ca ions, i.e., a time course of cytotoxic change. This study shows that the cytotoxic effect increases over time above a threshold drug concentration (O'Brien et al. 2006).
Furthermore, DDT- or CPF-mediated increased intracellular calcium concentrations [Ca2 +]i via calcium channel inhibition (Meijer et al. 2014; Stevens et al. 2011) may indirectly disrupt the circadian rhythm by increasing oxidative stress, as shown in the mechanistic diagram. In addition, elevated lipid peroxidation, which is induced by oxidative stress and calcium ions, is likely associated with mitochondrial damage, disrupting the circadian rhythm (Fig. 6). Collectively, the findings of the current study suggest that circadian rhythm disruption is associated with cytotoxicity, which progresses from abnormal MMP to oxidative stress and Ca2+ influx (Baev et al. 2022). It is well-established that abnormalities in mitochondrial function induce excessive ROS generation (Guo et al. 2013). Therefore, further mechanistic studies are warranted. The results of in vitro bioluminescence assays using circadian reporter cells may exhibit similar sensitivity to predictors of HCS cytotoxicity assays, potentially indicating a close relationship between cellular rhythm disturbances and cytotoxic mechanisms.
Schematic diagram illustrating the putative mechanistic relationship between circadian genes and cytotoxicity markers in mitochondria. The decrease in MMP and increase in calcium ions levels via the mitochondria-circadian rhythm axis result in circadian rhythm disruption and ROS generation via cell damage, thereby stimulating lipid peroxidation. ROS, reactive oxygen species
The putative interdependent function of PER2 and mitochondria suggests that molecular pathways in mammalian circadian clocks respond to chemicals, leading to dysfunctions, such as reduced MMP and increased calcium ion influx, during the early cell damage process and subsequent oxidative stress. Typically, neural mitochondrial dysfunction and ROS affect the accumulation of lipid droplets in glial cells (Liu et al. 2015). Furthermore, the SIRT3-NAD+ axis reportedly serves as a metabolic link between mitochondrial function and the circadian rhythm (de Goede et al. 2018; Manella and Asher 2016; Sardon Puig et al. 2018). Specifically, the interdependence of circadian rhythm disruption and reduced MMP induced by neurotoxic agents was confirmed by an increase in MMP owing to the restoration of the disrupted circadian rhythm (Kenche et al. 2021). In the current study, changes in PER2 in all tests showed a clear change in the circadian rhythm during the initial phase of the assay, along with a decrease in the MMP. Furthermore, we found that antioxidants (MSCs and NACs) known to reduce neurotoxicity could prevent the loss of cellular circadian rhythms or even restore normal patterns. These results suggest that antioxidant-mediated restoration of the disrupted circadian rhythm is associated with the normalization of the MMP and preventive effect on oxidative stress.
In addition, we identified other associations between alterations in circadian rhythm and intracellular Ca2+ following chemical treatment. The circadian rhythm phase was prolonged when CPF and DDT increased intracellular Ca2+ levels at 24 and 72 h, while CPX increased Ca2+ only at 72 h. These results revealed that neurotoxic chemicals could increase the movement of Ca2+ across the neuronal plasma membrane, enhancing intracellular calcium concentrations [Ca2 +]i (Bondy 1989). Considering persistent molecular rhythms in the suprachiasmatic nucleus, periodic transmembrane flux of Ca2+ due to circadian changes in membrane potential is an important process that functions as a circadian pacemaker (Lundkvist et al. 2005). Therefore, follow-up investigations are warranted to examine the relationship between the cellular circadian rhythm and elevated levels of cytosolic calcium.
In summary, our results suggest that the in vitro circadian reporter bioluminescence cell assay is highly sensitive and could allow the assessment and evaluation of neurotoxicity at trace levels of environmental chemicals, pesticides, and drugs. Furthermore, in this model system, disrupting the circadian rhythm of PER2 using a test item capable of inducing environmental toxicity was associated with mitochondrial toxicity, calcium ions, and oxidative stress in human neural cells (Meyer et al. 2018).
Moreover, we demonstrated that antioxidants could recover or prevent circadian rhythm disruption. Although the relationship between oxidative stress and cellular rhythm has been explored (Mezhnina et al. 2022; Sun et al. 2020), molecular mechanisms underlying the restorative and preventive effects of antioxidants on disrupted cellular rhythms need to be elucidated. If specific molecular mechanisms were identified and confirmed to be active in existing cytotoxicity evaluation methods, a high-throughput circadian rhythm neurotoxicity assay for neurotoxicity screening would be an efficient and appropriate alternative assay for in vivo toxicity tests.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Amo T, Sato S, Saiki S et al (2011) Mitochondrial membrane potential decrease caused by loss of PINK1 is not due to proton leak, but to respiratory chain defects. Neurobiol Dis 41(1):111–118. https://doi.org/10.1016/j.nbd.2010.08.027
Antoch MP, Gorbacheva VY, Vykhovanets O et al (2008) Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle 7(9):1197–1204. https://doi.org/10.4161/cc.7.9.5886
Audira G, Sampurna BP, Juniardi S et al (2019) Establishing simple image-based methods and a cost-effective instrument for toxicity assessment on circadian rhythm dysregulation in fish. Biol Open. https://doi.org/10.1242/bio.041871
Baev AY, Vinokurov AY, Novikova IN, Dremin VV, Potapova EV, Abramov AY (2022) Interaction of Mitochondrial Calcium and ROS in Neurodegeneration. Cells. https://doi.org/10.3390/cells11040706
Bal-Price AK, Suñol C, Weiss DG, van Vliet E, Westerink RH, Costa LG (2008) Application of in vitro neurotoxicity testing for regulatory purposes: Symposium III summary and research needs. Neurotoxicology 29(3):520–531. https://doi.org/10.1016/j.neuro.2008.02.008
Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93(6):929–937. https://doi.org/10.1016/s0092-8674(00)81199-x
Bondy SC (1989) Intracellular calcium and neurotoxic events. Neurotoxicol Teratol 11(6):527–531. https://doi.org/10.1016/0892-0362(89)90032-9
Boughattas NA, Lévi F, Fournier C et al (1989) Circadian rhythm in toxicities and tissue uptake of 1,2-diamminocyclohexane(trans-1)oxalatoplatinum(II) in mice. Cancer Res 49(12):3362–3368
Brown LA, Fisk AS, Pothecary CA, Peirson SN (2019) Telling the Time with a Broken Clock: Quantifying Circadian Disruption in Animal Models. Biology 8(1):18
Carter B, Justin HS, Gulick D, Gamsby JJ (2021) The Molecular Clock and Neurodegenerative Disease: A Stressful Time. Front Mol Biosci 8:644747. https://doi.org/10.3389/fmolb.2021.644747
Cavieres-Lepe J, Ewer J (2021) Reciprocal Relationship Between Calcium Signaling and Circadian Clocks: Implications for Calcium Homeostasis, Clock Function, and Therapeutics. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2021.666673
Chen Z, Yoo SH, Park YS et al (2012) Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A 109(1):101–106. https://doi.org/10.1073/pnas.1118034108
Choi AO, Cho SJ, Desbarats J, Lovrić J, Maysinger D (2007) Quantum dot-induced cell death involves Fas upregulation and lipid peroxidation in human neuroblastoma cells. J Nanobiotechnology 5:1. https://doi.org/10.1186/1477-3155-5-1
Costa LG, Giordano G, Guizzetti M, Vitalone A (2008) Neurotoxicity of pesticides: a brief review. Front Biosci 13:1240–1249. https://doi.org/10.2741/2758
Davidson PW, Myers GJ, Weiss B (2004) Mercury exposure and child development outcomes. Pediatrics 113(4 Suppl):1023–1029
de Goede P, Wefers J, Brombacher EC, Schrauwen P, Kalsbeek A (2018) Circadian rhythms in mitochondrial respiration. J Mol Endocrinol 60(3):R115–R130. https://doi.org/10.1530/JME-17-0196
Fagiani F, Di Marino D, Romagnoli A et al (2022) Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct Target Ther 7(1):41. https://doi.org/10.1038/s41392-022-00899-y
Fang M, Guo WR, Park Y, Kang HG, Zarbl H (2015) Enhancement of NAD(+)-dependent SIRT1 deacetylase activity by methylselenocysteine resets the circadian clock in carcinogen-treated mammary epithelial cells. Oncotarget 6(40):42879–42891. https://doi.org/10.18632/oncotarget.6002
Fang M, Kang HG, Park Y, Estrella B, Zarbl H (2017) In Vitro Bioluminescence Assay to Characterize Circadian Rhythm in Mammary Epithelial Cells. J vis Exp. https://doi.org/10.3791/55832
Fanjul-Moles ML, López-Riquelme GO (2016) Relationship between Oxidative Stress, Circadian Rhythms, and AMD. Oxid Med Cell Longev 2016:7420637. https://doi.org/10.1155/2016/7420637
Fifel K, Videnovic A (2020) Circadian alterations in patients with neurodegenerative diseases: Neuropathological basis of underlying network mechanisms. Neurobiol Dis 144:105029. https://doi.org/10.1016/j.nbd.2020.105029
Fu L, Lee CC (2003) The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer 3(5):350–361. https://doi.org/10.1038/nrc1072
Grill MF, Maganti RK (2011) Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol 72(3):381–393. https://doi.org/10.1111/j.1365-2125.2011.03991.x
Guenthner CJ, Luitje ME, Pyle LA et al (2014) Circadian rhythms of Per2: Luc in individual primary mouse hepatocytes and cultures. PLoS ONE 9(2):e87573. https://doi.org/10.1371/journal.pone.0087573
Guo C, Sun L, Chen X, Zhang D (2013) Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 8(21):2003–2014. https://doi.org/10.3969/j.issn.1673-5374.2013.21.009
Hastings MH, Goedert M (2013) Circadian clocks and neurodegenerative diseases: time to aggregate? Curr Opin Neurobiol 23(5):880–887. https://doi.org/10.1016/j.conb.2013.05.004
Hirota T, Kay SA (2009) High-throughput screening and chemical biology: new approaches for understanding circadian clock mechanisms. Chem Biol 16(9):921–927. https://doi.org/10.1016/j.chembiol.2009.09.002
Hwang JW, Sundar IK, Yao H, Sellix MT, Rahman I (2014) Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J 28(1):176–194. https://doi.org/10.1096/fj.13-232629
Izumo M, Johnson CH, Yamazaki S (2003) Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci U S A 100(26):16089–16094. https://doi.org/10.1073/pnas.2536313100
Izumo M, Sato TR, Straume M, Johnson CH (2006) Quantitative analyses of circadian gene expression in mammalian cell cultures. PLoS Comput Biol 2(10):e136. https://doi.org/10.1371/journal.pcbi.0020136
Jung CH, Kim EM, Park JK et al (2013) Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway. Oncol Rep 29(6):2109–2113. https://doi.org/10.3892/or.2013.2381
Kenche H, Singh M, Smith J, Shen K (2021) Neuronal mitochondrial dysfunction in a cellular model of circadian rhythm disruption is rescued by donepezil. Biochem Biophys Res Commun 567:56–62. https://doi.org/10.1016/j.bbrc.2021.06.029
Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20(14):1868–1873. https://doi.org/10.1101/gad.1432206
Kudo T, Loh DH, Truong D, Wu Y, Colwell CS (2011) Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol 232(1):66–75. https://doi.org/10.1016/j.expneurol.2011.08.003
Lee S, Donehower LA, Herron AJ, Moore DD, Fu L (2010) Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE 5(6):e10995. https://doi.org/10.1371/journal.pone.0010995
Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K (2019) Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 18(3):307–318. https://doi.org/10.1016/S1474-4422(18)30461-7
Li C, Zhang Y, Liu R, Mai Y (2021) Ramelteon ameliorated 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity in neuronal cells in a mitochondrial-dependent pathway. Bioengineered 12(1):4868–4877. https://doi.org/10.1080/21655979.2021.1960767
Liu L, Zhang K, Sandoval H et al (2015) Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160(1–2):177–190. https://doi.org/10.1016/j.cell.2014.12.019
Logan RW, McClung CA (2019) Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci 20(1):49–65. https://doi.org/10.1038/s41583-018-0088-y
Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD (2005) A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci 25(33):7682–7686. https://doi.org/10.1523/jneurosci.2211-05.2005
Manella G, Asher G (2016) The Circadian Nature of Mitochondrial Biology. Front Endocrinol (lausanne) 7:162. https://doi.org/10.3389/fendo.2016.00162
Meijer M, Dingemans MM, van den Berg M, Westerink RH (2014) Inhibition of voltage-gated calcium channels as common mode of action for (mixtures of) distinct classes of insecticides. Toxicol Sci 141(1):103–111. https://doi.org/10.1093/toxsci/kfu110
Meyer JN, Hartman JH, Mello DF (2018) Mitochondrial Toxicity. Toxicol Sci 162(1):15–23. https://doi.org/10.1093/toxsci/kfy008
Mezhnina V, Ebeigbe OP, Poe A, Kondratov RV (2022) Circadian Control of Mitochondria in Reactive Oxygen Species Homeostasis. Antioxid Redox Signal 37(10–12):647–663. https://doi.org/10.1089/ars.2021.0274
Nassan M, Videnovic A (2022) Circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 18(1):7–24. https://doi.org/10.1038/s41582-021-00577-7
National Toxicology P (2021) NTP cancer hazard assessment report on night shift work and light at night. Nation Toxicol Progr Res Triangle Park (NC)
Ndikung J, Storm D, Violet N et al (2020) Restoring circadian synchrony in vitro facilitates physiological responses to environmental chemicals. Environ Int. https://doi.org/10.1016/j.envint.2019.105265
Oakeshott S, Balci F, Filippov I et al (2011) Circadian abnormalities in motor activity in a BAC transgenic mouse model of huntington’s disease. PLoS Curr. https://doi.org/10.1371/currents.RRN1225
O’Brien PJ, Irwin W, Diaz D et al (2006) High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol 80(9):580–604. https://doi.org/10.1007/s00204-006-0091-3
Partch CL, Green CB, Takahashi JS (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol 24(2):90–99. https://doi.org/10.1016/j.tcb.2013.07.002
Richardson JR, Fitsanakis V, Westerink RHS, Kanthasamy AG (2019) Neurotoxicity of pesticides. Acta Neuropathol 138(3):343–362. https://doi.org/10.1007/s00401-019-02033-9
Rivkees SA (2007) The Development of Circadian Rhythms: From Animals To Humans. Sleep Med Clin 2(3):331–341. https://doi.org/10.1016/j.jsmc.2007.05.010
Saini C, Liani A, Curie T et al (2013) Real-time recording of circadian liver gene expression in freely moving mice reveals the phase-setting behavior of hepatocyte clocks. Genes Dev 27(13):1526–1536. https://doi.org/10.1101/gad.221374.113
Salgado-Delgado R, Tapia Osorio A, Saderi N, Escobar C (2011) Disruption of circadian rhythms: a crucial factor in the etiology of depression. Depress Res Treat. https://doi.org/10.1155/2011/839743
Sardon Puig L, Valera-Alberni M, Canto C, Pillon NJ (2018) Circadian Rhythms and Mitochondria: Connecting the Dots. Front Genet 9:452. https://doi.org/10.3389/fgene.2018.00452
Shen Y, Lv Q-k, Xie W-y et al (2023) Circadian disruption and sleep disorders in neurodegeneration. Translational Neurodegeneration 12(1):8. https://doi.org/10.1186/s40035-023-00340-6
Singh N, Lawana V, Luo J et al (2018) Organophosphate pesticide chlorpyrifos impairs STAT1 signaling to induce dopaminergic neurotoxicity: Implications for mitochondria mediated oxidative stress signaling events. Neurobiol Dis 117:82–113. https://doi.org/10.1016/j.nbd.2018.05.019
Slat EA, Sponagel J, Marpegan L et al (2017) Cell-intrinsic, Bmal1-dependent Circadian Regulation of Temozolomide Sensitivity in Glioblastoma. J Biol Rhythms 32(2):121–129. https://doi.org/10.1177/0748730417696788
Sterniczuk R, Dyck RH, Laferla FM, Antle MC (2010) Characterization of the 3xTg-AD mouse model of Alzheimer’s disease: part 1. Circadian Changes Brain Res 1348:139–148. https://doi.org/10.1016/j.brainres.2010.05.013
Stevens M, Peigneur S, Tytgat J (2011) Neurotoxins and Their Binding Areas on Voltage-Gated Sodium Channels. Front Pharmacol. https://doi.org/10.3389/fphar.2011.00071
Sun Q, Yang Y, Wang Z et al (2020) PER1 interaction with GPX1 regulates metabolic homeostasis under oxidative stress. Redox Biol. https://doi.org/10.1016/j.redox.2020.101694
Tolosa L, Pinto S, Donato MT et al (2012) Development of a multiparametric cell-based protocol to screen and classify the hepatotoxicity potential of drugs. Toxicol Sci 127(1):187–198. https://doi.org/10.1093/toxsci/kfs083
Wilsbacher LD, Yamazaki S, Herzog ED et al (2002) Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice invivo. Proc Natl Acad Sci U S A 99(1):489–494. https://doi.org/10.1073/pnas.012248599
Wong A, Cortopassi GA (2002) High-throughput measurement of mitochondrial membrane potential in a neural cell line using a fluorescence plate reader. Biochem Biophys Res Commun 298(5):750–754
Xiao Y, Xiong T, Meng X, Yu D, Xiao Z, Song L (2019) Different influences on mitochondrial function, oxidative stress and cytotoxicity of antibiotics on primary human neuron and cell lines. J Biochem Mol Toxicol. https://doi.org/10.1002/jbt.22277
Yamada S, Kubo Y, Yamazaki D, Sekino Y, Kanda Y (2017) Chlorpyrifos inhibits neural induction via Mfn1-mediated mitochondrial dysfunction in human induced pluripotent stem cells. Sci Rep 7:40925. https://doi.org/10.1038/srep40925
Yang X, Guo M, Wan YJ (2010) Deregulation of growth factor, circadian clock, and cell cycle signaling in regenerating hepatocyte RXRalpha-deficient mouse livers. Am J Pathol 176(2):733–743. https://doi.org/10.2353/ajpath.2010.090524
Yuan Y, Atchison WD (2016) Multiple Sources of Ca2+ Contribute to Methylmercury-Induced Increased Frequency of Spontaneous Inhibitory Synaptic Responses in Cerebellar Slices of Rat. Toxicol Sci 150(1):117–130. https://doi.org/10.1093/toxsci/kfv314
Zerin T, Kim JS, Gil HW, Song HY, Hong SY (2015) Effects of formaldehyde on mitochondrial dysfunction and apoptosis in SK-N-SH neuroblastoma cells. Cell Biol Toxicol 31(6):261–272. https://doi.org/10.1007/s10565-015-9309-6
Zhang Y (2018) Cell toxicity mechanism and biomarker. Clin Transl Med 7(1):34. https://doi.org/10.1186/s40169-018-0212-7
Zheng X, Zhang K, Zhao Y, Fent K (2021) Environmental chemicals affect circadian rhythms: An underexplored effect influencing health and fitness in animals and humans. Environ Int. https://doi.org/10.1016/j.envint.2020.106159
Acknowledgements
This work was supported by the SOT-Colgate Palmolive Grant for Alternative Research (M. Fang) and a U.S. NIEHS grant P30ES005022 (H. Zarbl). In addition, this work was supported by the research fund from the Animal and Plant Quarantine Agency, the Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea (H-G. Kang). Additional support was provided by the Research Institute of Veterinary Science, College of Veterinary Medicine, Seoul National University.
Funding
Open Access funding enabled and organized by Seoul National University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, Y., Kang, HG., Kang, SJ. et al. Combined use of multiparametric high-content-screening and in vitro circadian reporter assays in neurotoxicity evaluation. Arch Toxicol 98, 1485–1498 (2024). https://doi.org/10.1007/s00204-024-03686-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-024-03686-6